A New Genus of Sminthurididae (Collembola, Symphypleona) from Brazil, with Notes on the Systematics of the Family
Abstract
1. Introduction
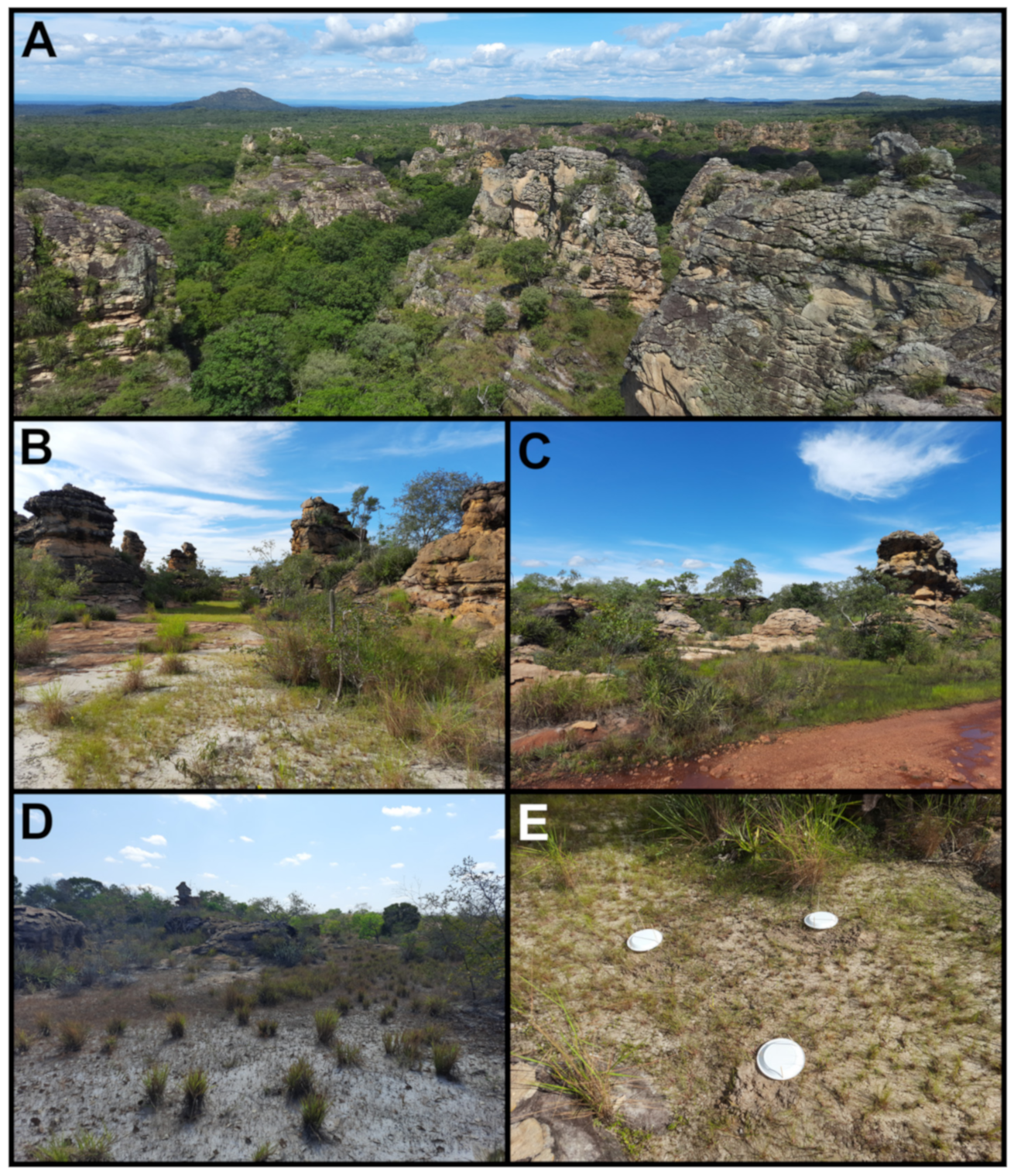
2. Materials and Methods
3. Results
3.1. Taxonomic Summary and Genus Diagnosis
- Order Symphypleona Börner, 1901 [36]
- Family Sminthurididae Börner, 1906 [37]
- Genus Parasminthurides gen. nov. Medeiros and Bellini
3.2. Identification Key and Distribution of the Extant Genera of Sminthurididae
- 1.
- Tibiotarsus III distal organ absent ……………………...….…………………………….. 2
- -
- Tibiotarsus III distal organ present ……………..……..………………………………… 4
- 2.
- -
- Males’ antennal clasper complex, Ant II with b1–b4, tra1 modified elements, Ant III with c1 and c3 (Figure 1C–D), mucronal chaeta present ………………………...……………………………………………………..………………. 3
- 3.
- -
- Males’ Ant II with b5–b7 and tra2 elements (Figure 1C), females Ant IV undivided, Abd II–III dorsal vesicles absent, ventral tube simple, without lateral projections, mucro narrow …….……………………….......... Denisiella Folsom and Mills, 1938 [20]; Americas (including Hawaii), French Polynesia, South Africa, United Arab Emirates
- 4.
- Males’ leg II with a clasping organ made by opposing strong spines on trochanter and femur ……………………………..……....…. Pedonides Bretfeld, 2010 [46]; Portugal
- -
- Males’ leg II without such morphology ……………………………………………….... 5
- 5.
- Males’ Abd V (small abdomen) dorsally with a pair of large modified processes ………………………..…………………………. Pygicornides Betsch, 1969 [48]; Australia
- -
- Males’ Abd V without such morphology ……………………………………………….. 6
- 6.
