Abstract
Cities are growing rapidly worldwide, with over half of the human population living in cities. Amphibians are the most threatened vertebrates on our planet and are particularly vulnerable to the effects of urbanization. While it is known that landscape features and scales are important for amphibians in urban areas, we do not adequately understand how the urban landscape affects diversity patterns, nor have we identified which spatial scale is most appropriate for evaluating how amphibians respond to urban environments. In this study, we examined the relationships between anuran abundance/richness and landscape features at four spatial scales in Shanghai, China. In order to determine the relative importance of landscape variables and the most appropriate spatial scale, a multi-model inference approach was used to evaluate and compare model weighted mean coefficients. Our results show that large spatial scales, i.e., 1500 m and 2000 m, best predicted relative anuran abundance and richness, while the total anuran abundance responded most strongly to landscape variables at smaller scales, i.e., 500 m and 1000 m. Patch richness and the interspersion and juxtaposition index play a large role in predicting the anuran species’ richness and abundance. The abundance of P. nigromaculatus, F. multistriata, and B. gargarizans increased with patch richness. Species richness and total abundance were most strongly related to the interspersion and juxtaposition index. Our research highlights the importance of identifying the most suitable spatial scale in urban environments because not all anuran respond to the same spatial scale. We found that the relationships between anuran relative abundance and species and urban habitat features are not consistent with the prediction of other landscapes (e.g., farmland, forest, and island). Additionally, constructing diverse habitat patches and more neighboring habitats may maintain or improve anuran communities in urbanizing landscapes.
1. Introduction
The continued expansion of urban environments is rapidly increasing worldwide, with more than 50% of the population now living in urban areas [1]. Urbanization is associated with numerous changes to the physical and biotic environments [2,3]. Anthropogenic activities in urban areas alter the structure and function of ecosystems and degrade biodiversity, by modifying land use and landscape heterogeneity [4,5,6]. Decades of research have often focused on pristine habitats or habitats that have undergone little intervention to save the last remnants of wildlife. In recent years, the focus on the changes in urban environments has increased worldwide [7,8,9]. A greater understanding of how environmental characteristics affect biodiversity in urban areas will provide insight into biodiversity conservation and facilitate the design of more sustainable cities.
Landscape heterogeneity is mainly driven by two factors, namely (i) the diversity of habitat types, and (ii) size, number, and configuration of habitat patches; these factors are defined as compositional heterogeneity and configurational heterogeneity [10,11], respectively. Achieving a higher compositional heterogeneity in landscapes by diversifying the crop can support higher levels of biodiversity [12,13]. Fahrig et al. [14] found that a complex spatial pattern (configurational heterogeneity) also has positive relationships with the diversity of birds, plants, and five different arthropod groups in agricultural landscapes. Studies have shown that compositional heterogeneity has a greater impact on biodiversity [15], while changes in configurational heterogeneity have a lower impact on biodiversity [16]. The diversity of plants, animals, and insects in natural areas and agricultural landscapes is often positively associated with landscape heterogeneity [11,14,17,18,19,20,21]. However, it remains uncertain whether landscape heterogeneity has consistent, positive relationships with biodiversity in urban environments.
Among the vertebrates in the world, amphibians appear to be particularly vulnerable to changes in the environment. Urbanization is regarded as a predominant threat to amphibian populations, largely resulting from habitat loss and degradation [22,23]. Shanghai is one of the most urbanized cities in China. The diversity and body condition of amphibians is rapidly declining in this area [24,25]. Due to the expansion of cities and changes in land-use types, remnant wetlands that can support amphibian populations have been considered priority conservation targets [26]. The heterogeneity of urban landscapes can be increased by diversifying remnant habitats, even artificial habitats that were recently created [27]. Identifying how urban landscape patterns relate to amphibian diversity would provide options for maintaining and enhancing amphibian diversity in urban areas.
Scale is a fundamental consideration in ecological and conservation studies, and can influence the results of complex landscape analyses [28]. Previous studies have shown that landscape heterogeneity plays an important role in biodiversity at different scales, and some ecologists found that landscape structures affect metapopulation dynamics and species richness at large scales [19,29]. As few anurans are likely to regularly disperse over a wider area than 2 km, researchers often measure the local habitat land variables to range from 500 to 2000 m radius [30,31,32,33,34]. Particularly in urbanized landscapes, there is no consensus as to which scale is the more appropriate, and the validity of the scales is unknown. We explored a new methodology to evaluate the impact of landscape metrics at different scales on anuran abundance or species richness in urban areas. Our objectives were to (i) identify the spatial scale that is most appropriate for predicting abundance patterns, and (ii) examine the relationships between anuran relative abundance and landscape heterogeneity in urban environments.
2. Methods
2.1. Study Sites
Shanghai is the largest city in East China, with a total population of approximately 25 million. At present, 80% of the city’s population resides in both urban and suburban areas. Shanghai is located on an alluvial plain formed by natural deposition of the Yangtze River. This region has many rivers, canals, streams, wetlands, and lakes, which serve as the primary habitats for breeding anurans. As a result of Shanghai’s urbanization, the native vegetation has been destroyed, leaving the final breeding habitats of amphibians limited to the ponds in urban parks and green areas, the temporary wetlands formed by rainfall, and the roadside drainage ditches and rice fields in the suburbs. These remaining wetlands support some amphibian populations in Shanghai.
