Microbial Communities of Ferromanganese Sedimentary Layers and Nodules of Lake Baikal (Bolshoy Ushkany Island)
Abstract
1. Introduction
2. Materials and Methods
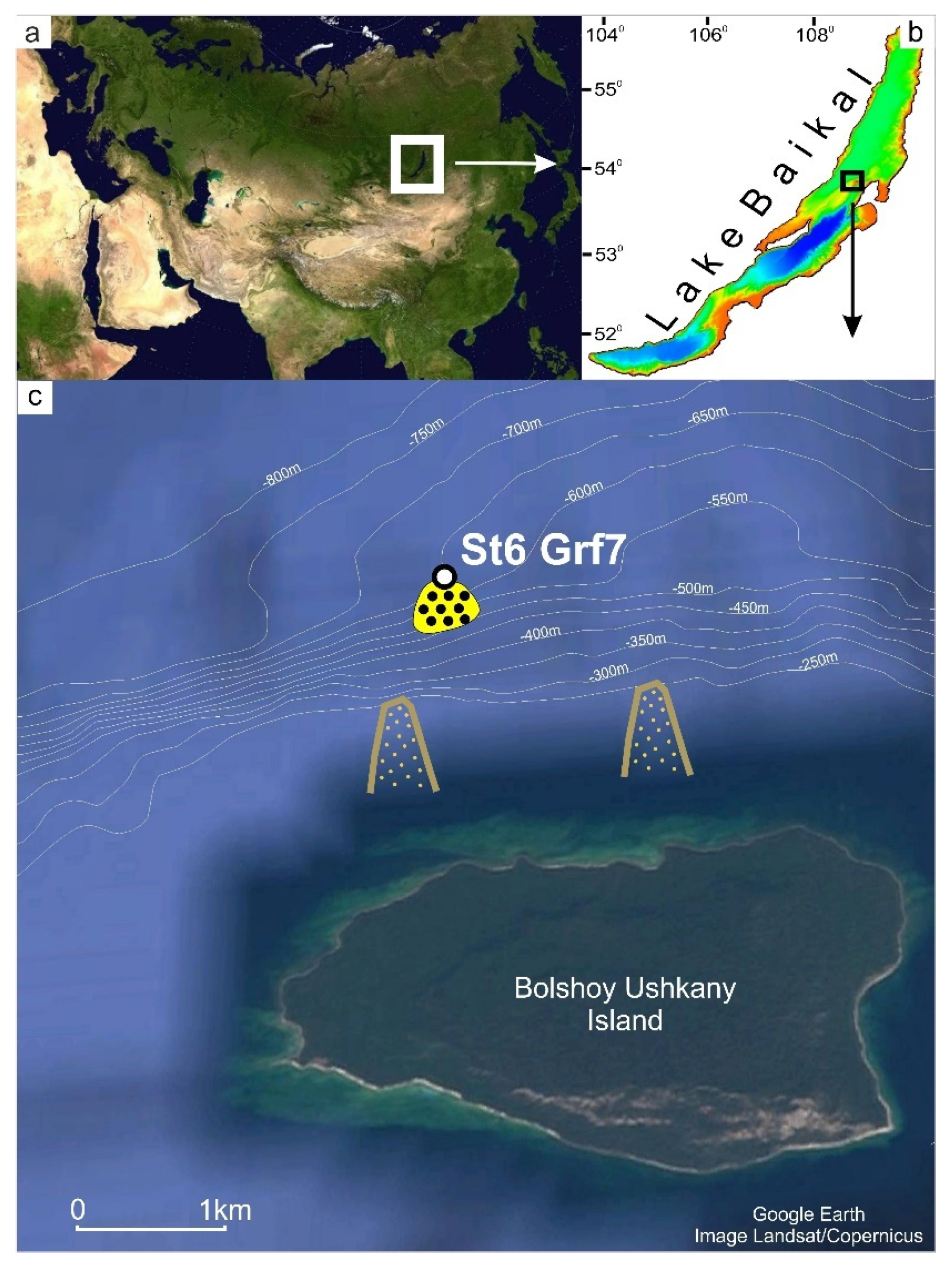
2.1. Sample Collection
2.2. Chemical Analysis
2.3. Age Dating
2.4. Methane Concentration
2.5. Molecular Microbiological Methods
2.6. Data Analysis
3. Results
3.1. Properties of Sediments
3.1.1. Lithology
3.1.2. Chemical Composition of Pore Water
3.2. Properties of Fe-Mn Nodules
3.2.1. Morphology
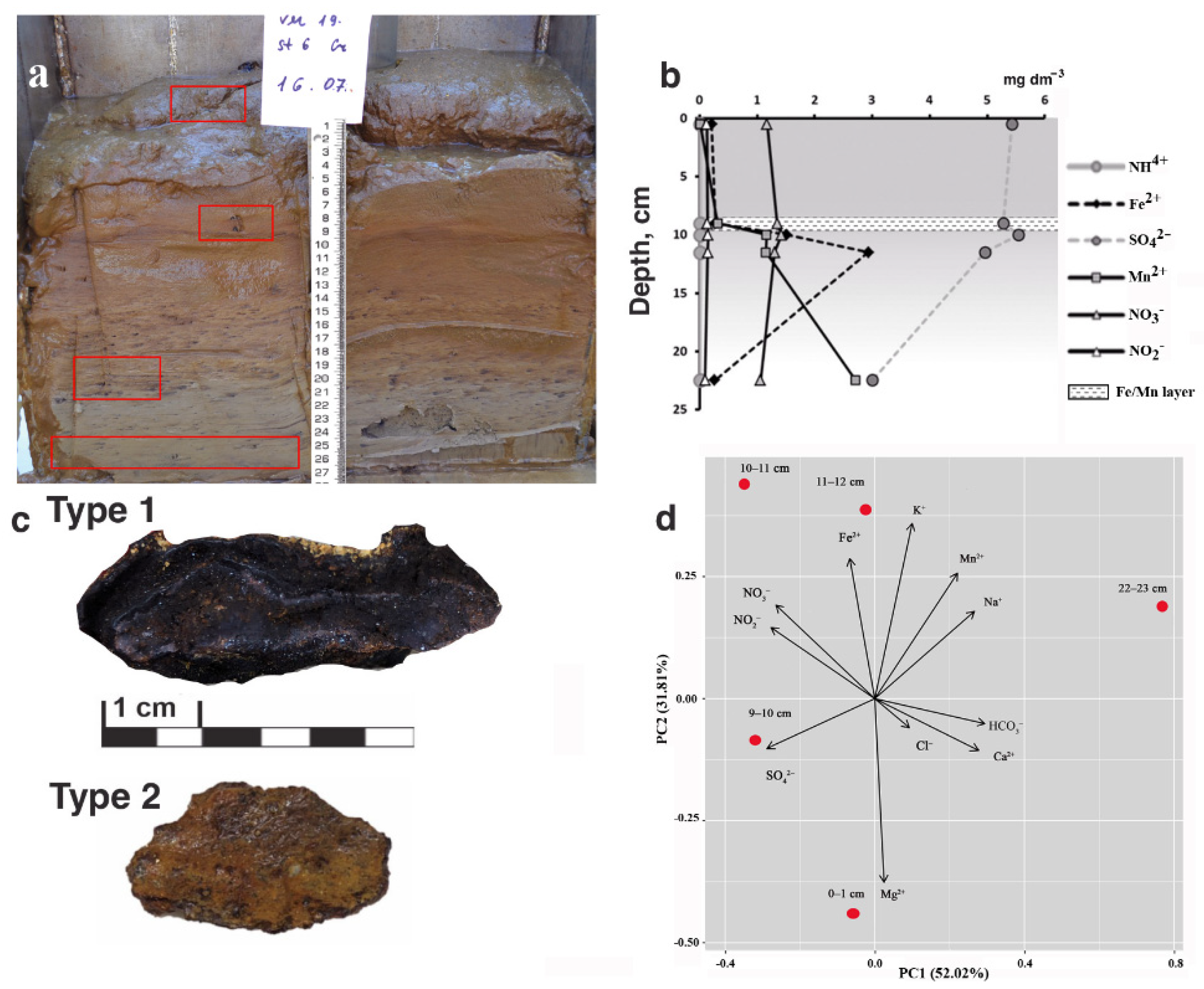
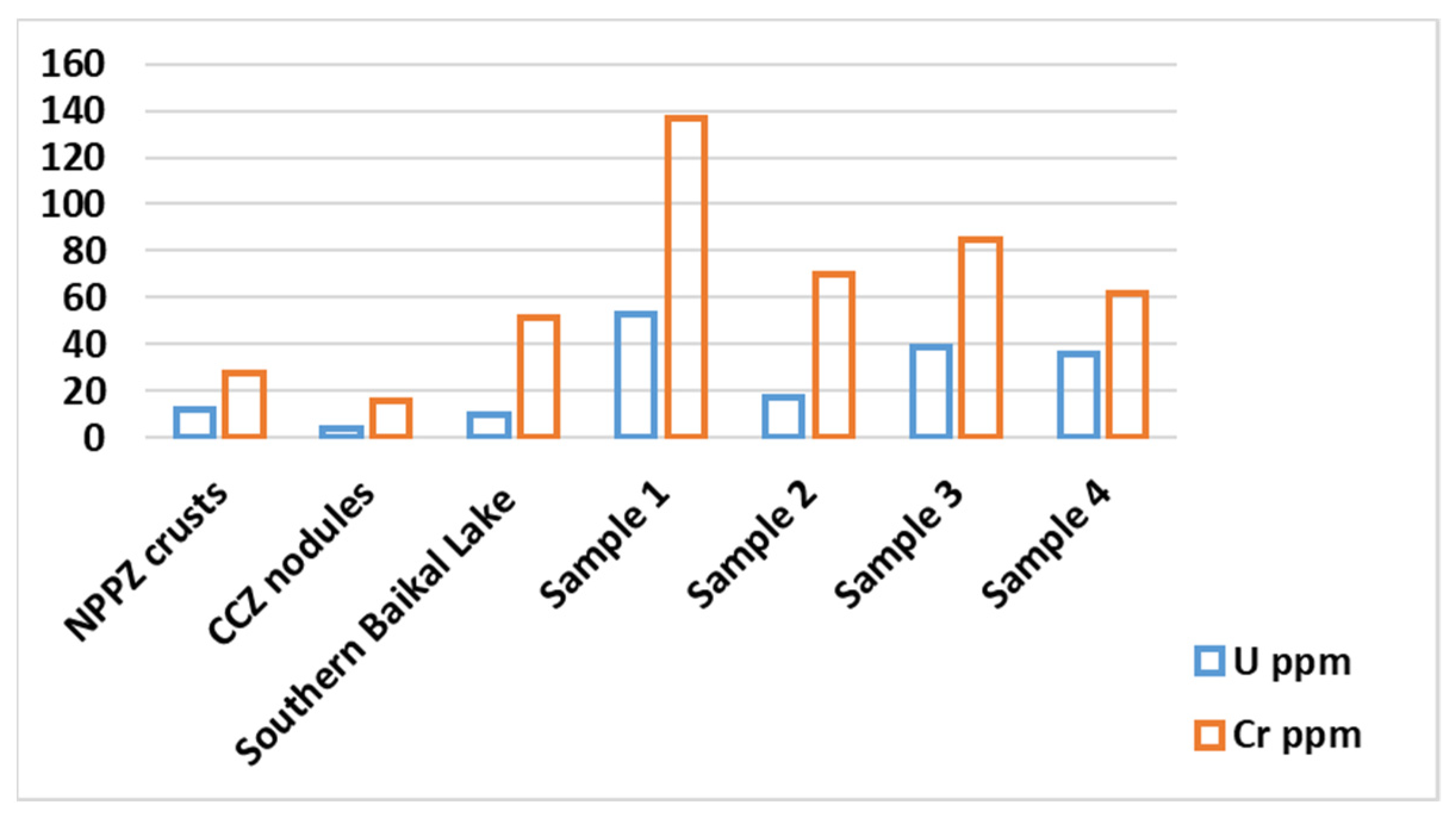
3.2.2. Chemical Composition
3.2.3. Age of Fe-Mn Nodules
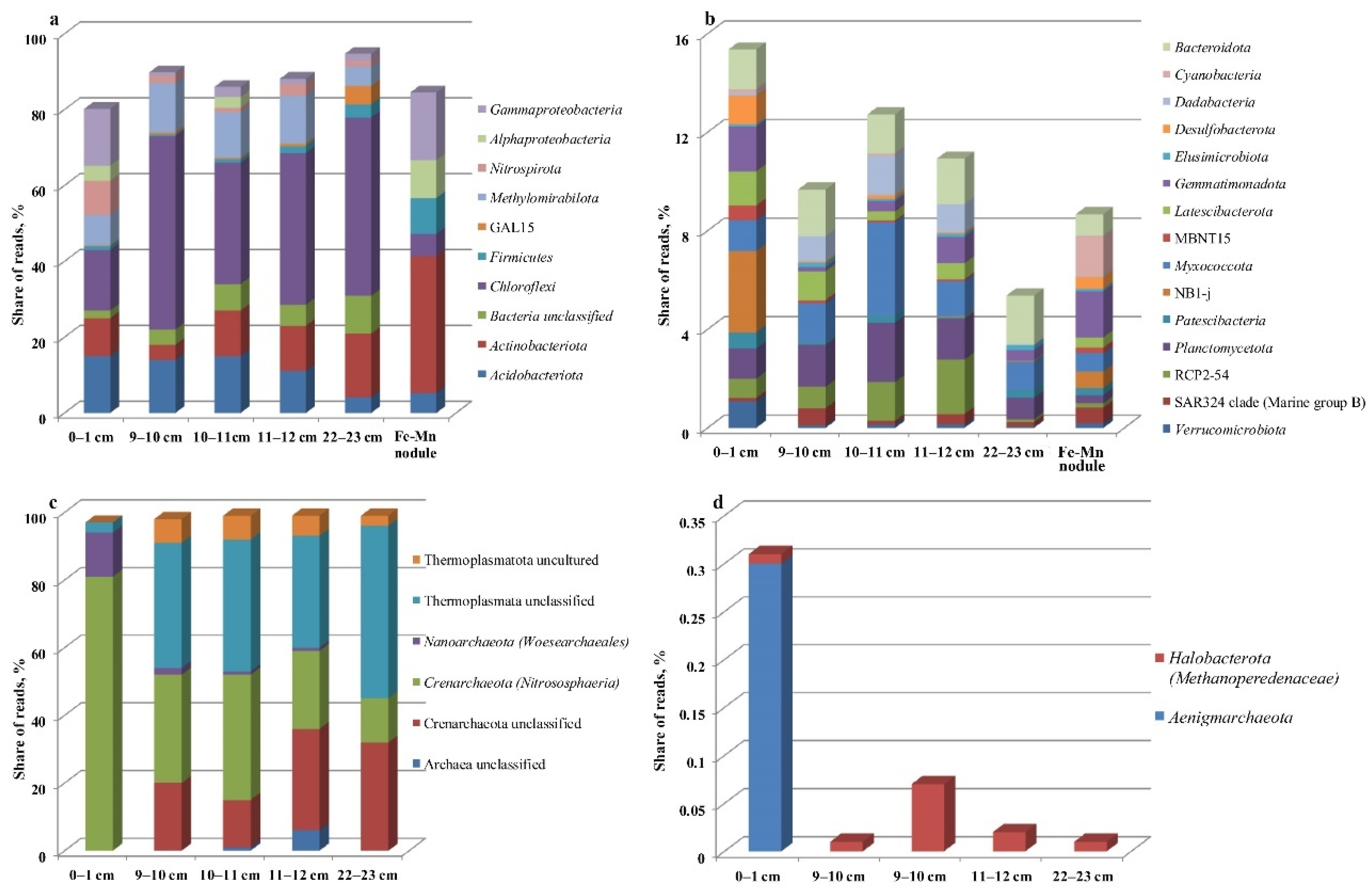
3.3. Microbial Communities
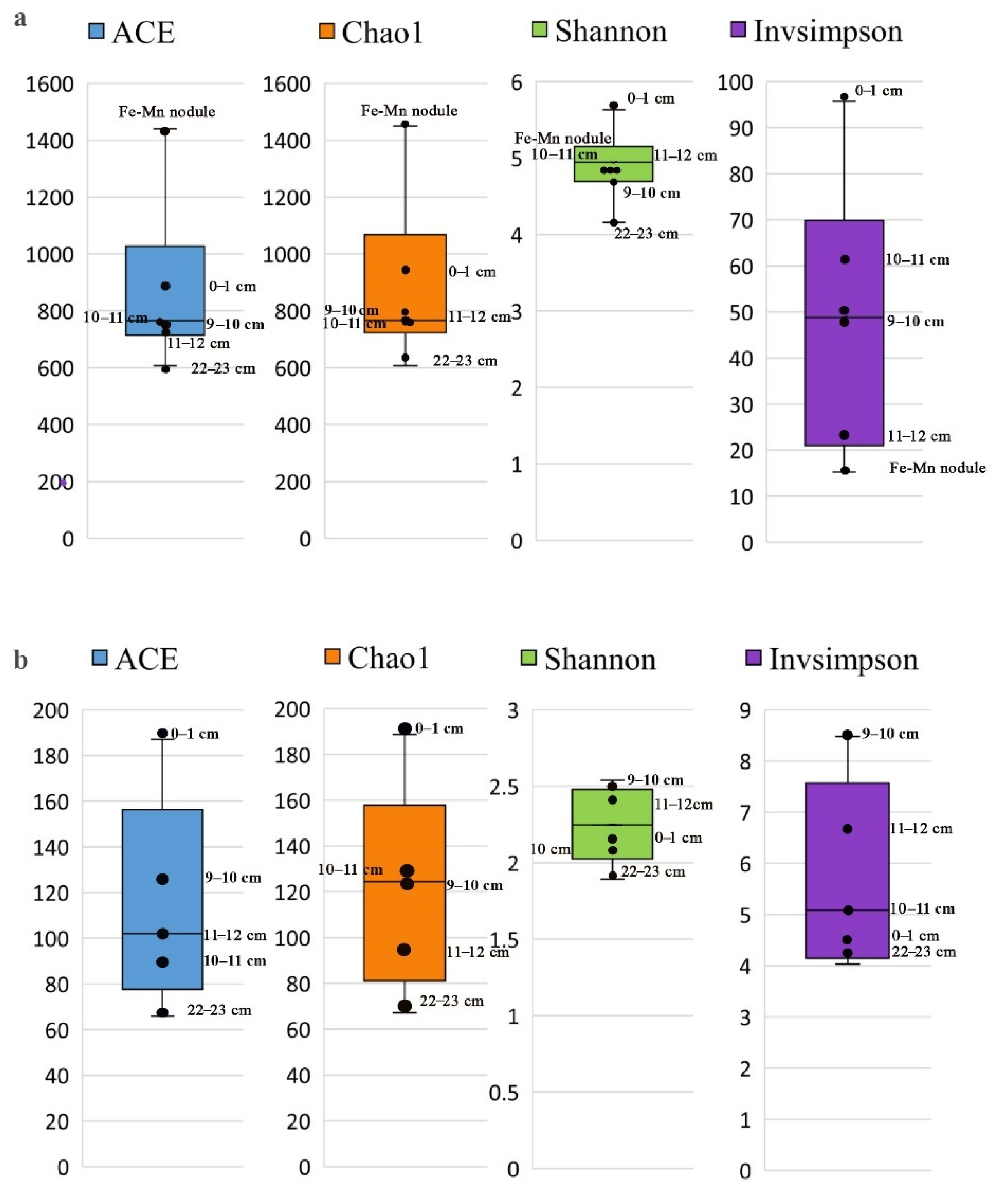
3.3.1. Alpha Diversity of Microbial Communities in the Sediments and Fe-Mn Nodules