- -
- Males’ antennal clasper complex, Ant II with further modified elements than b1–b3, Ant III also with c1 element, usually with extra modified chaetae ………….……..… 7
- 7.
- Females’ Ant II–III with apical robust spiniform chaetae, males with large dorsal vesicles on Abd II–III ………………………..………. Parasminthurides gen. nov.; Brazil
- -
- Females’ antennae without such chaetae, males without vesicles on Abd II–III ….... 8
- 8.
- -
- Males’ Ant III with c2 element (Figure 1E–F,H,K), mucronal chaeta mostly present ………………………………………………………………………………...……. 9
- 9.
- -
- Males with B1 or Tra2 element present on Ant II (Figure 1F,H,K), Ant IV mostly undivided, and with Th III dorsal vesicles …...………………………………. 10
- 10.
- Head and mouthparts elongated, dorso-posterior large abdomen with long chaetae in both sexes ….………...… Sinnamarides Betsch and Waller, 1991 [49]; French Guiana
- -
- Head and mouthparts normal, dorso-posterior large abdomen with long chaetae only in one of the sexes …………………………………………...……………………….... 11
- 11.
- Females’ Ant III and IV with large blunt chaetae, males with long chaetae on dorso-posterior large abdomen …………………………………… Stenacidia Börner, 1906 [37]; Holarctic, Cape Verde Islands, Australia, Kerguelen Islands
- -
- Females’ Ant III and IV without large blunt chaetae, at most Ant IV with sensillum-like chaetae, males with short chaetae on dorso-posterior large abdomen ………………………………………………. Sminthurides Börner, 1900 [19]; Worldwide
3.3. Parasminthurides spinosus gen. nov. sp. nov. Medeiros and Bellini
4. Discussion
Systematics of the Sminthurididae: Past, Present, and Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourlet, C. Mémoire sur les Podurides et les Sminthurides. Ann. Soc. Entomol. Fr. 1842, 10, 40–41. [Google Scholar]
- Krausbauer, T. Neue Collembola aus der Umgebung von Weilburg a. Lahn. Zool. Anz. 1898, 21, 495–504. [Google Scholar]
- Blancquaert, J.P.; Mertens, J. Mating behaviour in Sphaeridia pumilis (Collembola). Pedobiologia 1977, 17, 343–349. [Google Scholar]
- Betsch, J.M. Éléments pour une monographie des Collemboles Symplyplêones (Hexapodes, Aptérygotes). Mém. Mus. Natl. Hist. Nat. Sér. A Zool. 1980, 116, 1–227. [Google Scholar]
- Bretfeld, G. Synopses on Palaeartic Collembola, Volume 2. Symphypleona. Abh. Ber. Naturkundemus. Gorlitz 1999, 71, 1–318. [Google Scholar]
- Bretfeld, G. Phylogenetic systematics of the higher taxa of Symphypleona Börner, 1901 (Insecta Entognatha, Collembola). Proc. 2nd Intern. Sem. Apterygota 1986, 1, 302–311. [Google Scholar]
- Sánchez-García, A.; Engel, M.S. Long-term stasis in a diverse fauna of Early Cretaceous springtails (Collembola: Symphypleona). J. Syst. Palaeontol. 2016, 15, 513–537. [Google Scholar] [CrossRef]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World. Available online: http://www.collembola.org (accessed on 28 September 2022).
- Hammer, M. Investigations on the Microfauna of Northern Canada, Part II, Collembola. Acta Arct. 1953, 6, 1–108. [Google Scholar]
- Huther, W. Die systematische Stellung von Mackenziella psocoides Hammer (Collembola). Zool. Anz. 1964, 173, 119–126. [Google Scholar]
- Moen, P.; Ellis, W.N. Morphology and Taxonomic Position of Podura aquatica (Collembola). Entomol. Gen. 1984, 9, 193–204. [Google Scholar] [CrossRef]
- Yosii, R. Phylogenetische bedeutung der chaetotaxie bei den Collembolen. Contrib. Biol. Lab. Kyoto Univ. 1961, 12, 1–37. [Google Scholar]
- Fjellberg, A. Redescription of Mackenziella psocoides Hammer, 1953 and discussion of its systematic position (Collembola, Mackenziellidae). Proc. 2nd Intern. Sem. Apterygota 1989, 1, 93–105. [Google Scholar]
- Richards, W.R. Generic classification, evolution, and biogeography of the Sminthuridae of the world (Collembola). Mem. Ent. Soc. Can. 1968, 53, 1–54. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, F.; Stevens, M.I.; Yan, Q.; Liu, M.; Hu, F. New insight into the systematics of Tomoceridae (Hexapoda, Collembola) by integrating molecular and morphological evidence. Zool. Scr. 2015, 45, 286–299. [Google Scholar] [CrossRef]
- Sun, X.; Yu, D.; Xie, Z.; Dong, J.; Ding, Y.; Yao, H.; Greenslade, P. Phylomitogenomic analyses on collembolan higher taxa with enhanced taxon sampling and discussion on method selection. PLoS ONE 2020, 15, e0230827. [Google Scholar] [CrossRef]
- Cucini, C.; Fanciulli, P.P.; Frati, F.; Convey, P.; Nardi, F.; Carapelli, A. Re-evaluating the internal phylogenetic relationships of Collembola by means of Mitogenome Data. Genes 2021, 12, 44. [Google Scholar] [CrossRef]
- Linnaniemi, W.M. Die Apterygotenfauna Finlands, II. Spezieller Teil. Acta Soc. Sci. Fenn. 1912, 40, 1–359. [Google Scholar]
- Börner, C. Vorläufige Mittheilung zur Systematik der Sminthuridae Tullb. inbesondere der genus Sminthurinus Latr. Zool. Anz. 1900, 23, 609–618. [Google Scholar]
- Folsom, J.W.; Mills, H.B. Contribution to the knowledge of the genus Sminthurides Börner. Bull. Mus. comp. Zool. Harvard 1938, 82, 231–274. [Google Scholar]
- Mari-Mutt, J.A.; Bellinger, P.F. A Catalog of Neotropical Collembola, including Nearctic Areas of Mexico—Flora & Fauna Handbook 5, 1st ed.; Sandhill Crane Press: Gainesville, FL, USA, 1990; pp. 1–237. [Google Scholar]
- Mari-Mutt, J.A.; Bellinger, P.F. Supplement to the catalog of the Neotropical Collembola. Caribb. J. Sci. 1996, 32, 166–175. [Google Scholar]
- Mari-Mutt, J.A.; Bellinger, P.F.; Janssens, F. Checklist of the Collembola: Supplement to the Catalog of the Neotropical Collembola. Available online: http://www.collembola.org/publicat/neotrcat.htm (accessed on 28 September 2022).