All potential wetlands to be surveyed were marked in Google Earth Pro (June 2015; Google). The final survey sites were selected based on two criteria. First, considering the results of earlier studies [31,35], this study chose wetlands that were more than 2 km apart from each other. Second, wetland candidates were strictly limited to semi-permanent or permanent ponds with at least 50% of open water; most urban wetlands fall into this category. In contrast, temporary ponds and wetlands with excessively dense aquatic vegetation may increase the collinearity between habitats and landscape features.
We chose a 1 km radius as the landscape size because this is considered a reasonable size to represent the average dispersal and migration movements for amphibians [36,37], and landscape variables have been shown to affect anuran occupancy and diversity at this scale in agriculture-dominated regions [36,38,39,40]. Forty-four wetlands were selected in Shanghai as the study sites; these were distributed in various areas and subjected to different degrees of urbanization (Figure 1, Table S1).
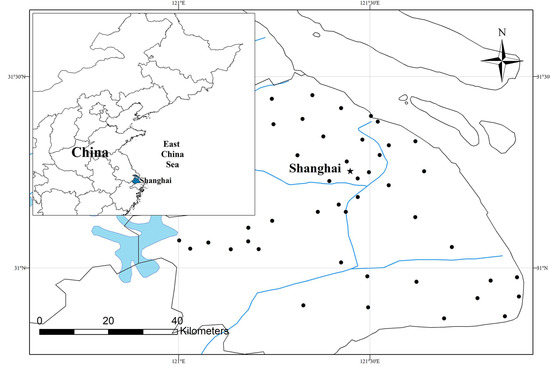
Figure 1.
Map of the study area in Shanghai. Solid dots indicate the location of 44 survey wetlands. Map shows the major rivers and lakes near the study sites in blue.
2.2. Amphibian Surveys
We conducted a series of surveys from April through August 2018 to cover the breeding seasons of most anurans present in Shanghai [41]. Survey methods followed Zhang et al. [25]. Surveys were conducted at least 0.5 h after sunset (at 19:00–24:00). We established a transect that was 5 m wide and at least 500 m long along the pond edge. Visual encounter surveys were performed to detect anurans [42]. Three people searched along the pond perimeter with spotlights and headlamps. We walked along the pond edge at a steady speed (1 km/h). We recorded the species, number of individuals (adults only), location, and the observed time. We surveyed each wetland on three different nights. We surveyed points in a randomized order.
Anuran species richness was the total number of anuran species detected at each site. The anuran population densities of anurans at each site were used to estimate their relative abundances according to the following equation:
where Di represents the population density of anuran at site i, Nik is the number of anuran individuals at site i in survey k, Li is the length of transect at site i, and Ki is the total number of surveys at site i [25].
In some cases, accounting for detection probability is important to avoid bias in the estimation of site occupancy [43,44]. Based on survey effort, we used site-occupancy modeling to assess anuran occurrence, and used a simple model of constant probability of occupancy and constant probability of detection [44,45]. The occupancy and detection probability of each species were estimated using the program PRESENCE 11.5 [46]. According to the amphibian survey results, a habitat occupancy model was adopted to estimate the presence of amphibians. In this simple model, the occupancy probability and detection probability took constant values that were estimated using the software PRESENCE 11.5. The detection probability was used to calculate the minimum number of surveys required to find each type of amphibian at a 95% confidence level [47], and the calculation formula was as follows:
where Nmin represents the minimum number of surveys, which can be derived by using the detection probability p and the formulas given by Pellet and Schmidt (2005).
2.3. Landscape Variables
The remote sensing images of FORMOSAT-2 were used as a data source to fuse panchromatic remote sensing images (June 2012; 2-m resolution) and multi-spectral remote sensing images (8-m resolution). The fused remote sensing image (2-m resolution) was used as the basis for classification of habitat landscapes. Exelis Visual Information Solutions (ENVI 5.1) was used to classify the landscapes at all amphibian survey sites and at the surrounding buffer zones over four different scales using the object-oriented image classification approach. Appropriate thresholds were first set via visual interpretation and field surveys as well as Google Earth images, and then the thresholds were used to divide landscapes at different scales and merge the same objects. After image segmentation and merging, image classification was performed, dividing the landscapes into eight types: (i) rivers; (ii) roads; (iii) buildings (all hard surface areas except roads, including residential buildings, hardened ground surfaces between buildings, parking lots, squares, and factories, as well as some urban infrastructure); (iv) ponds (immobile waters of small areas); (v) lakes (man-made lakes and natural lakes of large areas); (vi) farmland (land used for rice fields, for greenhouse cultivation, for planting Zizania latifolia, and for the cultivation of annual crops); (vii) forest land (parkland and urban green spaces, roadside shelterbelts, and orchards); (viii) grassland (land covered by herbaceous vegetation) (e.g., Figure 2).
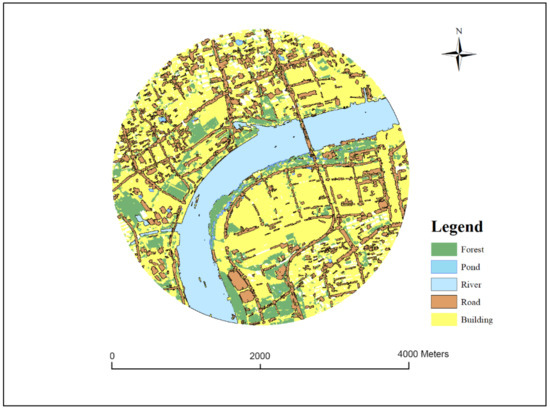
Figure 2.
Example land-use map of one of the 442 km radius urban landscapes in Shanghai, China.