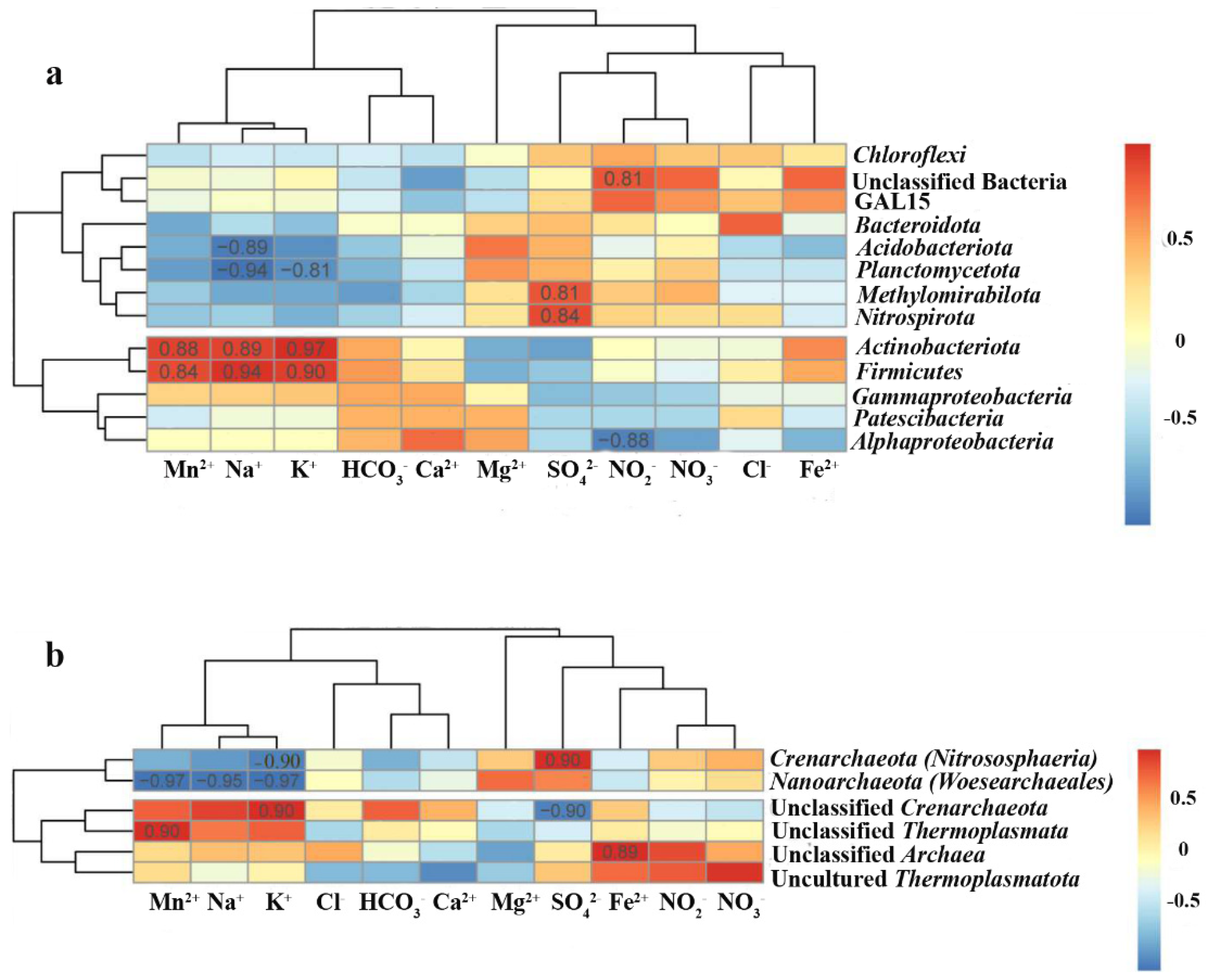
3.3.2. Beta Diversity of Microbial Communities
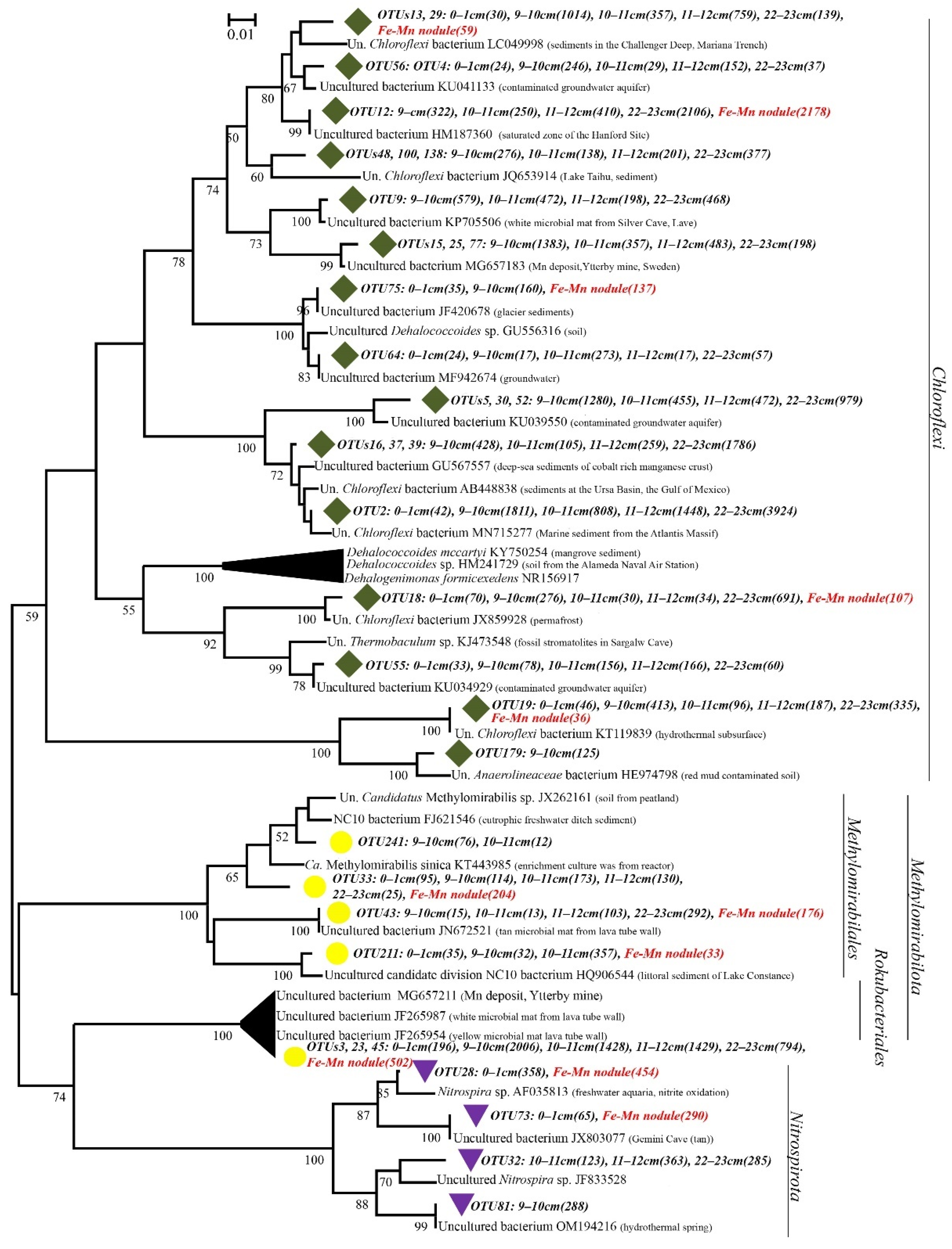
3.4. Community Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baturin, G. Geochemistry of Manganese and Manganese Nodules in the Ocean; Reidel, D., Ed.; Springer: Dordrecht, Netherlands, 1988; p. 342. [Google Scholar] [CrossRef]
- Baturin, G.; Yushina, I.; Zolotykh, E. Variations in the elemental composition of ferromanganese structures from Lake Baikal. Oceanology 2009, 49, 505–514. (In Russian) [Google Scholar] [CrossRef]
- Baturin, G. Distribution of elements in ferrum-manganese nodules in seas and lakes. Lithol. Miner. 2019, 5, 404–417. (In Russian) [Google Scholar] [CrossRef]