- Zeppelini, D.; Queiroz, G.C.; Bellini, B.C. Symphypleona in Catálogo Taxonômico da Fauna do Brasil. PNUD. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/470 (accessed on 28 September 2022).
- Arlé, R.; Mendonça, C. Estudo preliminar das espécies de Dicranocentrus Schött, 1893, ocorrentes no Parque Nacional da Tijuca, Rio de Janeiro (Collembola). Rev. Brasil. Biol. 1982, 42, 41–49. [Google Scholar]
- Jordana, R.; Arbea, J.I.; Simón, C.; Luciáñez, M.J. Fauna Iberica Volume 8: Collembola, Poduromorpha, 1st ed.; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 1997; pp. 1–807. [Google Scholar]
- Massoud, Z.; Betsch, J.M. Étude sur les Insectes Collemboles II.—Les caractères sexuels secondaires des antenes des Symphypléones. Rev. Ecol. Biol. Sol. 1972, 9, 55–97. [Google Scholar]
- Cipola, N.G.; Morais, J.W.; Bellini, B.C. A new species of Seira (Collembola: Entomobryidae: Seirini) from Northern Brazil, with the addition of new chaetotaxic characters. Zoologia 2014, 31, 489–495. [Google Scholar] [CrossRef]
- Betsch, J.M.; Waller, A. Chaetotaxic nomenclature of the head, thorax and abdomen in Symphypleona (Insecta, Collembola). Acta Zool. Fenn. 1994, 195, 5–12. [Google Scholar]
- Vargovitsh, R.S. New cave Arrhopalitidae (Collembola: Symphypleona) from the Crimea (Ukraine). Zootaxa 2009, 2047, 1–47. [Google Scholar] [CrossRef]
- Vargovitsh, R.S. New troglomorphic Arrhopalitidae (Collembola: Symphypleona) from the Western Caucasus. Zootaxa 2012, 3174, 1–21. [Google Scholar] [CrossRef]
- Vargovitsh, R.S. Cavernicolous Arrhopalites abchasicus sp. nov. (Collembola: Symphypleona: Arrhopalitidae) from the West Caucasus with a key to the World species of the genus. Zootaxa 2013, 3666, 16–30. [Google Scholar] [CrossRef]
- Betsch, J.M. An ontogenetically focused chaetotaxial scheme in Symphypleona (collembolan): The 6th abdominal segment. Pedobiologia 1997, 41, 13–18. [Google Scholar]
- Nayrolles, P. Chetotaxie tibiotarsale des collemboles symphyplèones. Trav. Lab. Ecobiol. Arthr. Édaph. Toulouse 1988, 5, 1–19. [Google Scholar]
- Bretfeld, G. Symphypleona from Northwest and West Africa, collected in the years 1979–1986 by Johan Mertens, Gent (Insecta, Collembola). Senckenb. Biol. 2001, 81, 87–131. [Google Scholar]
- Börner, C. Neue Collembolenformen und zur Nomenclatur der Collembola Lubbock. Zool. Anz. 1901, 24, 696–712. [Google Scholar]
- Börner, C. Das System der Collembolen nebst Beschreibung neuer Collembolen des Hamburger Naturhistorischen Museums. Mit. Natur. Mus. Hamburg 1906, 23, 147–188. [Google Scholar]
- Betsch, J.M.; Massoud, Z. Études sur les Insectes Collemboles. I.—Systématique, ultrastructure externe et écologie du genre Jeannenotia Stach, 1956 (Symphypléones, Sminthurididae n. comb.)—Description de deux Collemboles nouveaux (Proisotoma et Sminthurides). Rev. Ecol. Biol. Sol 1970, 7, 153–225. [Google Scholar]
- Dallai, R. Ricerche sui Collemboli. XVII. Le Isole Eolie. Lav. Soc. Ital. Biogeog. 1973, 3, 481–590. [Google Scholar] [CrossRef]
- Murphy, D.H. Deboutevillea marina n. gen., n. sp., (Collembola, Sminthuridae) from the inter-tidal zone of Singapore. Bull. Nat. Mus. Singapore 1965, 33, 31–34. [Google Scholar]
- Palacios-Vargas, J.G. A new species of Denisiella (Collembola: Sminthurididae) from Nicaragua. Rev. Nica. Entomol. 1995, 32, 25–33. [Google Scholar]
- Palacios-Vargas, J.G. A new species of Denisiella (Collembola: Sminthurididae) from Panama and new records for D. sexpinnata (Denis, 1938). Zootaxa 2007, 1637, 63–68. [Google Scholar] [CrossRef]
- Ospina, M.; Palacios-Vargas, J.G. A new Denisiella Folsom and Mills, 1938 (Collembola: Sminthurididae) from Colombia. Zootaxa 2009, 2168, 63–68. [Google Scholar] [CrossRef]
- Schulz, H.J.; Harten, A.V. Subclass Collembola, order Symphypleona. Arthropod Fauna UAE 2013, 5, 13–21. [Google Scholar]
