Abstract
Invasive species impact both the local biota and human economies and are a very serious threat to biodiversity. The American bullfrog was initially introduced into northwestern China and many other sites for aquacultural purposes. Then, the frogs escaped and established feral populations. Here, we investigated the variations in age, body size, and sexual size dimorphism of two breeding populations inhabiting the southern (warm and dry) and northern (cold and wet) regions of Xinjiang province in northwestern China. Both populations originated from a single source that was introduced into Xinjiang 54 years ago. In both populations, males were significantly smaller than females, indicating significant sexual size dimorphism. The mean age and body size of both sexes in the population in the colder region were lower than those of the population in the warmer region. Bullfrogs in the southern population had a higher degree of sexual size dimorphism. These results increase our understanding of the American bullfrog, which could help in the development of strategies to control this invasive species.
1. Introduction
Biological invasions seriously threaten native biodiversity [1]. Globalization has accelerated species invasion [2]. The American bullfrog, Rana catesbeiana, which is native to North America, is listed on the “100 of the World’s Worst Invasive Alien Species” list in the Global Invasive Species Database [3]. It has been introduced all over the world and has successfully invaded many countries [4]. These bullfrogs affect local amphibians through predation and competition for habitat and food and thus are a serious threat to native amphibian species [4].
Successful invaders typically show a high degree of variation in life history traits, while failed invaders have poor adaptability to geographical changes [5]. For example, the life history traits of Gambusia holbrooki, one of the most invasive fish species, vary in different environments. The population living in the warmer environment in the south allocates more energy to reproduction and matures to a larger size than the northern population [5]. Therefore, when evaluating the impacts of invasive species on native biota, we need to consider the geographical variations in morphology and their implications [6].
Age, body size, and sexual size dimorphism are important ecological traits. They are products of ecological and evolutionary plasticity and can vary geographically [7,8]. Many abiotic and biotic factors influence body size and sexual size dimorphism [9]. For anurans, such as bullfrogs, sexual size dimorphism is directly related to variations in life history strategy between sexes and variations in age and body size. To explore the variation in life history strategies, we need to assess age, body size, and sexual size dimorphism [10]. According to life history theories, in colder, harsher environments, anurans show reduced growth rates, increased time to maturity, reduced life expectancy, and increased female-biased sexual size dimorphism [6,10].
The invasive American bullfrog was introduced from a single source in Cuba to China in the 1950s as a food source [4,11]. They were introduced into Xinjiang in northwestern China in the 1960s [12,13]. After escaping from aquaculture farms, the bullfrog successfully invaded the environment in many areas of Xinjiang [14]. The diverse environments of Xinjiang provide excellent systems for studying variations related to habitat. In this study, we examined the age, body size, and sexual size dimorphism of adult bullfrog populations in the south (in Aksu, which has a warm climate) and the north (in Shihezi, which has a cold climate) and evaluated the life history traits related to the geographical variations in these non-native ranges. According to the life history theories and characteristics of anurans, we predicted that the bullfrog population in the southern region will be older, with a larger bigger body size and a higher degree of sexual size dimorphism.
2. Materials and Methods
2.1. Study Area and Field Survey
The study area was located in Xinjiang province of northwestern China. The Tianshan Mountains run through Xinjiang and divide the province into northern and southern regions. There are two populations of bullfrogs in Xinjiang, one in Aksu in the south (N 40.467610), and another in Shihezi in the north (N 44.481218). The southern population resides near a desert, where the climate is relatively warm and dry, with a mean annual temperature of 10.83 °C (range, −14–31 °C) and 74.4 mm of annual precipitation (mean over the last 10 years). The northern population lives near a lake, where it is relatively cold and wet compared to the southern region, with a mean annual temperature of 7.29 °C (range, −21–25 °C) and 206 mm of annual precipitation (mean over the last 10 years). We sampled these two bullfrog populations during the summer of 2015, collecting 131 adult frogs: 71 from the southern population (32 males and 39 females) and 60 from the northern population (31 males and 29 females). The bullfrogs were randomly collected by hand with the help of a dip-net during the night from 19:00 to 24:00 [10].
2.2. Sex Determination and Morphological Measurements
Males were identified based on the presence of swollen nuptial pads and yellow throat pigment [10]. A bullfrog without these characteristics was assumed to be female [15]. The snout to vent length (SVL) of each bullfrog was measured (to the nearest 0.1 mm) using a vernier caliper [10].