Upon landscape classification, ENVI 5.1 was used to create four circular buffer zones (500 m, 1000 m, 1500 m, and 2000 m) centered at the midpoint of each survey line. The landscape classification results in each buffer zone were extracted using the Area of Interest tool in ENVI 5.1 and exported as raster files. Landscape structural variables at each landscape scale were calculated using the software FRAGSTATS 4.2 [48].
Our two primary landscape variables were urban compositional and configurational heterogeneity. We measured compositional heterogeneity as diversity metrics (patch richness and Shannon evenness index) of urban land types in each landscape. We measured configurational heterogeneity as area and aggregation index in each landscape (Table 1). According to the results of Fahrig et al. [11] for agricultural landscapes, we predicted that anuran species richness and abundance would benefit from diversifying land use and complex spatial pattern in urban landscapes.

Table 1.
Landscape metrics calculated by FRAGSTATS 4.2 landscape analysis software as described by McGarigal et al. [48]. This table represents a subset (8 out of 15 metrics) that was found to not be highly correlated (|r| < 0.7).
2.4. Statistical Analysis
The response variables were: (i) the species richness, i.e., the total number of anuran species detected at each survey site; (ii) the anuran abundance at each site, i.e., each species’ density of anurans at each site; and (iii) the total anuran abundance at each site, i.e., the total anuran densities at each site. The predictor variables were patch density, largest patch index, mean patch size, Euclidean nearest neighbor distance, interspersion and juxtaposition index, Connectance index, patch richness, and Shannon’s evenness index for each landscape (refer to Table 1).
Spearman correlation analysis was performed on all landscape variables to obtain a pairwise correlation matrix for all predictors. It is generally considered that |r| = 0.7 is an appropriate maximum collinearity threshold [49]. To further quantify collinearity, variance inflation factors (VIFs) were also calculated for each predictor. Generally, VIF > 5 indicates severe collinearity [50].
Multiple linear regression was used to evaluate which predictor variables were the best predictors of anuran species richness and relative abundance at various spatial scales. The best models were selected according to Akaike’s information criterion corrected (AICc) for small sample sizes [51]. A multi-model inference approach was utilized to calculate and compare standardized model-weight mean coefficients to determine the direction and relative importance of the predictor variable on response variables. The difference between model i and the model with the smallest AICc value (Δi) was used to rank each model. The global model of each response variable which included all five predictor variables was used to perform model selection for each landscape spatial scale. Each candidate model represents a biologically relevant hypothesis. Therefore, all possible models were estimated, and model-averaged across estimates from all models containing that coefficient. All statistical analyses and data manipulations were performed in the R environment [52]. We performed model selection and averaging using the package MuMIn [53].
3. Results
A total of 44 habitat ponds and Bufo were surveyed. Bufo gargarizans was distributed in 97.7% of the ponds; Pelophylax nigromaculatus and Pelophylax plancyi were distributed in 90.9% the ponds; Fejervarya multistriata was distributed in 84.1% of the ponds; Microhyla fissipes was distributed in 40.9% of the ponds; Kaloula borealis was only distributed in three ponds (Table 2). Two surveys were enough to ensure that the species Bufo gargarizans, Pelophylax plancyi, Pelophylax nigromaculatus, and Fejervarya multistriata could be detected, while four surveys were required to ensure the detection of Microhyla fissipes. The model-estimated occupancy probability was slightly higher than the naïve occupancy and was within the error range (Table 2), indicating that the actual observations were close to the estimates. Thus, we can assume that detection bias has minimal or negligible effects in this study.

Table 2.
Summary results of the estimated probability of occupancy, detection probabilities under constant probability of occupancy and detection.
Pairwise correlation showed that the correlations between landscape variables were different at different landscape scales (Table S2). After excluding strongly correlated landscape variables (|r| > 0.7), a total of eight independent landscape variables were included for analysis, but not all landscape variables were investigated as predictor variables at the same scale (Table S3).
For the abundance of P. nigromaculatus, model selection identified a top model and multiple equivalent models at different scales. The top models (based on Wi) indicated that landscape metrics within 500 m, 1500 m, and 2000 m could predict the abundance of P. nigromaculatus. The model selection results indicate that PR was the most important predictor, as it was present in every model in the top set (Table S4a). The model-averaged coefficients and 95% confidence intervals identified PR as the only significant predictor for the abundance of P. nigromaculatus, with one exception: a 500 m radius (Figure 3, Table S5).
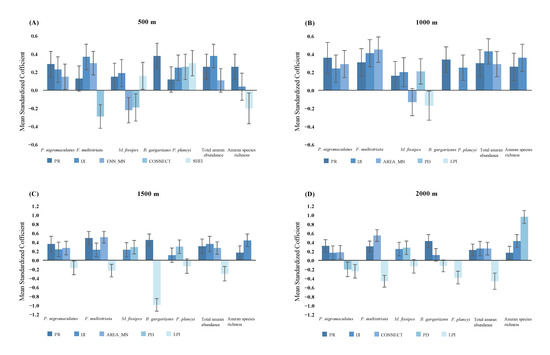
Figure 3.
Model-weighted mean standardized coefficients and 95% confidence intervals showing the direction and relative magnitude of the effects of landscape variables (PR, IJI, ENN_MN, CONNECT, SHEI) on 5 anuran species, anuran species richness and abundance at different radii in urban landscapes in Shanghai. All possible models were estimated, using generalized linear modeling. Each coefficient was model-averaged across estimates from all models containing that coefficient. Note that landscape variables differed between the models at different radii. (A) 500 m radius; (B) 1000 m radius; (C) 1500 m radius; (D) 2000 m radius.
