Dolioletta advena sp. nov., a New Species of Doliolid (Tunicata, Thaliacea) from the Adriatic Sea
Abstract
1. Introduction
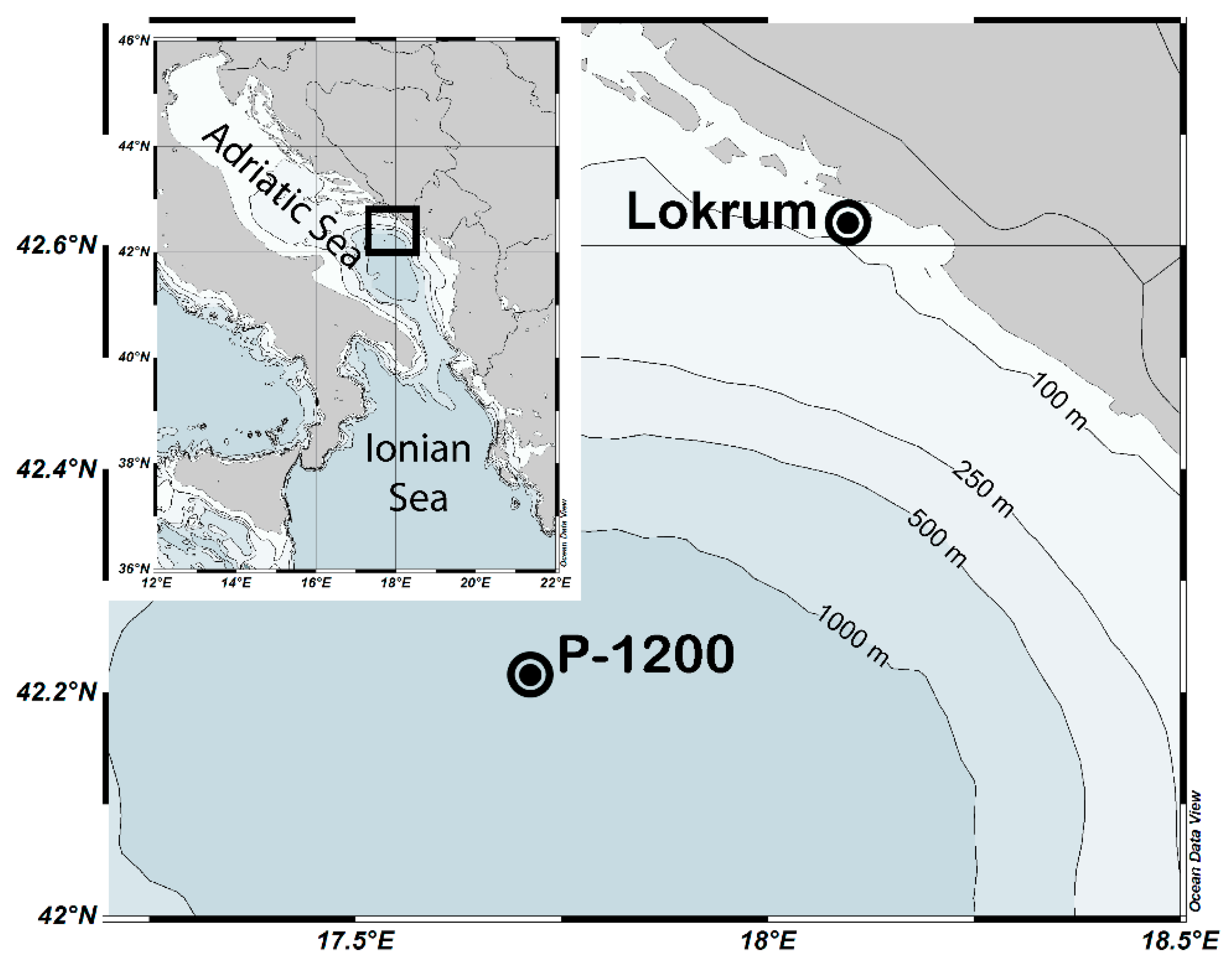
2. Materials and Methods
3. Results
3.1. Temperature and Salinity at Stations P-1200 and Lokrum
3.2. Abundance and Composition of Doliolid Communities at Stations P-1200 and Lokrum
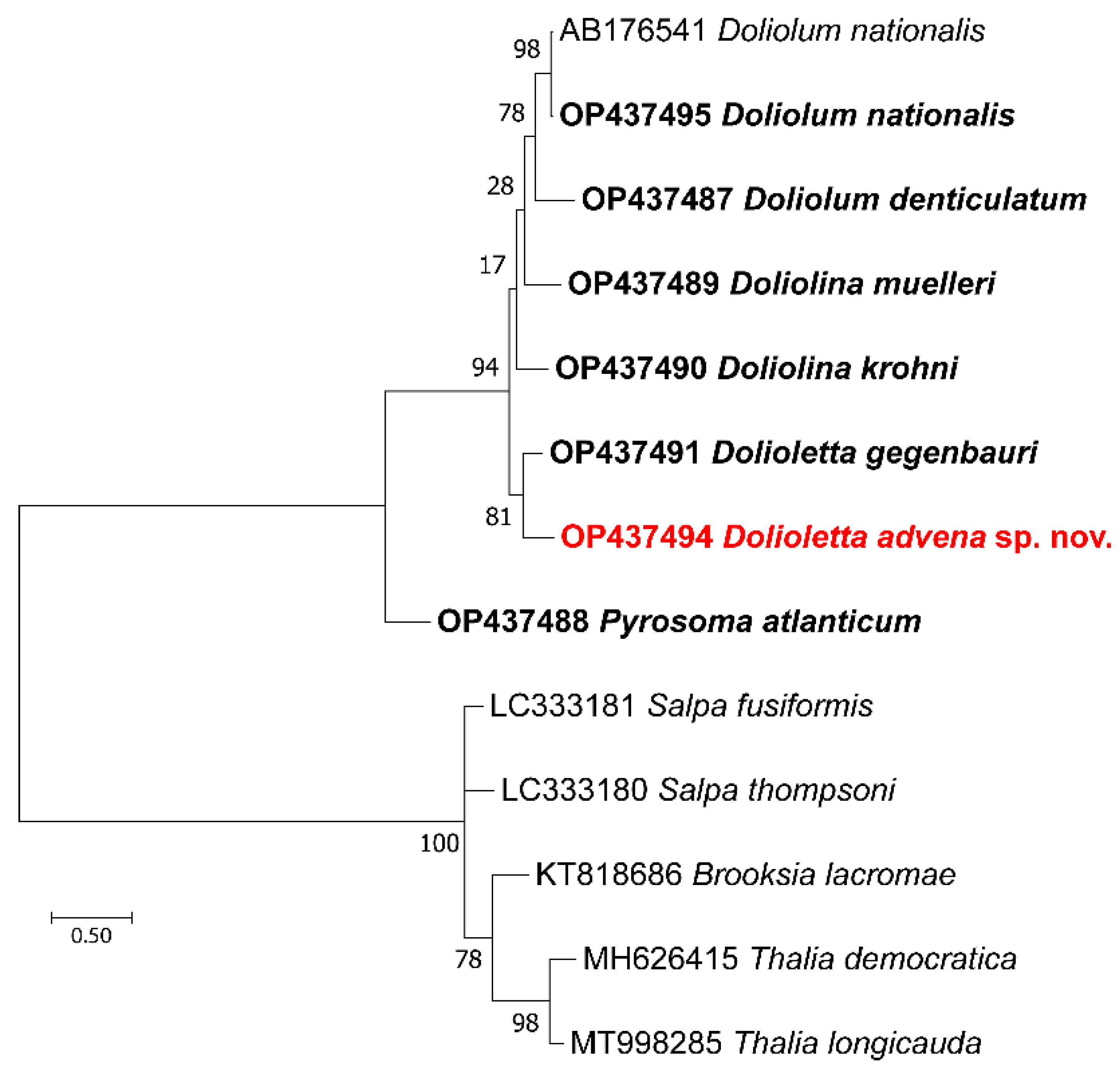
3.3. DNA Analysis
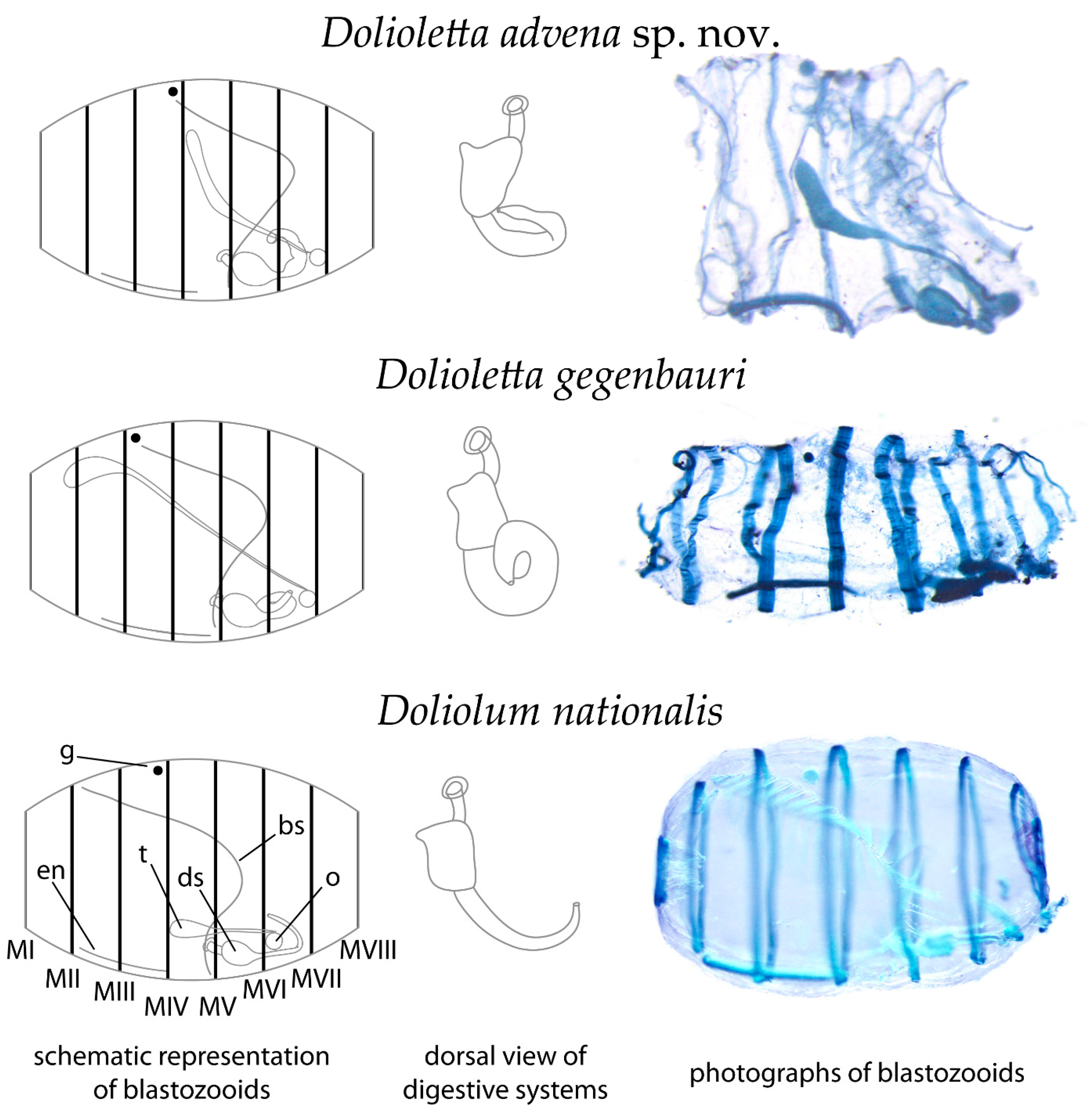
3.4. Species Description
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deibel, D. The Abundance, Distribution and Ecological Impact of Doliolids. In The Biology of Pelagic Tunicates; Oxford University Press: New York, NY, USA, 1998; pp. 171–186. [Google Scholar]
- Braconnot, J.-C. Étude Du Cycle Annuel Des Salpes et Dolioles En Rade de Villefranche-Sur-Mer. Ices J. Mar. Sci. 1963, 28, 21–36. [Google Scholar] [CrossRef]
- Braconnot, J.-C. Contribution a l’étude Biologique et Écologique Des Tuniciers Pélagiques Salpides et Doliolides—II. Écologie Des Doliolides, Biologie Des Deux Groupes. Vie Milieu 1971, 22, 437–467. [Google Scholar]
- Godeaux, J. A Contribution to the Knowledge of the Thaliacean Faunas of the Eastern Mediterranean and the Red Sea. Isr. J. Zool. 1973, 22, 39–50. [Google Scholar] [CrossRef]
- Godeaux, J. Thaliacés récoltés en Méditerranée centrale par le N.O. Atlantis II (Woods Hole). Bull. Soc. R. Sci. Liege 1987, 56, 107. [Google Scholar]
- Ménard, F.; Fromentin, J.-M.; Goy, J.; Dallot, S. Temporal Fluctuations of Doliolid Abundance in the Bay of Villefranche-Sur-Mer (Northwestern Mediterranean Sea) from 1967 to 1990. Oceanol. Acta 1997, 20, 733–742. [Google Scholar]
- Godeaux, J.E.A. The Thaliaceans, a Group of Animals Refractory to Lessepsian Migration: An Updated Survey of Their Populations in the Levantine Basin and the Red Sea. Isr. J. Zool. 1999, 45, 91–100. [Google Scholar] [CrossRef]