- Palacios-Vargas, J.G.; Ferreira, A.S.; Zeppelini, D. Redefinition of Denisiella Folsom & Mills, 1938 (Collembola: Sminthurididae) with description of three new species from Brazil. Zootaxa 2018, 4434, 111–129. [Google Scholar] [CrossRef]
- Bretfeld, G. Pedonides alcochetensis n. g. n. sp. (Insecta, Collembola, Sminthurididae) with two clasping organs from the mainland of Portugal. Soil Org. 2010, 82, 317–323. [Google Scholar]
- Womersley, H. The Collembola-Symphypleona of Australia: A preliminary account. J. Counc. Sci. Ind. Res. 1932, 34, 8–47. [Google Scholar]
- Betsch, J.M. Contribution à l’étude des Sminthuridinae (Collemboles Symphypléones) un genre nouveau d’Australie: Pygicornides. Rev. Ecol. Biol. Sol 1969, 6, 349–355. [Google Scholar]
- Betsch, J.M.; Waller, A. Collemboles Symphypléones de Guyane. I. Un nouveau genre de Sminthurididae. Rev. Ecol. Biol. Sol 1991, 28, 229–235. [Google Scholar]
- Ferreira, A.S.; Arango, A.; Palacios-Vargas, J.G. Two new species of Sminthurides Börner, 1900 (Collembola: Sminthurididae) from Mexico with comments on the genus and a key to the members from the Americas. Neotrop. Entomol. 2021, 50, 408–429. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Vargas, J.G. New aquatic Sminthurides Börner species (Collembola: Sminthurididae) from Tabasco, Mexico. Folia Entomol. Mex. 2022, 8, e0081001. [Google Scholar] [CrossRef]
- Murphy, D.H. Collembola Symphypleona from the Gambia, with a note on the biogeography of some characteristic savanna forms. Proc. Zool. Soc. Lond. 1960, 134, 557–594. [Google Scholar] [CrossRef]
- Arlé, R. O gênero Sphaeridia Linnaniemi, 1912 no Brasil com descrição de novas espécies (Collembola-Symphypleona). Bol. Mus. Para. Emílio Goeldi Zool. 1984, 1, 229–255. [Google Scholar]
- Bretfeld, G.; Gauer, U. Diagnostic description of the males of new Sphaeridia species (Insecta, Collembola) from South America. Andrias 1994, 13, 113–136. [Google Scholar]
- Bretfeld, G.; Schulz, H.J. New species of Sphaeridia Linnaniemi, 1912, sensu Bretfeld & Trinklein 2000 (Insecta, Collembola) from Peru; with short descriptions of the males and a key to the Sphaeridia species described from South America. Soil Org. 2012, 84, 513–527. [Google Scholar]
- Yosii, R. Collembola of Himalaya. J. Coll. Art. Sci. Chiba U. 1966, 4, 461–531. [Google Scholar]
- Itoh, R.; Zhao, L.J. Two new species of Symphypleona (Collembola) from the Tian-mu Mountains in East China. Edaphologia 1993, 50, 31–36. [Google Scholar]
- Jeannenot, F. Contribution à l’étude des Collemboles. Sminthurides (Stenacidia) stachi spec. nov. Trav. Lab. Zool. Fac. Sci. Dijon 1955, 8, 1–25. [Google Scholar]
- Reuter, O.M. För Finland nya Collembola. Medd. Soc. Fauna Flora Fenn. 1881, 6, 203–205. [Google Scholar]
- Instituto Brasileiro de Desenvolvimento Florestal. Plano de Manejo: Parque Nacional de Sete Cidades; IBDF: Brasilia, Brazil, 1979; pp. 1–61. [Google Scholar]
- Oliveira, M.; Castro, A.A.J.F.; Martins, F.R. Classificação e caracterização dos tipos vegetacionais do Parque Nacional de Sete Cidades, Piauí, Brasil. In Biodiversidade e Ecótonos da Região Setentrional do Piauí; Castro, A.A.J.F., Castro, N.M.C.F., Arzabe, C., Eds.; Editora EDUFPI: Teresina, Brazil, 2010; pp. 66–89. [Google Scholar]
- Andrade, E.; Araújo, K.; Costa, C.; Sena, F.; Santos, A.; Araújo, S.; Uchôa, L.; Rodrigues, N.; Ferreira, J.; Benício, R.; et al. Anfíbios Anuros do Parque Nacional de Sete Cidades, Guia Ilustrado; IFPI: Teresina, Brazil, 2022; pp. 1–109. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorologia. Clima/Normais Climatológicas. Available online: http://www.inmet.gov.br/portal/index.php/r=clima/normaisClimatologicas (accessed on 11 October 2022).
