Chemical Cues Used by the Weevil Curculio chinensis in Attacking the Host Oil Plant Camellia oleifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Extraction and GC-MS Identification of Host Plant Chemicals
2.3. GC-EAD of Cu. Chinensis Volatiles
2.4. Behavioral Bioassays to Single Compound with Y-Tube Olfactometer
2.5. Statistical Analyses
3. Results
3.1. Identification of Plant Volatiles with GC-MS
3.2. GC-EAD Analysis
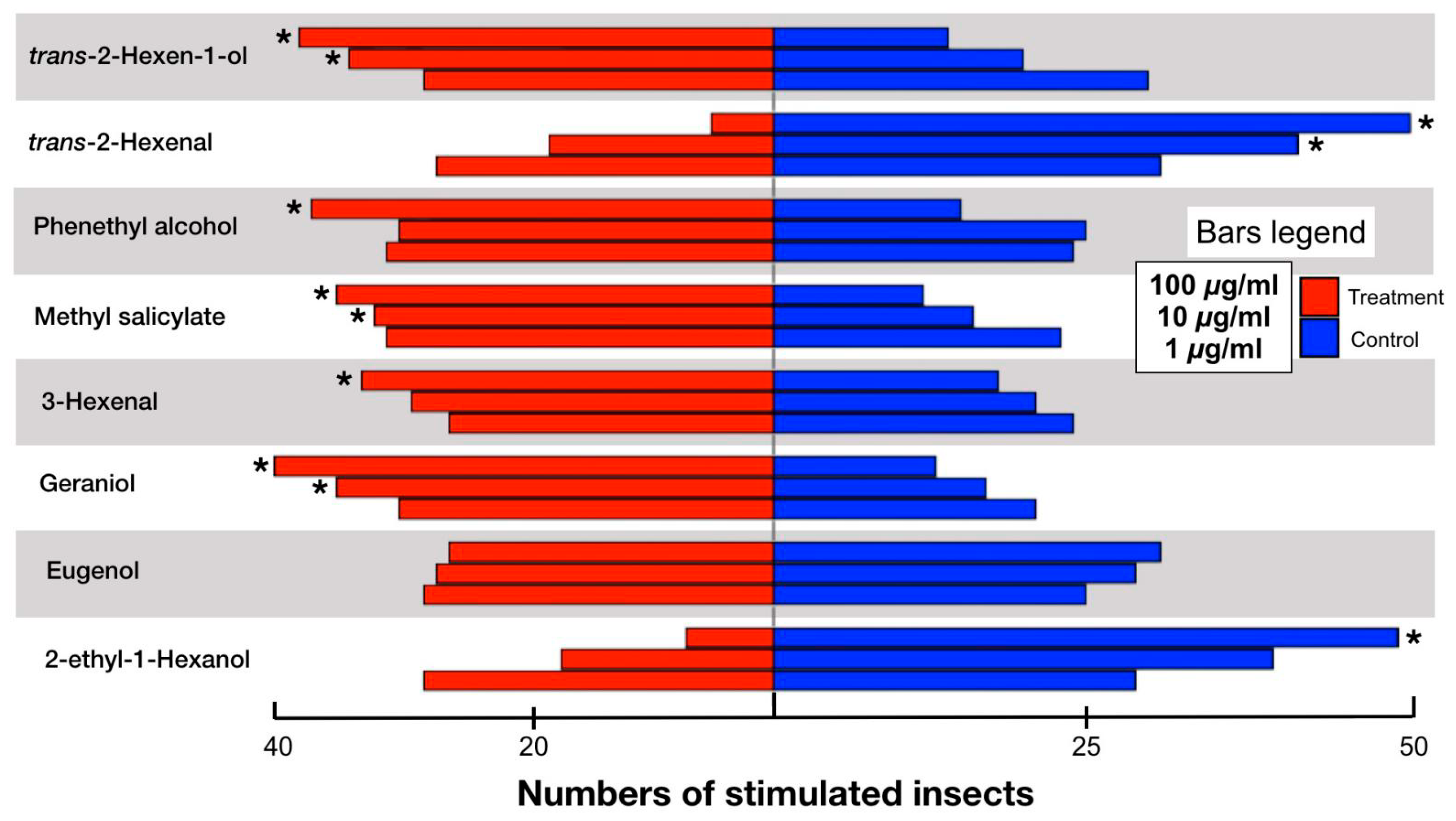
3.3. Behavioral Responses in Cu. chinensis to the GC-EAD Active Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Ciou, Y.P.; Huang, Y.C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Intern. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.J.; Wang, C.Z.; Chen, H.X.; Zhou, H.; Ye, J.Z. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, L.C.; Chen, D.; Li, M.; Wen, C.J. Soil quality assessment of different Camellia oleifera stands in mid-subtropical China. Appl. Soil Ecil. 2017, 113, 29–35. [Google Scholar] [CrossRef]
- Deng, X.; Tang, J.; Qin, Q.; Qin, Z.; Yan, Q.; Yang, W.; Deng, Y.; Song, X.; He, Y. Primary investigation of diseases and pests on oil Camellia (Camellia oleifera Abel) in Guangxi province of China and suggestions for prevention and control. Plant Dis. Pest 2013, 4, 35–38. [Google Scholar]
- Cai, S.P.; He, X.Y.; Li, Z.Z.; Xiong, Y.; Huang, J.S.; Zhou, S.Y. Study on damage of Curculio chinensis on Camellia oleifera fruit. Chinses. J. Fujian For. Sci. Technol. 2011, 38, 14–16. (In Chinese) [Google Scholar]
- Zhou, S. Biology and control of the Camellia weevil Curculio chinensis Chevrolat. Acta Entomol. Sin. 1981, 24, 48–52. (In Chinese) [Google Scholar]
- Xu, L.; Pan, Y.Z.; Huang, Y.Y. Predicting the potential geographical distribution of Curculio chinensis Cheveolat. J. Fujian For. Sci. Technol. 2011, 38, 55–58. (In Chinese) [Google Scholar]