- Weikert, H.; Godeaux, J.E.A. Thaliacean Distribution and Abundance in the Northern Part of the Levantine Sea (Crete and Cyprus) during the Eastern Mediterranean Climatic Transient, and a Comparison with the Western Mediterranean Basin. Helgol. Mar. Res. 2008, 62, 377–387. [Google Scholar] [CrossRef]
- Katavić, I. Qualitative and Quantitative Composition of Thaliacean Populations of the Adriatic Sea in the Autumn 1974 and Spring 1975 (Kvalitativni i Kvantitativni Sastav Populacije Taliacea Jadranskog Mora u Jeseni 1974. i Proljeće 1975. Godine). Master’s Thesis, University of Zagreb, Zagreb, Croatia, 1974. [Google Scholar]
- Sigl, A. Adriatische Thaliaceenfauna. Sitz. Akad. Wiss. Math.-Nat. Kl. 1912, 121, 463–508. [Google Scholar]
- Katavić, I. Cruises of the Research Vessel “Vila Velebita” in the Kvarner Region of the Adriatic Sea. XIV. Thaliacea. Thalass. Jugosl 1979, 15, 217–230. [Google Scholar]
- Garić, R.; Batistić, M. Description of Brooksia Lacromae sp. nov. (Tunicata, Thaliacea) from the Adriatic Sea. Eur. J. Taxon. 2016, 196, 1–13. [Google Scholar] [CrossRef][Green Version]
- Batistić, M.; Jasprica, N.; Carić, M.; Čalić, M.; Kovačević, V.; Garić, R.; Njire, J.; Mikuš, J.; Bobanović-Ćolić, S. Biological Evidence of a Winter Convection Event in the South Adriatic: A Phytoplankton Maximum in the Aphotic Zone. Cont. Shelf Res. 2012, 44, 57–71. [Google Scholar] [CrossRef]
- Uljanin, B. Die Arten der Gattung Doliolum Im Golfe von Neapel und den Angrenzenden Meeresabschnitten; Fauna und Flora des Golfes von Neapel und der angrenzenden Meeres-Abschnitte, Monographie 10; W. Engelmann: Leipzig, Germany, 1884; Volume VIII. [Google Scholar]
- Graeffe, E. Ubersicht Der Fauna Des Golfes von Triest Nebst Notizen Uber Vorkommen Lebensweise, Erscheinungs- Und Laichzeit Der Einzelnen Arten. Arb. Aus. Zool. Inst. Univ. Wien Zool. Stn. Triest 1905, 15, 97–112. [Google Scholar]