- Börner, C. Die Familien der Collembolen. Zool. Anz. 1913, 61, 315–322. [Google Scholar]
- Stach, J. The Apterygotan Fauna of Poland in Relation to the World-Fauna of this Group of Insects. Family: Sminthuridae, 1ed.; Panstwowe Wydawnictwo Naukowe: Kraków, Poland, 1956; pp. 1–287. [Google Scholar]
- Deharveng, L. Recent advances in Collembola systematics. Pedobiologia 2004, 48, 415–433. [Google Scholar] [CrossRef]
- D’Haese, C.A. Morphological appraisal of Collembola phylogeny with special emphasis on Poduromorpha and a test of the aquatic origin hypothesis. Zool. Scr. 2003, 32, 563–586. [Google Scholar] [CrossRef]
- Salmon, J.T. An Index to the Collembola. R. Soc. N. Z. Bull. 1964, 7, 1–144. [Google Scholar]
- Cassagnau, P. La phylogénie des Collemboles à la lumière des structures endocrines rétrocérébrales. Proc. 1st. Symp. Inter. Zoolfilogenia 1971, 1, 333–349. [Google Scholar]
- Massoud, Z. Essai de synthèse sur la phylogénie des Collemboles. Rev. Ecol. Biol. Sol 1976, 13, 241–252. [Google Scholar]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, 20–26. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, C.A. Were the first springtails semi-aquatic? A phylogenetic approach by means of 28S rDNA and optimization alignment. Proc. R. Soc. Lond. 2002, 269, 1143–1151. [Google Scholar] [CrossRef]
- Luan, Y.X.; Mallatt, J.M.; Xie, R.D.; Yang, Y.M.; Yin, W.Y. The phylogenetic positions of three basal-hexapod groups (Protura, Diplura, and Collembola) based on ribosomal RNA gene sequences. Mol. Biol. Evol. 2005, 22, 1579–1592. [Google Scholar] [CrossRef]