- Zhang, S.; Shu, J.; Xue, H.; Zhang, W.; Wang, Y.; Liu, Y.; Wang, H. Genetic diversity in the camellia weevil, Curculio chinensis Chevrolat (Coleoptera: Curculionidae) and inferences for the impact of host plant and human activity. Entomol. Sci. 2018, 21, 447–460. [Google Scholar] [CrossRef]
- Li, Z.W.; He, L.H.; Yang, L.J.; He, B.; Zeng, A.P. Opposition strategy of the camellia weevil, Curculio chinensis (Coleoptera: Curculionidae), on oil tea (Camellia meiocarpa). Acta Entomol. Sin. 2015, 58, 981–988. [Google Scholar]
- Zhao, D.Y.; Qin, C.S.; Xu, J.Z.; Liao, F.Y.; Jie, Y.Z. Relative preferences of Curculio chinensis adults for different varieties of tea-oil trees. Chin. Agric. Sci. Bull. 2015, 31, 100–104. (In Chinese) [Google Scholar]
- Bruce, T.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulou, M.A.; Krokos, F.D.; Mazomenos, B.E. Chemical stimuli from corn plants affect host selection and oviposition behavior of Sesamia nonagrioides (Lepidoptera: Noctuidae). J. Econ. Entomol. 2003, 95, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Wolfin, M.S.; Chilson, R.R.; Thrall, J.; Liu, Y.; Linn, C.E. Proximate mechanisms of host plant location by a specialist phytophagous insect, the grape berry moth, Paralobesia Viteana. J. Chem. Ecol. 2019, 45, 946–958. [Google Scholar] [CrossRef]
- Wang, H.M.; Bai, P.H.; Zhang, J.; Zhang, X.M.; Hui, Q.; Zheng, H.X.; Zhang, X.H. Attraction of bruchid beetles Callosobruchus chinensis (L) (Coleoptera: Bruchidae) to host plant volatiles. J. Integr. Agric. 2020, 19, 3035–3044. [Google Scholar] [CrossRef]
- Joseph, C.; Dickens, J.C. Green leaf volatiles enhance aggregation pheromone of boll weevil, Anthonomus grandis. Entomol. Exp. Appl. 2011, 52, 191–203. [Google Scholar]
- Eller, F.J.; Bartelt, R.J.; Shasha, B.S.; Schuster, D.J.; Riley, D.G.; Stansly, P.A.; Mueller, T.F.; Shuler, K.D.; Johnson, B.; Davis, J.H.; et al. Aggregation pheromone for the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae): Identification and field activity. J. Chem. Ecol. 1994, 20, 1537–1556. [Google Scholar] [CrossRef]