The best supported models suggested that landscape metrics within 2000 m best predicted the abundance of F. multistriata. Additionally, the model had the best reliability in this study (2000 m: Wi = 0.46; Figure 3D, Table S4b) within a radius of 2000 m. CONNECT and PR were significantly positively correlated with F. multistriata, while LPI was significantly negatively correlated with the number of F. multistriata (Figure 3D, Table S5).
Compared to the model sets for all spatial scales, landscape metrics within 1500 m poorly predicted the abundance of M. fissipes, but the top models were weak, with little differences in the weight of the best model among four spatial scales (Table S4c). Moreover, there were no significant correlations between landscape variables and the abundance of M. fissipes at any spatial scale (Figure 3, Table S5). The top models indicated that the landscape metrics obtained from four scales could predict the abundance of B. gargarizans (500 m: Wi = 0.32; 1000 m: Wi = 0.28; 1500 m: Wi = 0.32; 2000 m: Wi = 0.28). The model selection results indicate that PR was the most important predictor, as it was present in every model in the top set (Table S4d). The model selection results indicate that landscape variables could better predict the abundance of P. plancyi within a radius of 2000 m than that within other radii (Table S4e). The model-averaged coefficients and 95% confidence intervals identified that SHEI was significantly positively correlated with P. plancyi within a radius of 500 m, while LPI was significantly negatively correlated with P. plancyi within a radius of 2000 m (Figure 3A,D, Table S5).
The model selection results indicate that landscape variables best predicted the total anuran abundance within 500 m or 1000 m (500 m: Wi = 0.25; 1000 m: Wi = 0.27; Table S4f). IJI and PR were present in every model in the top set, with the exception of the 2000 m scales. IJI was significantly positively correlated with the total anuran abundance within 500 m, 1000 m, and 1500 m, while LPI displays a significant negative correlation within 2000 m.
The number of anuran species could be better predicted by landscape variables within 1500 m and 2000 m (Figure 3C,D). IJI was present in every model in the top set, and it was the most important predictor. IJI also was significantly positively correlated with the total number of anuran species at four scales.
4. Discussion
The results confirmed that factors at multiple spatial scales influence patterns of anuran abundance. The abundance and richness of anurans showed strong relationships with larger scales (1500 m and 2000 m) (Figure 3C,D), while the total anuran abundance responded most strongly to variables at smaller scales (500 m and 1000 m) (Figure 3C,D). Previous studies suggested that scales vary widely among studies. Many ecologists believe that distances from 200 m to 10 km are appropriate scales for examining relationships between anuran species and landscape feature [54,55]. Due to their low dispersal ability, most studies assumed that 1 km (or <l km) spatial scales were identified to be the most appropriate to measure the impact of agricultural and urban landscapes on anurans [21,23,31,56]. However, our results are not consistent with the hypotheses in the literature. Anurans were best predicted by variables at the 1500 m and 2000 m spatial scales, an outcome that is likely due to differences among species or geographical areas. In light of the previous findings, we can only speculate as to why our results are different. The current literature on urbanization and amphibians has a strong geographic bias, and the overwhelming majority of studies have been conducted in North America, Europe, and Australia [22,57]. China is rapidly urbanizing, but the spatial scale at which urbanization impacts amphibians is unknown outside of China. Thus, the differences may be explained by regional and species-specific heterogeneity. It could likely be that large spatial scales could be better predictors of the abundance/richness of anurans in this area.
Whatever the case, most researchers assume that population transitions in anurans occur within areas in a radius of 1 km and tailor the spatial scale for their measurement of the environment accordingly [31,58]. Our study in urban areas is surprising, despite the early studies suggesting that the landscape changes and environmental variables at far greater scales influence anuran species diversity [54,59]. We hypothesized that this is caused by a combination of two factors. On the one hand, in cities, anurans may adapt to fragmentation by developing better dispersal abilities, as has been shown for an “urban adapter”. On the other hand, cities may preselect for species with good dispersal abilities. This idea has mostly been studied for birds and invertebrates, but yielded ambiguous results, probably largely due to the difficulties in classifying movement behavior [60,61]. In addition, the model selection results of B. gargarizans revealed small differences at different spatial scales. This could be because the Zhoushan toad (B. gargarizans) is known to be mobile, and to use a wide range of human-modified habitats, such as gardens, crop fields and artificial wetlands [62]. Our study and that of Browne et al. [55] suggest that habitat land-use changes at surprisingly large spatial scales, i.e., 5 km, which influences variation in anuran diversity. We suggest that wetland buffers protect the terrestrial habitat for amphibians within 2000 m in Shanghai. For future studies in Shanghai, a wider range of spatial scales, for example, local (<1 km) and landscape (1–10 km) is necessary to fully understand diversity patterns and define an appropriate landscape scale for the study of amphibians.