- Xiong, Y.; Gao, Y.; Yin, W.Y.; Luan, Y.X. Molecular phylogeny of Collembola inferred from ribosomal RNA genes. Mol. Phylogenet. Evol. 2008, 49, 728–735. [Google Scholar] [CrossRef]
- Yosii, R. Springschwänze des Ozé-Naturschutzgebietes. Sci. R. Ozegahara Moor 1954, 1, 777–830. [Google Scholar]
- Nardi, F.; Cucini, C.; Leo, C.; Frati, F.; Fanciulli, P.P.; Carapelli, A. The complete mitochondrial genome of the springtail Allacma fusca, the internal phylogenetic relationships and gene order of Symphypleona. Mitochondrial DNA Part B Resour. 2020, 5, 3121–3123. [Google Scholar] [CrossRef]

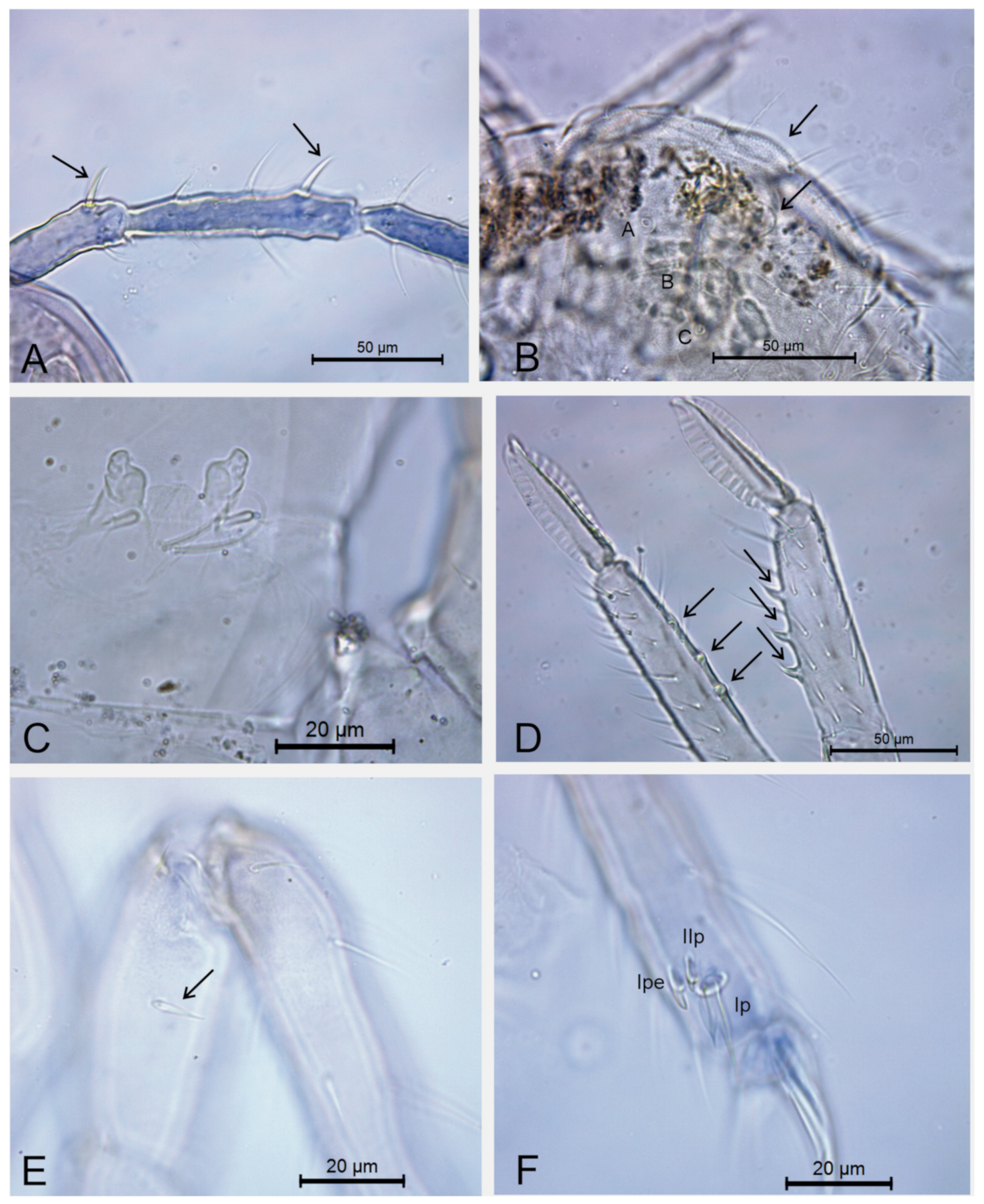
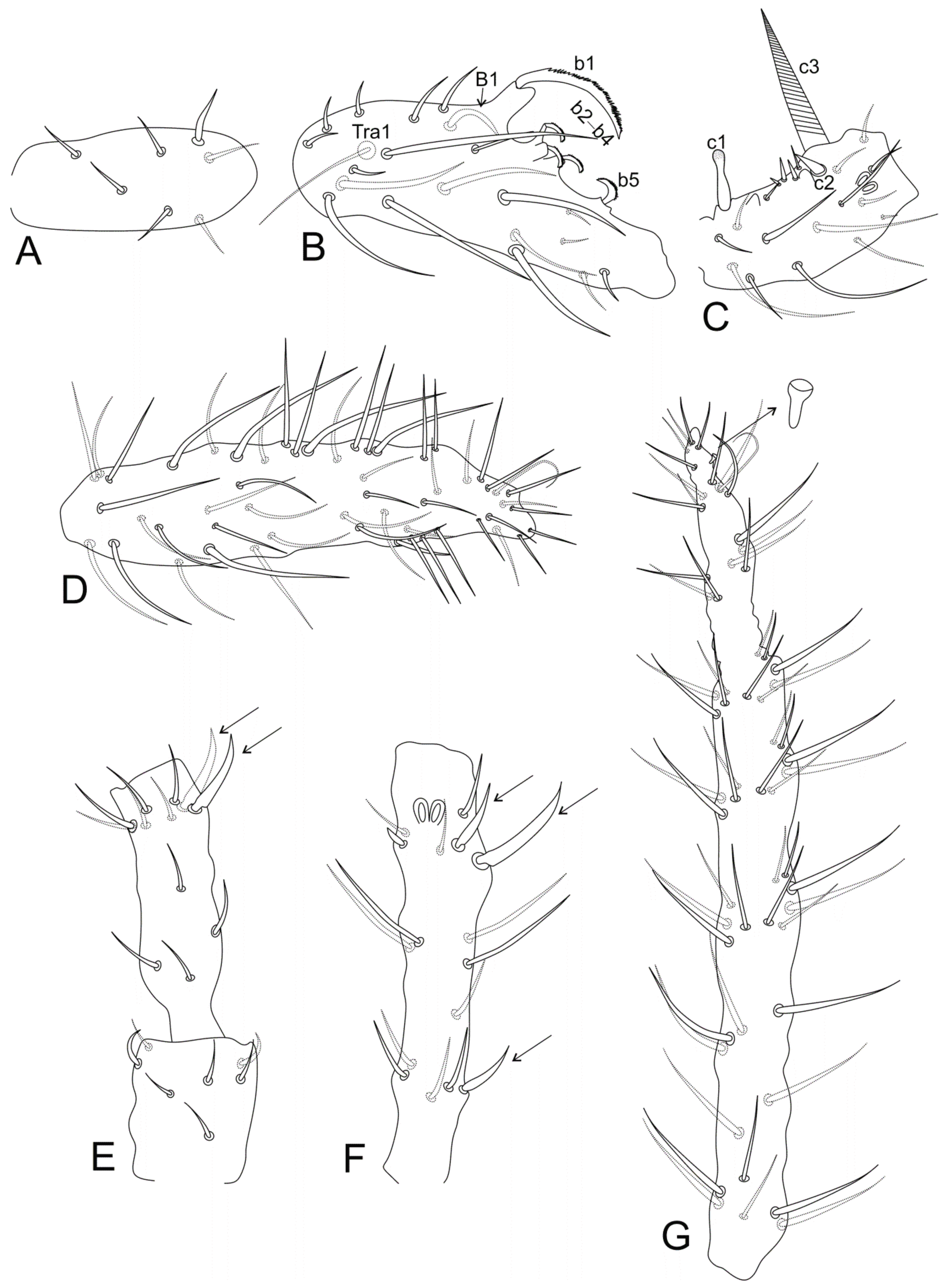

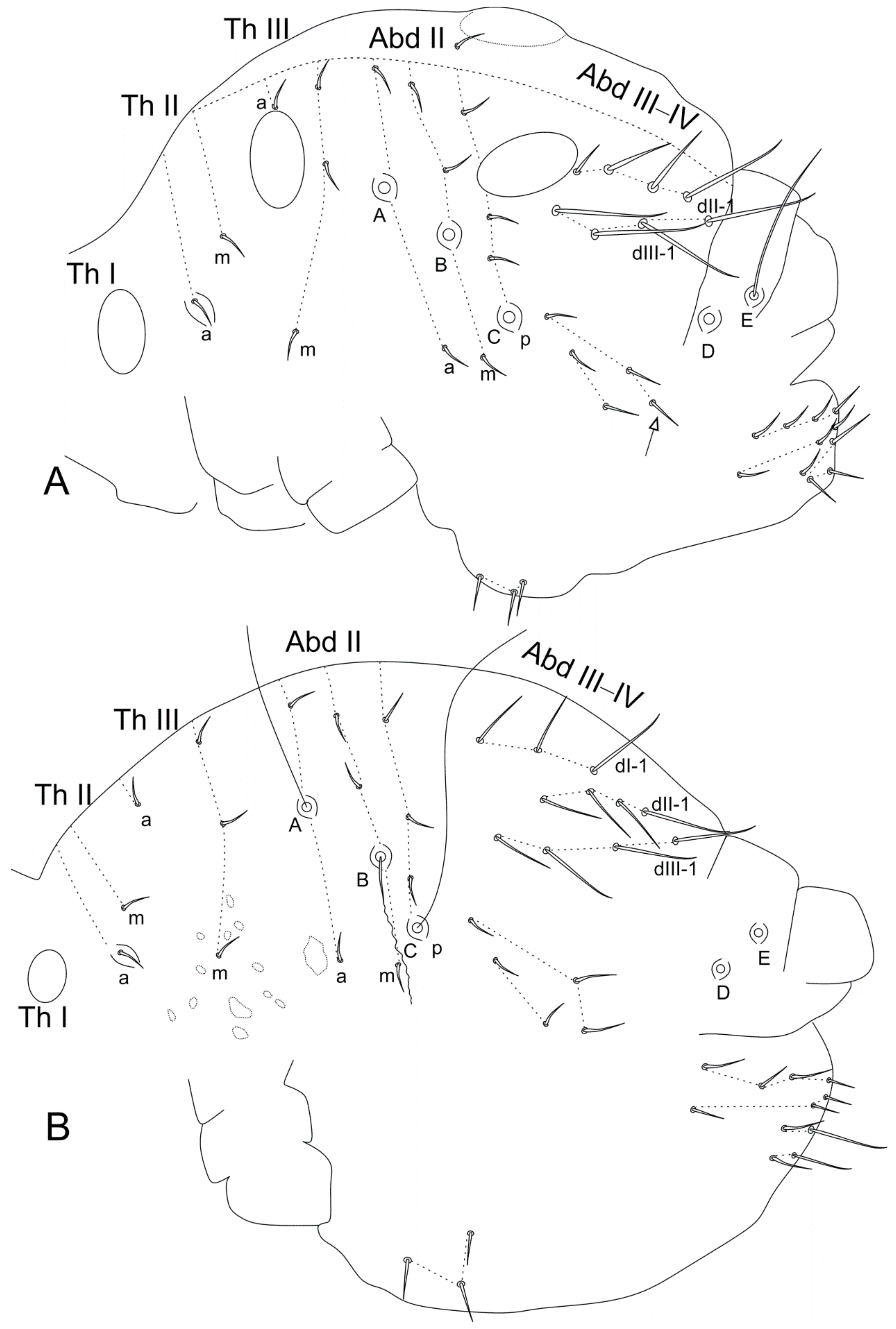
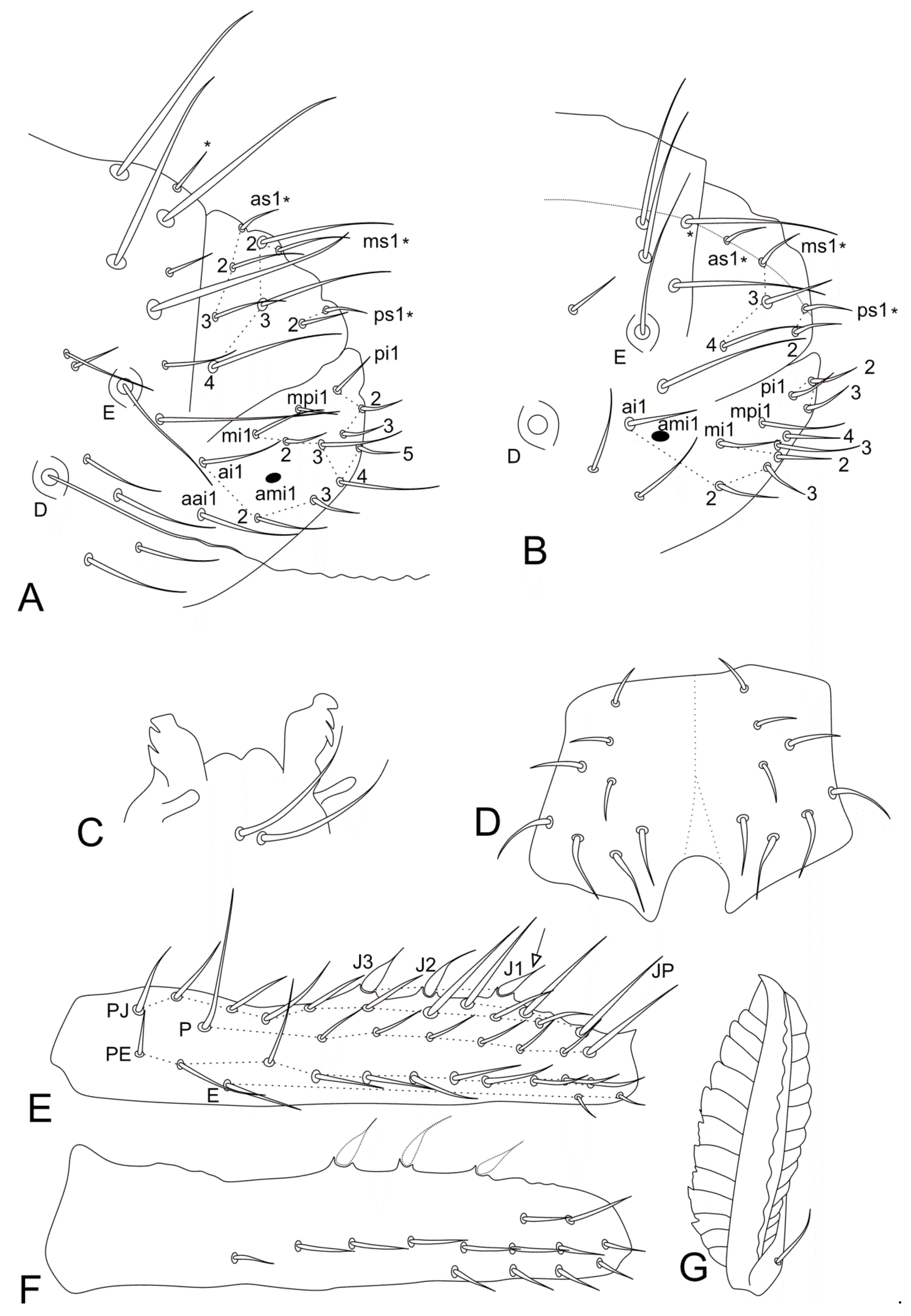


| Genera/Characters | Number of Described Species | Ant II Mod. Chaetae (♂) | Ant III Main Mod. Chaetae (♂) | Ant II Mod. Chaetae (♀) | Ant III Mod. Chaetae (♀) | Ant IV Mod. Chaetae (♀) | Ant IV Subdivisions (♀) | Ant IV Subdivisions (♂) | Elongated Maxillae | Th III Dorsal Vesicles (♂) | Abd II–III Dorsal Vesicles | Large Abdomen Dorsal Long Chaetae | Abd V Dorsal Processes (♂) | Mod. Ventral Tube (♂) | Proximal Tibiotarsus I Organ (♂) | Proximal Tibiotarsus II Mod Chaetae | Distal Tibiotarsus III Organ | Leg II Clasping Organ (♂) | Ungues I–II/III Ratio | Dental Spiniform Chaetae (J Line) | Mucro Shape | Mucronal Chaeta |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boernerides [5,39] | 1 | b1–b6, tra1 | c1,c3 | − | − | − | − | − | − | + | − | + | − | − | − | − | + | − | >? | + | na | − |