- Jin, X.F.; Qin, C.S.; Zhao, D.Y.; Xu, J.Z.; Tian, L.Y.; Yand, H.; Qiu, H.L. The research and application progress of the Curculionidae sex pheromones and aggregation pheromone. Forest. Environ. Sci. 2019, 35, 102–110. (In Chinese) [Google Scholar]
- Szendrei, Z.; Malo, E.; Stelinski, L.; Rodriguez-Saona, C. Response of cranberry weevil (Coleoptera: Curculionidae) to host plant volatiles. Environ. Entomol. 2009, 38, 861–869. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Q.; Shi, L.; Chen, G.; Zeng, Y.; Shi, C.; Zhang, Y. Electrophysiological and behavioral responses of Bradysia odoriphaga (Diptera: Sciaridae) to volatiles from its host plant, Chinese chives (Allium tuberosum Rottler ex Spreng). J. Econ. Entomol. 2019, 4, 1638–1644. [Google Scholar] [CrossRef]
- Fenemore, P.G. Host-plant location and selection by adult potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae)—A review. J. Insect. Physiol. 1988, 34, 175–177. [Google Scholar] [CrossRef]
- Leal, W.S.; Uchida, K. Application of GC-EAD to the determination of mosquito repellents derived from a plant, Cymbopogon citratus. J. Asia-Pac. Entomol. 1998, 1, 217–221. [Google Scholar] [CrossRef]
- Visser, J.H. Electroantennogram responses of the colorado beetle, Leptinotarsa decemlineata, to plant volatiles. Entomol. Exp. Appl. 1979, 25, 86–97. [Google Scholar] [CrossRef]
- Sun, X.L.; Li, X.W.; Xin, Z.J.; Han, J.J.; Ran, W.; Lei, S. Development of synthetic volatile attractant for male Ectropis obliqua moths. J. Integr. Agric. 2016, 15, 1532–1539. [Google Scholar] [CrossRef]
- Fill, A.; Barrett, B.A. Electroantennographic and behavioral responses of the lesser chestnut weevil, Curculio sayi (Coleoptera: Curculionidae), to mixtures of selected key host plant volatiles. J. Kans. Entomol. Soc. 2016, 89, 128–137. [Google Scholar] [CrossRef]