- Hayles, S.; Al, T.; Cornett, J.; Harrison, A.; Zhao, J. Growth rates for freshwater ferromanganese concretions indicate regional climate change in eastern Canada at the Northgrippian-Meghalayan boundary. Holocene 2021, 31, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.R.; Koschinsky, A. Deep-ocean ferromanganese crusts and nodules. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 13, pp. 273–291, Chapter 11. [Google Scholar]
- Kholodov, V.N.; Nedumov, R.I.; Golubovskaya, E.V. Facies types of sedimentary iron ore deposits and their geochemical features: Communication 1. Facies groups of sedimentary ores, their lithology, and genesis. Lithol. Miner. Resour. 2012, 47, 447–472. [Google Scholar] [CrossRef]
- Konstantinova, N.; Cherkashov, G.; Hein, J.R.; Mirão, J.; Dias, L.; Madureira, P.; Kuznetsov, V. Composition and characteristics of the ferromanganese crusts from the western Arctic Ocean. Ore Geol. Rev. 2017, 87, 88–99. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Perova, E.N.; Brusnitsyn, A.I.; Ershova, V.B.; Khudoley, A.K.; Shilovskikh, V.V.; Molchanova, E.V. Ferro-manganese nodules from the Kara Sea: Mineralogy, geochemistry and genesis. Ore Geol. Rev. 2019, 106, 192–204. [Google Scholar] [CrossRef]
- Bergo, N.M.; Bendia, A.G.; Neiva Ferreira, J.C.; Murton, B.J.; Brandini, F.P.; Pellizari, V.H. Microbial diversity of deep-sea ferromanganese crust field in the rio Grande Rise, Southwestern Atlantic Ocean. Microb. Ecol. 2021, 82, 344–355. [Google Scholar] [CrossRef]
- Emerson, D.; Field, E.K.; Chertkov, O.; Davenport, K.W.; Goodwin, L.; Munk, C.; Nolan, M.; Woyke, T. Comparative genomics of freshwater Fe-oxidizing bacteria: Implications for physiology, ecology, and systematic. Front. Microbiol. 