We examined the relationship between urban patch richness and anuran diversity. We found that compositional heterogeneity was significantly positively related to anuran relative abundance (Table S5). The abundance of P. nigromaculatus, F. multistriata, and B. gargarizans increased with PR at different landscape scales. The Shannon evenness index (SHEI) showed a significant correlation with anuran richness and abundance, with one exception: P. plancyi (Table S5). There are no studies describing the relationships between patch richness and Shannon evenness index, and anuran richness and abundance at present. Studies of other species have shown that the compositional heterogeneity of the landscape has a positive effect on biodiversity. For example, research on the diversity of butterfly communities revealed the phenomenon of landscape complementation [15,63]. Different land-use types provide different complementary resources for organisms to meet their needs at different life history stages [64]. The higher compositional heterogeneity should result in higher anuran richness and abundance in farmlands, which should provide a more temporally stable prey resource for anurans. However, different species from different habitat landscapes may respond differently. Collins and Fahrig [21] found that a higher compositional heterogeneity did not support higher anuran richness and abundance in farmlands with more diverse crops, while Li et al. [26] found that compositional heterogeneity was significantly positively correlated with anuran species richness in urban parks. Our results are consistent with the prediction that anuran abundance is positively associated with patch richness in urban landscapes. As anurans require aquatic and terrestrial habitats during their complex life cycles [65,66], a higher PR provides a complementary and switchable habitat environment that is beneficial to the survival and reproduction of anurans, especially in urban landscapes. An alternative explanation may be that some anuran species (e.g., toads) develop better dispersal abilities or adapt to urban environments. They can move around in urban landscapes, and benefit from the prey resources provided by the use of different land types.
We found that mean patch size (AREA_MN) is positively associated with anuran total abundance (Figure 3B,C). However, to our knowledge, no one has investigated the relationship between mean patch size and anuran abundance in urban landscapes. However, studies have reported negative effects of crop field size on birds [12], anurans [21], and invertebrates [13]. Li et al. [26] found that landscape configurational heterogeneity has a significant positive effect on anuran abundance in urban parks. Some scholars believe that species benefit from smaller field sizes as this offers easier access to field edge habitats (i.e., landscape complementation) which facilitates movement through the landscape [13,14,21] (Fahrig et al. 2015, Novotný et al. 2015, Collins and Fahrig 2017). Our results are not consistent with the prediction that high configurational heterogeneity (low mean patch size) likely facilitate anurans. The dispersal ability of most known anuran species was low. Therefore, it is very difficult to move around in urban landscapes, and species are not likely to benefit from the prey resources provided by different anuran land types. An anuran species can occur in urban landscapes determined by the size of the remaining habitat. Accessible refuge habitats in urban landscapes typically play the role of “islands”, and larger areas generally contain more anurans. Similar to our reasoning for anurans, Lima et al. [67] and Almeidagomes et al. [68] found that larger forest remnants and islands have higher levels of amphibian diversity.
The proportion of the largest patch (LPI) was significantly negatively correlated with F. multistriata and total anuran abundance at the 2000 m scale (Figure 3D, Table S5). Species richness and abundance were most strongly related to the amount of the interspersion and juxtaposition index (IJI), but they were positively correlated with anuran species richness. In urban environments, the largest patches are usually buildings, roads, and impervious surfaces in the city. Therefore, LPI usually reflects the degree of urbanization and anthropogenic disturbance in this study. According to previous studies, F. multistriata that prefer to live in crop fields decline with increasing urban density, and the total anuran abundance exhibits a negative relationship with urban density [25,69]. Anurans could benefit from a higher IJI due to there being more neighboring habitats. A similar reasoning applies to the other cases, such as Fahrig et al. [14] and Novotný et al. [13], who hypothesize that smaller field sizes facilitate movement through the landscape, because species have easier access to neighboring habitats.
Due to the fact that this is a correlational study, the relationships that we found are not necessarily driven by the mechanisms we hypothesized. There is also the possibility of unmeasured confounding factors that are representative of true causal relationships. These sites in the center of Shanghai have higher pesticides, noise, and light exposure; therefore, the positive effect of configurational heterogeneity on the anuran abundance we observed may be indirectly caused by an associated increase in these practices. Amphibians are known to be negatively affected by exposure to pesticides, as well as artificial noise and light [70,71,72]. It is unclear to us whether amphibian populations and pesticides, noise, and light exposure are related in our study region, but human activities like these are associated with altered frog sex ratios, hatching success and susceptibility stressors. We propose the following for future studies on amphibians in urban areas. Studies should consider identifying and quantifying the relative impacts of human activities and landscape heterogeneity in urban amphibian populations, which will provide a better understanding of the relation between complexity and mechanism. Fully understanding the complexity of these impacts is fundamental for outlining the priority management and conservation actions needed to preserve urban amphibian biodiversity.
We were surprised to find that the large spatial scales provide a better explanation of which factors influenced the variation in anuran species’ communities. Based on our results, we suggest that the most suitable landscape scale to assess the relationship between anurans and landscape variables varies among species, which can mainly be attributed to differences in dispersal abilities or environmental filtering. Our results have important implications for the management of urban landscapes for anuran conservation. We found that anuran richness and abundance were most strongly related to landscape variables within 1500 m or 2000 m, suggesting that the spatial scales are critical for maintaining or increasing urban anuran richness and abundance. In contrast, the maximum government-recommended wetland buffer size (300 m) in Ontario is far less than our results. Sawatzky et al. [73] suggested that the buffers are too narrow to encompass the landscape context anurans experience, and have limited benefits for many anurans. We recommend that the relevant government departments create wetland buffers that protect terrestrial habitat for amphibians within 2000 m.