- Hori, M.; Ohuchi, K.; Matsuda, K. Role of host plant volatile in the host-finding behavior of the strawberry leaf beetle, Galerucella vittaticollis Baly (Coleoptera: Chrysomelidae). Appl. Entomol. Zool. 2006, 41, 357–363. [Google Scholar] [CrossRef][Green Version]
- Xu, W.; Ma, Y.; Zhang, Y.; Dong, Y.; Sun, X. Electroantennographic and behavioral responses of four spotted beetle Popillia quadriguttata to 12 plant volatiles. Chin. J. Plant Prot. 2018, 45, 1028–1034. (In Chinese) [Google Scholar]
- Xiu, C.L.; Pan, H.S.; Liu, B.; Luo, Z.X.; Lu, Y.H. Perception of and behavioral responses to host plant volatiles for three Adelphocoris species. J. Chem. Ecol. 2019, 45, 779–788. [Google Scholar] [CrossRef]
- Pan, H.; Lu, Y.; Xiu, C.; Geng, H.; Cai, X.; Sun, X.; Zhang, Y.; Wyckhuys, K.; Wu, K. Volatile fragrances associated with flowers mediate host plant alternation of a polyphagous mirid bug. Sci. Rep. 2015, 5, 14805. [Google Scholar] [CrossRef]

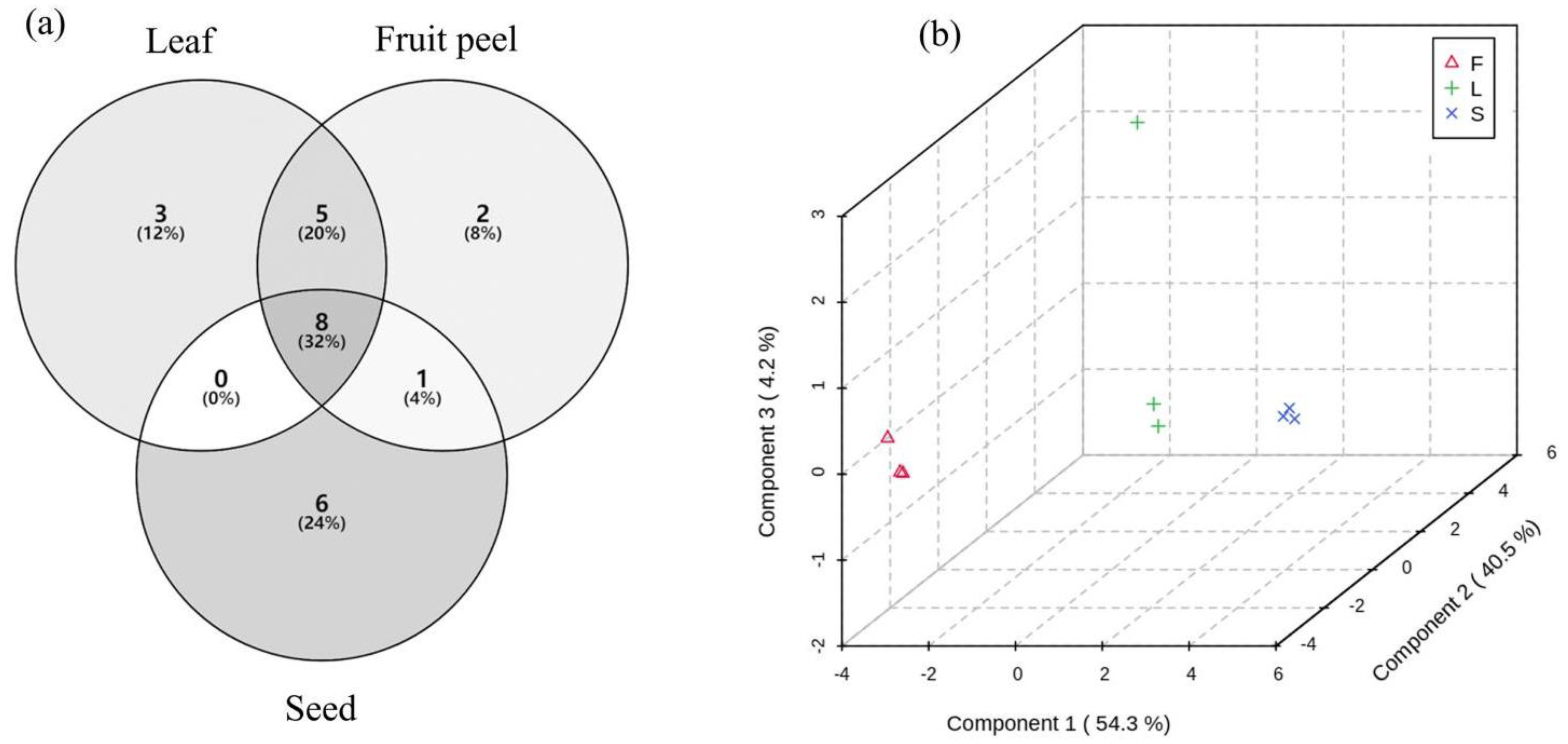

| No. | Chemical Name | RT (min) | Relative Proportion % (Mean ± SE) | ||
|---|---|---|---|---|---|
| Leaves | Fruit Peel | Seeds | |||
| 1 | 3-Hexenal * | 3.34 | 18.99 ± 1.06 | 2.70 ± 0.15 | 4.50 ± 0.22 |
| 2 | trans-2-hexenal * | 4.04 | 10.09 ± 0.54 | 15.62 ± 0.58 | 13.18 ± 1.03 |
| 3 | trans-2-Hexen-1-ol * | 4.27 | 3.90 ± 0.32 | 5.93 ± 1.64 | 3.77 ± 0.06 |
| 4 | α-Pinene | 5.13 | / | 4.90 ± 0.25 | / |
| 5 | Fumaric acid | 6.35 | 3.46 ± 0.78 | 5.50 ± 0.15 | 1.42 ± 0.67 |
| 6 | 2-Ethyl-1-hexanol * | 6.57 | / | / | 3.44 ± 0.11 |
| 7 | γ-Terpinene | 7.08 | 3.83 ± 0.25 | 9.01 ± 0.23 | 3.13 ± 0.22 |
| 8 | Linalool | 7.65 | / | / | 1.98 ± 0.08 |
| 9 | Phenethyl alcohol * | 7.88 | / | / | 10.09 ± 0.11 |
| 10 | Terpinen-4-ol | 8.90 | 2.94 ± 0.18 | 5.34 ± 0.12 | 1.66 ± 0.92 |
| 11 | Methyl salicylate * | 9.14 | / | / | 2.49 ± 0.13 |