2013, 4, 254. [Google Scholar] [CrossRef]
- González, F.J.; Rincón-Tomás, B.; Somoza, L.; Santofimia, E.; Medialdea, T.; Madureira, P.; López-Pamo, E.; Hein, J.R.; Marino, E.; de Ignacio, C.; et al. Low-temperature, shallow-water hydrothermal vent mineralization following the recent submarine eruption of Tagoro volcano (El Hierro, Canary Islands). Mar. Geol. 2020, 430, 106333. [Google Scholar] [CrossRef]
- Kato, S.; Okumura, T.; Uematsu, K.; Hirai, M.; Iijima, K.; Usui, A.; Suzuki, K. Heterogeneity of microbial communities on deep-sea ferromanganese crusts in the Takuyo-Daigo seamount. Microbes Environ. 2018, 33, 366–377. [Google Scholar] [CrossRef]
- Kato, S.; Hirai, M.; Ohkuma, M.; Suzuki, K. Microbial metabolisms in an abyssal ferromanganese crust from the Takuyo-Daigo Seamount as revealed by metagenomics. PloS ONE 2019, 14, e0224888. [Google Scholar] [CrossRef] [PubMed]
- Jianga, X.-D.; Sun, X.-M.; Yao, G. Biogenic mineralization in the ferromanganese nodules and crusts from the South China Sea. J. Asian Earth Sci. 2019, 171, 46–59. [Google Scholar] [CrossRef]
- Wang, X.H.; Schlossmacher, U.; Natalio, F.; Schröder, H.C.; Wolf, S.E.; Tremel, W.; Müller, W.E. Evidence for biogenic processes during formation of ferromanganese crusts from the Pacific Ocean: Implications of biologically induced mineralization. Micron 2009, 40, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Muller, W.E.G. Marine biominerals: Perspectives and challenges for polymetallic nodules and crusts. Trends Biotechnol. 