- Lo Bianco, S. Le Pesce Abissali Eseguita Da F.A. Krupp Col Yacht Puritan Nelle Adiacenze de Capri Ed in Altre Localita Del Mediterraneo. Mitth. Zool. Stn. Zu Neapel 1903, 16, 109–280. [Google Scholar]
- Benović, A.; Fonda-Umani, S.; Malej, A.; Specchi, M. Net-Zooplankton Biomass of the Adriatic Sea. Mar. Biol. 1984, 79, 209–218. [Google Scholar] [CrossRef]
- Aubry, F.B.; Cossarini, G.; Acri, F.; Bastianini, M.; Bianchi, F.; Camatti, E.; De Lazzari, A.; Pugnetti, A.; Solidoro, C.; Socal, G. Plankton Communities in the Northern Adriatic Sea: Patterns and Changes over the Last 30 Years. Estuar. Coast. Shelf Sci. 2012, 115, 125–137. [Google Scholar] [CrossRef]
- Miloslavić, M.; Lučić, D.; Njire, J.; Gangai, B.; Onofri, I.; Garić, R.; Žarić, M.; Miri Osmani, F.; Pestorić, B.; Nikleka, E.; et al. Zooplankton Composition and Distribution across Coastal and Offshore Waters off Albania (Southern Adriatic) in Late Spring. Acta Adriat. 2012, 53, 163–178. [Google Scholar]
- Ljubimir, S.; Jasprica, N.; Čalić, M.; Hrustić, E.; Dupčić Radić, I.; Car, A.; Batistić, M. Interannual (2009–2013) Variability of Winter-Spring Phytoplankton in the Open South Adriatic Sea: Effects of Deep Convection and Lateral Advection. Cont. Shelf Res. 2017, 143, 311–321. [Google Scholar] [CrossRef]
- Batistić, M.; Viličić, D.; Kovačević, V.; Jasprica, N.; Garić, R.; Lavigne, H.; Carić, M. Occurrence of Winter Phytoplankton Bloom in the Open Southern Adriatic: Relationship with Hydroclimatic Events in the Eastern Mediterranean. Cont. Shelf Res. 2019, 174, 12–25. [Google Scholar] [CrossRef]
- Jasprica, N.; Čalić, M.; Kovačević, V.; Bensi, M.; Dupčić Radić, I.; Garić, R.; Batistić, M. Phytoplankton Distribution Related to Different Winter Conditions in 2016 and 2017 in the Open Southern Adriatic Sea (Eastern Mediterranean). J. Mar. Syst. 2022, 226, 103665. [Google Scholar] [CrossRef]