| 12 | Geraniol * | 9.92 | / | 2.39 ± 0.30 | 27.64 ± 0.74 |
| 13 | Eugenol * | 11.40 | / | / | 17.94 ± 0.36 |
| 14 | n-Tetradecane | 11.89 | 11.65 ± 0.76 | 7.97 ± 0.70 | 2.23 ± 0.14 |
| 15 | α-Cedrene | 12.25 | 2.60 ± 0.23 | 3.49 ± 0.82 | / |
| 16 | β-Caryophyllene | 12.33 | / | 9.16 ± 0.13 | / |
| 17 | Tetradecane, 2,6,10-trimethyl- | 12.69 | 2.67 ± 0.07 | / | / |
| 18 | α-Farnesene | 13.30 | / | / | 3.93 ± 0.52 |
| 19 | Butylated hydroxytoluene | 13.41 | 2.51 ± 0.27 | 2.79 ± 0.16 | / |
| 20 | n-Hexadecane | 14.35 | 12.26 ± 1.23 | 12.32 ± 0.87 | 2.60 ± 0.09 |
| 21 | Hexadecane, 2,6,11,15-tetramethyl- | 15.63 | 4.87 ± 0.17 | 3.67 ± 0.09 | / |
| 22 | n-Octadecane | 16.57 | 10.09 ± 0.55 | 5.79 ± 0.13 | / |
| 23 | Heptadecane, 2,6,10,15-tetramethyl- | 17.88 | 4.78 ± 0.22 | 3.41 ± 0.07 | / |
| 24 | Eicosane, 2-methyl- | 18.31 | 2.80 ± 0.11 | / | / |
| 25 | n-Eicosane | 18.59 | 3.26 ± 0.14 | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Zhao, D.; Fox, E.G.P.; Ling, S.; Qin, C.; Xu, J. Chemical Cues Used by the Weevil Curculio chinensis in Attacking the Host Oil Plant Camellia oleifera. Diversity 2022, 14, 951. https://doi.org/10.3390/d14110951
Qiu H, Zhao D, Fox EGP, Ling S, Qin C, Xu J. Chemical Cues Used by the Weevil Curculio chinensis in Attacking the Host Oil Plant Camellia oleifera. Diversity. 2022; 14(11):951. https://doi.org/10.3390/d14110951
Chicago/Turabian StyleQiu, Hualong, Danyang Zhao, Eduardo G. P. Fox, Siquan Ling, Changsheng Qin, and Jinzhu Xu. 2022. "Chemical Cues Used by the Weevil Curculio chinensis in Attacking the Host Oil Plant Camellia oleifera" Diversity 14, no. 11: 951. https://doi.org/10.3390/d14110951
APA StyleQiu, H., Zhao, D., Fox, E. G. P., Ling, S., Qin, C., & Xu, J. (2022). Chemical Cues Used by the Weevil Curculio chinensis in Attacking the Host Oil Plant Camellia oleifera. Diversity, 14(11), 951. https://doi.org/10.3390/d14110951