| Debouttevillea [4,27,40] | 1 | B1, b1–b4, tra1 | c1–c3 | − | − | − | + | − | − | + | +(♂,♀) | − | − | + | − | +(♀) | − | − | >? | − | wi | + |
| Denisiella [4,14,20,27,41,42,43,44,45] | 13 | b1–7, tra1–2 | c1,c2(+/−), c3, tra3 | − | − | +/−(se) | − | − | − | − | − | +/− | − | − | +/− | +/− | − | − | >/= | − | na | + |
| Pedonides * [46] | 1 | b1–b5 | c1–c3 | ? | ? | ? | ? | − | − | + | − | − | − | − | + | + (♂) | + | + | = | + | na | − |
| Pseudosminthurides§ [7] | 1 | ? | ? | ? | ? | ? | ? | − | −? | ? | ? | ? | ? | ? | ? | ? | ? | ? | > | ? | wi | − |
| Pygicornides [4,27,47,48] | 2 | b1–b6, tra1–2 | c1–c3 | ? | ? | ? | + | +/− | − | + | − | + | + | − | − | −? | + | − | >? | − | wi | + |
| Sinnamarides [49] | 1 | B1, b1–b5, tra1 | c1–c3 | − | − | − | − | − | + | + | − | + | − | − | − | − | + | − | = | − | na | + |
| Sminthurides [4,5,14,19,27,39,50,51] | 56 | B1(+/−), b1–b6(+/−), tra1, tra2(+/−) | c1–c3 | − | − | +/−(se) | +/− | +/− | − | + | − | +(♀) | − | − | − | − | + | − | > | −/+ | wi/ na | +/− |
| Sminthuridia [4,27,52] | 1 | b1–b3 | c3 | −? | − | +(bl) | + | + | − | + | − | + | − | − | −? | −? | + | − | < | − | wi | + |
| Sphaeridia [4,5,14,18,27,53,54,55] | 69 | b1 | c3 | − | − | − | − | − | − | − | − | + | − | + | − | −? | − | − | >/= | +/− | na | − |