- Civitarese, G.; Gačić, M.; Lipizer, M.; Eusebi Borzelli, G.L. On the Impact of the Bimodal Oscillating System (BiOS) on the Biogeochemistry and Biology of the Adriatic and Ionian Seas (Eastern Mediterranean). Biogeosciences 2010, 7, 3987–3997. [Google Scholar] [CrossRef]
- Gačić, M.; Borzelli, G.L.E.; Civitarese, G.; Cardin, V.; Yari, S. Can Internal Processes Sustain Reversals of the Ocean Upper Circulation? The Ionian Sea Example. Geophys. Res. Lett. 2010, 37, L09608. [Google Scholar] [CrossRef]
- Batistić, M.; Garić, R.; Molinero, J.C. Interannual Variations in Adriatic Sea Zooplankton Mirror Shifts in Circulation Regimes in the Ionian Sea. Clim. Res. 2014, 61, 231–240. [Google Scholar] [CrossRef]
- Bonacci, O.; Bonacci, D.; Patekar, M.; Pola, M. Increasing Trends in Air and Sea Surface Temperature in the Central Adriatic Sea (Croatia). J. Mar. Sci. Eng. 2021, 9, 358. [Google Scholar] [CrossRef]
- Dulčić, J.; Grbec, B.; Lipej, L. Information on the Adriatic Ichthyofauna—Effect of Water Warming? Acta Adriat. 1999, 40, 33–43. [Google Scholar] [CrossRef]
- Iveša, N.; Piria, M.; Gelli, M.; Trnski, T.; Špelić, I.; Radočaj, T.; Kljak, K.; Jug-Dujaković, J.; Gavrilović, A. Feeding Habits of Predatory Thermophilic Fish Species and Species with Subtropical Affinity from Recently Extended Distributional Range in Northeast Adriatic Sea, Croatia. Diversity 2021, 13, 357. [Google Scholar] [CrossRef]
- Wada, H. Evolutionary History of Free-Swimming and Sessile Lifestyles in Urochordates as Deduced from 18S RDNA Molecular Phylogeny. Mol. Biol. Evol. 1998, 15, 1189–1194. [Google Scholar] [CrossRef][Green Version]
- Yokobori, S.; Oshima, T.; Wada, H. Complete Nucleotide Sequence of the Mitochondrial Genome of Doliolum Nationalis with Implications for Evolution of Urochordates. Mol. Phylogenet. Evol. 2005, 34, 273–283. [Google Scholar] [CrossRef]