Many have confirmed the importance of landscape heterogeneity for biodiversity in agricultural landscapes, and a mechanistic understanding of the impact of landscape heterogeneity in urban areas. Is habitat fragmentation good or bad for biodiversity? Different scholars have different views [57,74]. Though the topic is presently being debated, our results support that compositional heterogeneity and configurational heterogeneity could benefit anuran diversity in urban areas. These data provide important insights that will be useful in the controversy. Furthermore, we suspect that constructing diverse habitats, more neighboring habitats, and fragmenting impervious surface in urban areas may be an effective management strategy for maintaining and increasing anuran biodiversity. However, future research is needed to evaluate other mechanisms, especially those acting at landscape scales (e.g., increased habitat diversity, spreading of risk, landscape complementation), and a large-scale pattern.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14110968/s1, Table S1: Latitude and longitude coordinates and degree of urbanization for 44 study sites; Table S2: Correlation coefficient matrices for 15 landscape variables used to quantify the relationships between anuran communities and the urban environment of 44 wetlands in Shanghai. Landscape matrices as described by McGarigal et al. [48]; Table S3: Correlation coefficient matrices for eight independent landscape variables at different spatial scales; Table S4: The best models (Δi ≤ 2) examining relationships between multi-scale landscape variables and the relative abundances of five anuran species, total anuran relative abundance, and anuran species richness in 44 urban ponds in Shanghai. The best models predicting P. nigromaculatus are shown in Table S4a; the best models predicting F. multistriata are shown in Table S4b; the best models predicting M. fissipes are shown in Table S4c; the best models predicting B. gargarizans are shown in Table S4d; the best models predicting P. plancyi are shown in Table S4e; the best models predicting total anuran abundance are shown in Table S4f; the best models predicting anuran species richness are shown in Table S4g; Table S5: Model-averaged coefficients and 95% confidence intervals showing the direction relative magnitude of the effects of landscape variables on anuran relative abundance, and species at different spatial scales in urban landscapes in Shanghai. All possible models were estimated, using generalized linear modeling. Each coefficient was model-averaged across estimates from all models containing that coefficient. Bold font indicates p < 0.05.
Author Contributions
Conceptualization, W.Z. and G.Y.; Data curation, W.Z. and B.L.; Formal analysis, G.Y.; Funding acquisition, W.Z. and B.L.; Investigation, W.Z. and B.L.; Methodology, W.Z.; Project administration, W.Z.; Resources, W.Z.; Software, G.Y.; Supervision, G.Y.; Validation, W.Z.; Visualization, G.Y.; Writing—Original draft, W.Z.; Writing—Review and editing, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the National Natural Science Foundation of China (No. 31800350, 31901099), Science and Technology Commission of Shanghai Municipality (No. 20ZR1437100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to the fact that the project through which the research was funded is not yet completed.
Acknowledgments
We warmly thank Yingmin Mo for providing help in calculating land use, Xiaoxiao Shu in East China Normal University for their assistance with fieldwork. We thank Yuyi Liu and Shunqi, Bo from Shanghai Wild Plant and Animal Protection, Department for Issuance of a Research Permit. We are also grateful to the managers of urban parks and wetlands, and the private landowners involved in this study, without which this work would not have been possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations, DEAS. World Urbanization Prospects: The 2014 Revision; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2015. [Google Scholar]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.J.; MacGregor-Fors, I. The ecological future of cities. Science 2016, 352, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, S.; Lin, J.; Li, H.; Lin, Q.; Han, B. Urbanization increases biotic homogenization of zooplankton communities in tropical reservoirs. Ecol. Indic. 2020, 110, 105899. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Seto, K.C.; Sánchez-Rodríguez, R.; Fragkias, M. The New Geography of Contemporary Urbanization and the Environment. Annu. Rev. Environ. Resour. 2010, 35, 167–194. [Google Scholar] [CrossRef]
- Dobbs, C.; Nitschke, C.R.; Kendal, D. Assessing the drivers shaping global patterns of urban vegetation landscape structure. Sci. Total Environ. 2017, 592, 171–177. [Google Scholar] [CrossRef]
- Duelli, P. Biodiversity evaluation in agricultural landscapes: An approach at two different scales. Agric. Ecosyst. Environ. 1997, 62, 81–91. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Lindsay, K.; Kirk, D.A.; Bergin, T.M.; Best, L.B.; Sifneos, J.C.; Smith, J. Farmland Heterogeneity Benefits Birds in American Mid-west Watersheds. Am. Midl. Nat. 2013, 170, 121–143. [Google Scholar] [CrossRef]
- Novotný, D.; Zapletal, M.; Kepka, P.; Benes, J.; Konvicka, M. Large moths captures by a pest monitoring system depend on farmland heterogeneity. J. Appl. Entomol. 2015, 139, 390–400. [Google Scholar] [CrossRef]
- Fahrig, L.; Girard, J.; Duro, D.C.; Pasher, J.; Smith, A.C.; Javorek, S.; King, D.J.; Lindsay, K.F.; Mitchell, S.; Tischendorf, L. Farmlands with smaller crop fields have higher within-field biodiversity. Agric. Ecosyst. Env. 2015, 200, 219–234. [Google Scholar] [CrossRef]
- Dunning, J.B.; Danielson, B.J.; Pulliam, H.R. Ecological processes that affect populations in complex landscapes. Oikos 1992, 65, 169–175. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological responses to habitat edges: Mechanisms, models, and variability explained. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Zeleny, D.; Li, C.F.; Chytry, M. Pattern of local plant species richness along a gradient of landscape topographical heterogeneity: Result of spatial mass effect or environmental shift? Ecography 2010, 33, 578–589. [Google Scholar] [CrossRef]