2.3. Age Estimation
Skeletochronology, a reliable method for determining the age of many amphibian species [7,10,16], was used to estimate bullfrog age [10]. Briefly, the third toe phalanx of the hind foot was clipped, dehydrated with ethyl alcohol, and embedded in paraffin for sectioning. Then, the sections were stained with Ehrlich’s hematoxylin and observed under a microscope. Age was determined by counting the number of lines of arrested growth (LAGs), and each LAG was counted as 1 year of age. LAGs were independently counted by two different people using the same counting rule. Any disagreement between the two counters was resolved by a third counter; and the count as determined by two people was used as the final result [7,10,16].
2.4. Statistical Analysis
Normality was assessed using the Kolmogorov–Smirnov test. Because all variables were normally distributed, two-way ANOVA was used to compare mean age between sexes and air temperature at the two sites and to evaluate the interactive effects of these two factors. Partial correlation analysis was used to evaluate the relationship between age and body size after controlling for the effects of site and sex before comparing body size according to temperature and sex. Since age was positively correlated with body size (r = 0.974, df = 127, and p = 0.000), we used ANCOVA with age as the covariate to evaluate the variation in SVL between sexes and sites after controlling for the effects of age [10].
The sexual dimorphism index (SDI) was used to estimate sexual size dimorphism for each age group at each site, where SDI = (mean size of the larger sex/mean size of the smaller sex) − 1 [10,16]. Then, the sex ratio (male:female) was calculated for each age group and each population. ANOVA was used to compare sex ratios and SDI between the southern and northern populations. All analyses were conducted using SPSS 21.0, and two-tailed significance level was set at p < 0.05 [10,16].
3. Results
3.1. Age
There were significant differences in the mean age between the sexes in both populations, and the mean age of males was significantly lower than that of females (ANOVA; F [1,127] = 5.429, p = 0.021). In addition, the mean age of the northern population was lower than that of the southern population (F [1,127] = 5.146, p = 0.025). However, there was no interactive effect of latitude and sex (F [1,127] = 0.000, p = 0.996; Figure 1).
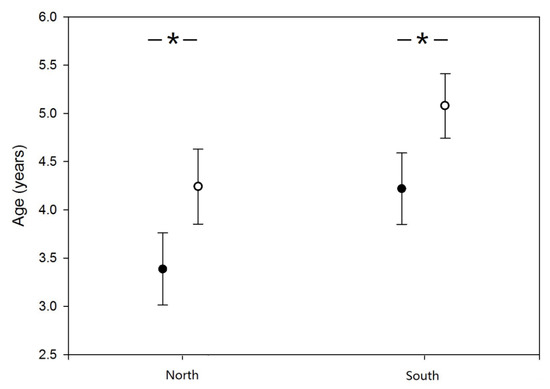
Figure 1.
Sex-specific variation in age of American bullfrogs in the northern and southern populations in northwestern China. Open and closed circles represent females and males, respectively. “*” represent significant difference.
3.2. Body Size
Bullfrogs in both populations exhibited significant female-biased sexual size dimorphism after controlling for the effect of age (ANCOVA; F [1,127] = 16.469, p < 0.001). The mean body size of bullfrogs in the northern population was significantly smaller than that of frogs in the southern population (F [1,127] = 18.256, p < 0.001). However, there was no interactive effect between latitude and sex on body size (F [1,127] = 1.292, p = 0.258; Figure 2).
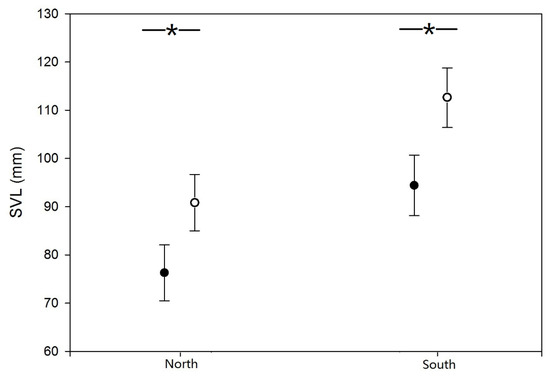
Figure 2.
Sex-specific variation in body size (SVL) of American bullfrogs in the northern and southern populations in northwestern China. Open and closed circles represent females and males, respectively. “*” represent significant difference.
3.3. Sex Ratio
There was no significant difference in the sex ratio between the two populations (ANOVA; F [1,13] = 0.499, p = 0.492). The sex ratio was negatively correlated with age in the southern population but not in the northern population (Spearman rho; Southern = −0.964, p < 0.001; Northern = −0.643, p = 0.119; Figure 3).