- Tsagkogeorga, G.; Turon, X.; Hopcroft, R.R.; Tilak, M.-K.; Feldstein, T.; Shenkar, N.; Loya, Y.; Huchon, D.; Douzery, E.J.; Delsuc, F. An Updated 18S RRNA Phylogeny of Tunicates Based on Mixture and Secondary Structure Models. BMC Evol. Biol. 2009, 9, 187. [Google Scholar] [CrossRef]
- Govindarajan, A.F.; Bucklin, A.; Madin, L.P. A Molecular Phylogeny of the Thaliacea. J. Plankton Res. 2010, 33, 843–853. [Google Scholar] [CrossRef]
- Frischer, M.E.; Lamboley, L.M.; Walters, T.L.; Brandes, J.A.; Arneson, E.; Lacy, L.E.; López-Figueroa, N.B.; Rodríguez-Santiago, Á.E.; Gibson, D.M. Selective Feeding and Linkages to the Microbial Food Web by the Doliolid Dolioletta Gegenbauri. Limnol. Oceanogr. 2021, 66, 1993–2010. [Google Scholar] [CrossRef]
- Delsuc, F.; Philippe, H.; Tsagkogeorga, G.; Simion, P.; Tilak, M.-K.; Turon, X.; López-Legentil, S.; Piette, J.; Lemaire, P.; Douzery, E.J.P. A Phylogenomic Framework and Timescale for Comparative Studies of Tunicates. BMC Biol. 2018, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Bucklin, A.; Peijnenburg, K.T.C.A.; Kosobokova, K.N.; O’Brien, T.D.; Blanco-Bercial, L.; Cornils, A.; Falkenhaug, T.; Hopcroft, R.R.; Hosia, A.; Laakmann, S.; et al. Toward a Global Reference Database of COI Barcodes for Marine Zooplankton. Mar. Biol. 2021, 168, 78. [Google Scholar] [CrossRef]
- Robison, B.H.; Raskoff, K.A.; Sherlock, R.E. Adaptations for Living Deep: A New Bathypelagic Doliolid, from the Eastern North Pacific. J. Mar. Biol. Assoc. UK 2005, 85, 595–602. [Google Scholar] [CrossRef][Green Version]
- Garić, R.; Batistić, M. Description of Aurelia Pseudosolida sp. nov. (Scyphozoa, Ulmaridae) from the Adriatic Sea. Water 2022, 14, 135. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Goodall-Copestake, W.P. NrDNA:MtDNA Copy Number Ratios as a Comparative Metric for Evolutionary and Conservation Genetics. Heredity 2018, 121, 105–111. [Google Scholar] [CrossRef]