- Ekroos, J.; Kuussaari, M.; Tiainen, J.; Heliola, J.; Seimola, T.; Helenius, J. Correlations in species richness between taxa depend on habitat, scale and landscape context. Ecol. Indic. 2013, 34, 528–535. [Google Scholar] [CrossRef]
- Perovic, D.; Gamez-Virues, S.; Borschig, C.; Klein, A.M.; Krauss, J.; Steckel, J.; Rothenwohrer, C.; Erasmi, S.; Tscharntke, T.; Westphal, C. Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J. Appl. Ecol. 2015, 52, 505–513. [Google Scholar] [CrossRef]
- Collins, S.J.; Fahrig, L. Responses of anurans to composition and configuration of agricultural landscapes. Agric. Ecosyst. Env. 2017, 239, 399–409. [Google Scholar] [CrossRef]
- Hamer, A.J.; McDonnell, M.J. Amphibian ecology and conservation in the urbanising world: A review. Biol. Conserv. 2008, 141, 2432–2449. [Google Scholar] [CrossRef]
- Smallbone, L.T.; Luck, G.W.; Wassens, S. Anuran species in urban landscapes: Relationships with biophysical, built environment and socio-economic factors. Landsc. Urban Plan. 2011, 101, 43–51. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Shu, X.X.; Pei, E.L.; Yuan, X.; Sun, Y.J.; Wang, T.H.; Wang, Z.H. The Impacts of Urbanization on the Distribution and Body Condition of the Rice-paddy Frog (Fejervarya multistriata) and Gold-striped Pond Frog (Pelophylax plancyi) in Shanghai, China. Asian Herpetol. Res. 2016, 7, 200–209. [Google Scholar]
- Zhang, W.; Li, B.; Shu, X.X.; Pei, E.L.; Yuan, X.; Sun, Y.J.; Wang, T.H.; Wang, Z.H. Responses of anuran communities to rapid urban growth in Shanghai, China. Urban For. Urban Green. 2016, 20, 365–374. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Shu, X.; Pei, E.; Yuan, X.; Wang, T.; Wang, Z. Influence of breeding habitat characteristics and landscape heterogeneity on anuran species richness and abundance in urban parks of Shanghai, China. Urban For. Urban Green. 2018, 32, 56–63. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, W.; Li, B.; Li, B.; Zhang, Y.; Wang, T. Construction technology of amphibian habitat and the evaluation of its effectiveness. Chin. J. Appl. Ecol. 2018, 29, 2771–2777. [Google Scholar]
- Sodhi, N.S.; Butler, R.; Laurance, W.F.; Gibson, L. Conservation successes at micro-, meso- and macroscales. Trends Ecol. Evol. 2011, 26, 585–594. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Luna, J.M. Multi-function agricultural biodiversity: Pest management and other benefits. Basic Appl. Ecol. 2003, 4, 107–116. [Google Scholar] [CrossRef]
- Gagne, S.A.; Fahrig, L. Effect of landscape context on anuran communities in breeding ponds in the National Capital Region, Canada. Landsc. Ecol. 2007, 22, 205–215. [Google Scholar] [CrossRef]
- Gagne, S.A.; Fahrig, L. Effects of time since urbanization on anuran community composition in remnant urban ponds. Environ. Conserv. 2010, 37, 128–135. [Google Scholar] [CrossRef]
- Hamer, A.J.; Parris, K.M. Local and landscape determinants of amphibian communities in urban ponds. Ecol. Appl. 2011, 21, 378–390. [Google Scholar] [CrossRef]
- Kruger, D.J.D.; Hamer, A.J.; Du Preez, L.H. Urbanization affects frog communities at multiple scales in a rapidly developing African city. Urban Ecosyst. 2015, 18, 1333–1352. [Google Scholar] [CrossRef]
- Hamer, A.J. Accessible habitat delineated by a highway predicts landscape-scale effects of habitat loss in an amphibian community. Landsc. Ecol. 2016, 31, 2259–2274. [Google Scholar] [CrossRef]
- Pillsbury, F.C.; Miller, J.R. Habitat and landscape characteristics underlying anuran community structure along an urban–rural gradient. Ecol. Appl. 2008, 18, 1107–1118. [Google Scholar] [CrossRef]
- Guerry, A.D.; Hunter, M.L. Amphibian distributions in a landscape of forests and agriculture: An examination of landscape composition and conf iguration. Conserv. Biol. 2002, 16, 745–754. [Google Scholar] [CrossRef]
- Wagner, N.; Züghart, W.; Mingo, V.; Lötters, S. Are deformation rates of anuran developmental stages suitable indicators for environmental pollution? Possibilities and limitations. Ecol. Indic. 2014, 45, 394–401. [Google Scholar] [CrossRef]
- Knutson, M.G.; Sauer, J.R.; Olsen, D.A.; Mossman, M.J.; Hemesath, L.M.; Lannoo, M.J. Effects of Landscape Composition and Wetland Fragmentation on Frog and Toad Abundance and Species Richness in Iowa and Wisconsin, U.S.A. Conserv. Biol. 1999, 13, 1437–1446. [Google Scholar] [CrossRef]
- Van Buskirk, J. Local and landscape influence on amphibian occurrence and abundance. Ecology 2005, 86, 1936–1947. [Google Scholar] [CrossRef]
- Vos, C.C.; Stumpel, A.H.P. Comparison of habitat-isolation parameters in relation to fragmented distribution patterns in the tree frog (Hyla arborea). Landsc. Ecol. 1996, 11, 203–214. [Google Scholar] [CrossRef]
- Fei, L.; Hu, S.; Ye, C.; Huang, Y. Fauna Sinica. In Amphibia Vol. 2 Anura; Science Press: Beijing, China, 2009. [Google Scholar]
- Crump, M.L.; Scott, N.J. Visual encounter survey. In Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Heyer, W.R., Donnelly, M.A., McDiarmid, R.W., Hayek, L.C., Foster, M.S., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1994; pp. 84–92. [Google Scholar]
- Mazerolle, M.J.; Desrochers, A.; Rochefort, L. Landscape characteristics influence pond occupancy by frogs after accounting for detectability. Ecol. Appl. 2005, 15, 824–834. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Moreno, M.; Lele, S.R. Improved estimation of site occupancy using penalized likelihood. Ecology 2010, 91, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.E. PRESENCE2-Software to Estimate Patch Occupancy and Related Parameters. USGS-PWRC: Laurel, MS, USA, 2006. [Google Scholar]
- Pellet, J.; Schmidt, B.R. Monitoring distributions using call surveys: Estimating site occupancy, detection probabilities and inferring absence. Biol. Conserv. 2005, 123, 27–35. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 15 June 2021).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- O’Brien, C.M. Analysing Ecological Data; Zuur, A.F., Ieno, E.N., Smith, G.M., Eds.; Springer: New York, NY, USA, 2007; Volume 75, pp. 426–427. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer Science & Business Media: Berlin, Germany, 2003. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. Available online: http://CRAN.R-project.org/package=MuMIn (accessed on 1 September 2022).