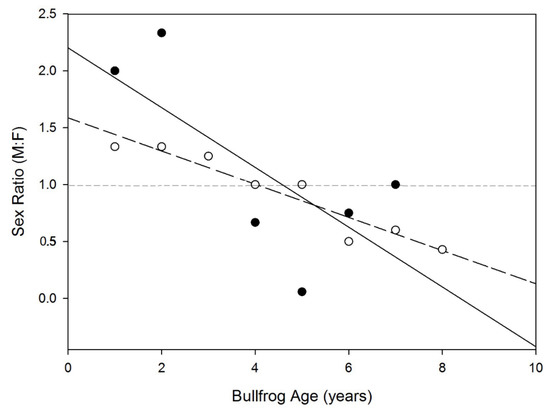
Figure 3.
Age-specific variation in the sex ratio of American bullfrogs in the northern and southern populations of northwestern China. Open circle and dash line represent the southern population. Filled circle and solid line represent the northern population. The horizontal line in the figure represent the equal abundance of both sexes in the population.
3.4. Sexual Size Dimorphism
Sexual size dimorphism was significantly greater in the southern population (ANOVA; F [1,13] = 4.944, p = 0.045) and was consistent with older age of females in this population. Sexual size dimorphism was not significantly correlated with age in either population (Spearman rho; Southern = −0.095, p = 0.823; Northern = 0.071, p = 0.879; Figure 4).
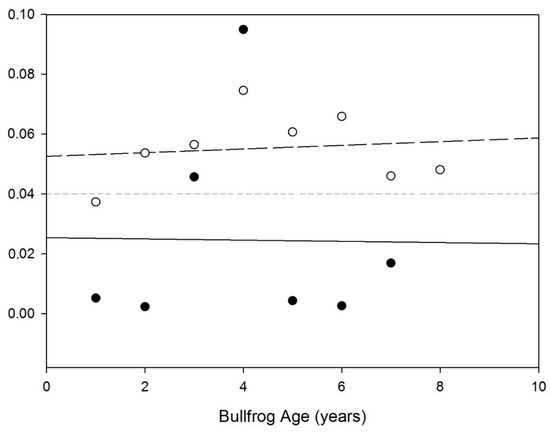
Figure 4.
Age-specific variation in the degree of sexual size dimorphism index (SDI) of American bullfrogs in the northern and southern populations in northwestern China. Open circle and dash line represent the southern population. Filled circle and solid line represent the northern population. The horizontal line in the figure represent the median SDI value.
4. Discussion
Geographical variations in body size are common for vertebrates, and body size may change with latitude or altitude [8,17], although temperature is thought to be the main factor related to size variation [18]. Geographical variations in age, body size, and sexual size dimorphism have been commonly reported for anurans [7,8], and the mechanism underlying this variation is complex compared to that for endotherms [6,19]. Geographical variability in body size is particularly interesting, as it is an important index of fitness and can influence the animal’s life history [20]. This knowledge will help us understand how to better manage biological invasions.
This study showed the geographical variations in age, body size, and sexual size dimorphism of the invasive American bullfrog in northwestern China; such data have also been reported for other invasive bullfrog populations [10] and other similar invasive species [21]. The geographical variations in the life history traits observed in this study could be caused by many factors, such as evolutionary adaptation and fitness differences between the populations. Since we have not conducted a genetic analysis of adaptation, our study data do not support evolutionary adaptation. Therefore, differences in the fitness of the two populations may be the main reason for the geographical variations. Difference in mortality between the sexes can cause variations in the age and sex ratio and influence sexual size dimorphism. Similar results have been reported for other studies of amphibians [6,10].
The difference in age structure is the main cause of the variation in mean body size between both the two populations and the sexes. Both mean age and body size were lower in males in the northern population, suggesting that geographical variations in mean body size and sexual size dimorphism may be caused by the difference in age distribution.
Studies have shown that temperature is important for variations in bullfrog life history traits, regardless of altitude or latitude [10]. In contrast, precipitation has limited effects on bullfrog life history traits. Although we found significant differences in the life history traits of two populations that live in areas with different precipitation levels, we cannot conclude that these variations were caused by a difference in precipitation rather than a difference in temperature. Thus, the effects of precipitation on the life history traits of the American bullfrog require further study.
It is important to determine the reasons why alien species can successfully invade and spread into new habitats. In our study, we found that the bullfrog living in different environments and descended from a single source population could have geographical variations in life history traits. This partly explains why the American bullfrog is one of the most invasive species in the world. To minimize the impact of any invasive species on native habitats, further study is needed to explain the mechanisms driving these geographical variations.