- Leray, M.; Yang, J.Y.; Meyer, C.P.; Mills, S.C.; Agudelo, N.; Ranwez, V.; Boehm, J.T.; Machida, R.J. A New Versatile Primer Set Targeting a Short Fragment of the Mitochondrial COI Region for Metabarcoding Metazoan Diversity: Application for Characterizing Coral Reef Fish Gut Contents. Front. Zool 2013, 10, 34. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Garstang, W. Report on the Tunicata. Part I. Doliolida. British Antarctic (‘Terra Nova’) Expedition, 1910. Nat. Hist. Rep. Zool. 1933, 4, 195–252. [Google Scholar]
- Godeaux, J.E.A. History and Revised Classification of the Order Cyclomyaria (Tunicata, Thaliacea, Doliolida). Bull. L’institut R. Sci. Nat. Belgique. Biol. 2003, 73, 191–222. [Google Scholar]
- WoRMS. WoRMS Editorial Board World List of Thaliacea. Doliolida; World Register of Marine Species: Ostend, Belgium, 2022. [Google Scholar]
- Godeaux, J. Thaliacés méditerranéens: Une synthèse. Bull. Soc. R. Sci. Liege 1988, 57, 359. [Google Scholar]
- Van Soest, R.W.M. The Cladistic Biogeography of Salps and Pyrosomes. In The Biology of Pelagic Tunicates; Oxford University Press: New York, NY, USA, 1998; pp. 231–249. [Google Scholar]
- Garić, R.; Batistić, M. Fritillaria Ragusina sp. Nov., a New Species of Appendicularia (Tunicata) from the Adriatic Sea. J. Mar. Biol. Assoc. UK 2011, 91, 555–559. [Google Scholar] [CrossRef]
- Piraino, S.; Aglieri, G.; Martell, L.; Mazzoldi, C.; Melli, V.; Milisenda, G.; Scorrano, S.; Boero, F. Pelagia Benovici sp. nov. (Cnidaria, Scyphozoa): A New Jellyfish in the Mediterranean Sea. Zootaxa 2014, 3794, 455–468. [Google Scholar] [CrossRef]
- Godeaux, J. The Relationships and Systematics of the Thaliacea, with Keys for Identification. In The Biology of Pelagic Tunicates; Oxford University Press: New York, NY, USA, 1998; pp. 273–294. [Google Scholar]



| Species | Isolate | GenBank Acession | Date of Collection (DD-MM-YYYY) | Station | Forward COI Primer | Reverse COI Primer |
|---|---|---|---|---|---|---|