| Stenacidia ** [4,5,14,37,38] | 1 | B1, b1–b6, tra1 | c1–c3 | − | +(bl) | +(bl) | − | − | − | + | − | +(♂) | − | − | − | − | + | − | </> | +(♂) | na | + |
| Yosiides [4,27,56,57] | 2 | b1–b6, tra1(+/−) | c1–c3 | − | − | − | + | + | − | − | − | + | − | − | −? | ? | + | − | > | − | na | + |
| Parasminthurides gen. nov. | 1 | B1, b1–b5, tra1 | c1–c3 | +(sp) | +(sp) | − | − | − | + | + | +(♂) | + | − | − | − | − | + | − | = | + | wi | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, G.d.S.; Nunes, R.C.; Zhang, F.; Godeiro, N.N.; Bellini, B.C. A New Genus of Sminthurididae (Collembola, Symphypleona) from Brazil, with Notes on the Systematics of the Family. Diversity 2022, 14, 960. https://doi.org/10.3390/d14110960
Medeiros GdS, Nunes RC, Zhang F, Godeiro NN, Bellini BC. A New Genus of Sminthurididae (Collembola, Symphypleona) from Brazil, with Notes on the Systematics of the Family. Diversity. 2022; 14(11):960. https://doi.org/10.3390/d14110960
Chicago/Turabian StyleMedeiros, Gleyce da Silva, Rudy Camilo Nunes, Feng Zhang, Nerivânia Nunes Godeiro, and Bruno Cavalcante Bellini. 2022. "A New Genus of Sminthurididae (Collembola, Symphypleona) from Brazil, with Notes on the Systematics of the Family" Diversity 14, no. 11: 960. https://doi.org/10.3390/d14110960
APA StyleMedeiros, G. d. S., Nunes, R. C., Zhang, F., Godeiro, N. N., & Bellini, B. C. (2022). A New Genus of Sminthurididae (Collembola, Symphypleona) from Brazil, with Notes on the Systematics of the Family. Diversity, 14(11), 960. https://doi.org/10.3390/d14110960