Author Contributions
Conceptualization, F.X.; methodology, F.X. and J.L; software, F.X.; validation, F.X. and J.L.; formal analysis, F.X.; investigation, F.X. and J.L.; resources, F.X., J.L. and W.Y.; data curation, F.X. and J.L.; writing—original draft preparation, F.X.; writing—review and editing, F.X., J.L. and W.Y.; supervision, F.X. and W.Y.; project administration, F.X. and W.Y.; funding acquisition, F.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Third Xinjiang Scientific Expedition Program (grant number 2021xjkk0600), a Grant for High Quality Economic and Social Development in South Xinjiang (grant number NFS2101), and the China Biodiversity Observation Network (Sino-BON).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences (20220620-1, 2022.06.20).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Xinyun Li, Rui Yu, Yuliang Pan, Yunyun Zhang, Liying Yin, and Hongkui Zhang for their help with the field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Floerl, O.; Inglis, G.J.; Dey, K.; Smith, A. The importance of transport hubs in stepping-stone invasions. J. Appl. Ecol. 2009, 46, 37–45. [Google Scholar] [CrossRef]
- ISSG. Invasive Species Specialist Group. 2008. Available online: http://www.issg.org/database/welcome/ (accessed on 6 June 2020).
- Li, Y.M.; Ke, Z.; Wang, Y.; Blackburn, T.M. Frog community responses to recent American bullfrog invasions. Curr. Zool. 2011, 57, 83–92. [Google Scholar] [CrossRef]
- Benejam, L.; Alcaraz, C.; Sasal, P.; Simon-Levert, G.; García-Berthou, E. Life history and parasites of the invasive mosquito fish (Gambusia holbrooki) along a latitudinal gradient. Biol. Invasions 2009, 11, 2265–2277. [Google Scholar] [CrossRef]
- McGarrity, M.E.; Johnson, S.A. Geographic trend in sexual size dimorphism and body size of Osteopilus septentrionalis (Cuban treefrog): Implications for invasion of the southeastern United States. Biol. Invasions 2009, 11, 1411–1420. [Google Scholar] [CrossRef]
- Liao, W.B.; Lu, X. Adult body size = f (initial size + growth rate × age): Explaining the proximate cause of Bergman’s cline in a toad along altitudinal gradients. Evol. Ecol. 2016, 26, 579–590. [Google Scholar] [CrossRef]
- Liao, W.B.; Luo, Y.; Luo, S.L.; Lu, D.; Robert, J. Geographic variation in life-history traits: Growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi). Front. Zool. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Travis, J. Evaluating the adaptive role of morphological plasticity. In Ecological Morphology: Integrative Organismal Biology; Wainright, P.C., Reilly, S.M., Eds.; University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Liu, X.; Li, Y.M.; McGarrity, M. Geographical variation in body size and sexual size dimorphism of introduced American bullfrogs in southwestern China. Biol. Invasions 2010, 12, 2037–2047. [Google Scholar] [CrossRef][Green Version]
- Bai, C.M.; Ke, W.Z.; Sofia, C.; Liu, X.; Li, Y.M. The role of founder effects on the genetic structure of the invasive bullfrog (Lithobates catesbeianaus) in China. Biol. Invasions 2012, 14, 1785–1796. [Google Scholar] [CrossRef]
- Xu, H.; Qiang, S.; Han, Z.; Gu, J.; Huang, Z.; Sun, H.; He, S.; Ding, H.; Wu, H.; Wan, F. The status and causes of alien species invasion in China. Biodivers. Conserv. 2006, 15, 2893–2904. [Google Scholar] [CrossRef]
- Zeng, X.J. Prospect and strategy of bullfrog farming. Inland Fish 1998, 10, 4–5. [Google Scholar]
- Xu, F.; Yang, W.K. The alien invasive American bullfrogs (Lithobates catesbeianus = Rana catesbeiana) in Xinjiang Uygur Autonomous Region, China. Chin. J. Zool. 2019, 54, 195. [Google Scholar]
- Howard, R.D. Evolution of mating strategies in bullfrogs, Rana-Catesbeiana. Evolution 1978, 32, 850–871. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Xu, F.; Guo, Z.W.; Liu, X.; Jin, C.N.; Wang, Y.P.; Wang, S.P. Reduced predator species richness drives the body gigantism of a frog species on the Zhoushan Archipelago in China. J. Anim. Ecol. 2011, 80, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ashton, K.G.; Feldman, C.R. Bergmann’s rule in non-avian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution 2003, 57, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.M.; Gaston, K.J.; Loder, N. Geographic gradients in body size: A clarification of Bergmann’s rule. Divers. Distrib. 1999, 5, 165–174. [Google Scholar] [CrossRef]
- Morrison, C.; Hero, J.M. Geographic variation in life-history characteristics of amphibians: A review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef]
- Stearn, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Bohn, T.; Sandlund, O.T.; Amundsen, P.A.; Primicerio, R. Rapidly changing life history during invasion. Oikos 2004, 106, 138–150. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).