| Pyrosoma atlanticum | pyrver | OP437488 | 08-04-2016 | P-1200 | coi-70f | od1140coir |
| Dolioletta advena sp. nov. | gega | OP437493 | 23-08-2021 | dollco | dol1150coir | |
| gegb | OP437492 | 23-08-2021 | ||||
| dcgen | OP437494 | 18-08-2021 | Lokrum | |||
| Dolioletta gegenbauri | gegpr2 | OP437491 | 16-09-2021 | |||
| Doliolina krohni | dkroh | OP437490 | 18-08-2021 | |||
| Doliolina muelleri | dcfmul1 | OP437489 | 18-08-2021 | |||
| Doliolum denticulatum | dolden11 | OP437487 | 16-04-2021 | |||
| Doliolum nationalis | dolnat11 | OP437495 | 18-10-2021 | jgHCO2198 |
| Stations | P-1200 | Lokrum | ||
|---|---|---|---|---|
| Layers | 0–100 m | 0–50 m | 50–90 m | |
| Species | ||||
| Dolioletta advena sp. nov. blastozooids | 0.80 | 2.24 | 0.80 | |
| Dolioletta sp. oozooids + nurses | 0.54 | 0.48 | 1.60 | |
| Doliolina krohni blastozooids | 0.01 | 0 | 0.80 | |
| Doliolina muelleri blastozooids | 0 | 0.32 | 2.80 | |
| Doliolina sp. oozooids + nurses | 0 | 0 | 13.60 | |
| Doliolida larvae | 0 | 0 | 3.20 | |
| Doliolida ova | 0 | 1.28 | 0 | |
| Doliolida unidentified | 0.38 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garić, R.; Batistić, M. Dolioletta advena sp. nov., a New Species of Doliolid (Tunicata, Thaliacea) from the Adriatic Sea. Diversity 2022, 14, 959. https://doi.org/10.3390/d14110959
Garić R, Batistić M. Dolioletta advena sp. nov., a New Species of Doliolid (Tunicata, Thaliacea) from the Adriatic Sea. Diversity. 2022; 14(11):959. https://doi.org/10.3390/d14110959
Chicago/Turabian StyleGarić, Rade, and Mirna Batistić. 2022. "Dolioletta advena sp. nov., a New Species of Doliolid (Tunicata, Thaliacea) from the Adriatic Sea" Diversity 14, no. 11: 959. https://doi.org/10.3390/d14110959
APA StyleGarić, R., & Batistić, M. (2022). Dolioletta advena sp. nov., a New Species of Doliolid (Tunicata, Thaliacea) from the Adriatic Sea. Diversity, 14(11), 959. https://doi.org/10.3390/d14110959