- Gibbs, J.P.; Whiteleather, K.K.; Schueler, F.W. Changes in frog and, toad populations over 30 years in New York State. Ecol. Appl. 2005, 15, 1148–1157. [Google Scholar] [CrossRef]
- Browne, C.L.; Paszkowski, C.A.; Foote, A.L.; Moenting, A.; Boss, S.M. The relationship of amphibian abundance to habitat features across spatial scales in the Boreal Plains. Ecoscience 2009, 16, 209–223. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Paszkowski, C.A. Amphibian use of urban stormwater wetlands: The role of natural habitat features. Landsc. Urban Plan. 2013, 113, 139–149. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Knutson, M.G.; Richardson, W.B.; Reineke, D.M.; Gray, B.R.; Parmelee, J.R.; Weick, S.E. Agricultural ponds support amphibian populations. Ecol. Appl. 2004, 14, 669–684. [Google Scholar] [CrossRef]
- Houlahan, J.E.; Findlay, C.S. The effects of adjacent land use on wetland amphibian species richness and community composition. Can. J. Fish. Aquat. Sci. 2003, 60, 1078–1094. [Google Scholar] [CrossRef]
- Evans, K.L.; Chamberlain, D.E.; Hatchwell, B.J.; Gregory, R.D.; Gaston, K.J. What makes an urban bird. Glob. Change Biol. 2011, 17, 32–44. [Google Scholar] [CrossRef]
- Turrini, T.; Knop, E. A landscape ecology approach identifies important drivers of urban biodiversity. Glob. Change Biol. 2015, 21, 1652–1667. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Wang, I.J.; Comes, H.P.; Peng, H.; Qiu, Y.-X. Contributions of historical and contemporary geographic and environmental factors to phylogeographic structure in a Tertiary relict species, Emmenopterys henryi (Rubiaceae). Sci. Rep. 2016, 6, 24041. [Google Scholar] [CrossRef]
- Devictor, V.; Jiguet, F. Community richness and stability in agricultural landscapes: The importance of surrounding habitats. Agric. Ecosyst. Environ. 2007, 120, 179–184. [Google Scholar] [CrossRef]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batary, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes—eight hypotheses. Biol. Rev. Camb. Philos. Soc. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Bodie, J.R. Biological Criteria for Buffer Zones around Wetlands and Riparian Habitats for Amphibians and Reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Lima, J.R.; Galatti, U.; Lima, C.; Faveri, S.B.; Vasconcelos, H.L.; Neckeloliveira, S. Amphibians on Amazonian Land-Bridge Islands are Affected More by Area Than Isolation. Biotropica 2015, 47, 369–376. [Google Scholar] [CrossRef]
- Almeida-Gomes, M.; Vieira, M.V.; Rocha, C.F.D.; Metzger, J.P.; De Coster, G. Patch size matters for amphibians in tropical fragmented landscapes. Biol. Conserv. 2016, 195, 89–96. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Wang, T.; Zhou, L. Breeding habitat influences abundance and body condition of rice frog (Fejervarya multistriata) in agricultural landscape of Shanghai, China. Agric. Ecosyst. Env. 2019, 279, 74–79. [Google Scholar] [CrossRef]
- May, D.; Shidemantle, G.; Melnick-Kelley, Q.; Crane, K.; Hua, J. The effect of intensified illuminance and artificial light at night on fitness and susceptibility to abiotic and biotic stressors. Environ. Pollut. 2019, 251, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.J.D.; Du Preez, L.H. The effect of airplane noise on frogs: A case study on the Critically Endangered Pickersgill’s reed frog (Hyperolius pickersgilli). Ecol. Res. 2016, 31, 393–405. [Google Scholar] [CrossRef]
- Lambert, M.R.; Giller, G.S.J.; Barber, L.B.; Fitzgerald, K.C.; Skelly, D.K. Suburbanization, estrogen contamination, and sex ratio in wild amphibian populations. Proc. Natl. Acad. Sci. USA 2015, 112, 11881–11886. [Google Scholar] [CrossRef] [PubMed]
- Sawatzky, M.E.; Martin, A.E.; Fahrig, L. Landscape context is more important than wetland buffers for farmland amphibians. Agric Ecosyst Env. 2019, 269, 97–106. [Google Scholar] [CrossRef]
- Fletcher, R.J.; Didham, R.K.; Banks-Leite, C.; Barlow, J.; Ewers, R.M.; Rosindell, J.; Holt, R.D.; Gonzalez, A.; Pardini, R.; Damschen, E.I.; et al. Is habitat fragmentation good for biodiversity? Biol. Conserv. 2018, 226, 9–15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).