2009, 27, 375–383. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.R.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halbach, P.E.; Manheim, F.T.; Bau, M.; Kang, J.; Lubick, N. Iron and manganese oxide mineralization in the Pacific. Geol. Soc. Spec. Publ. 1997, 119, 123–138. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.T.; Kang, J.-K.; Roberts, L. Cobalt-rich ferromanganese crusts in the Pacific. In Handbook of Marine Mineral Deposits; Cronan, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 239–279. [Google Scholar]
- Orcutt, B.N.; Bradley, J.A.; Brazelton, W.J.; Estes, E.R.; Goordial, J.M.; Huber, J.A.; Jones, R.M.; Mahmoudi, N.; Marlow, J.J.; Murdock, S.; et al. Impacts of deep-sea mining on microbial ecosystem services. Limnol. Oceanogr. 2020, 65, 1489–1510. [Google Scholar] [CrossRef]
- Molari, M.; Janssen, F.; Vonnahme, T.; Wenzhöfer, F.; Boetius, A. The contribution of microbial communities in polymetallic nodules to the diversity of the deep-sea microbiome of the Peru Basin (4130–4198 m depth). Biogeosciences 2020, 17, 3203–3222. [Google Scholar] [CrossRef]
- Strakhovenko, V.; Subetto, D.; Ovdina, E.; Belkina, N.; Efremenko, N. Distribution of elements in iron-manganese formations in bottom sediments of Lake Onego (NW Russia) and small lakes (Shotozero and Surgubskoe) of Adjacent Territories. Minerals 2020, 10, 440. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Zhang, L.-M.; He, J.-Z.; Liu, F. Comparison of archaeal populations in soil and their encapsulated iron-manganese nodules in four locations spanning from North to South China. Geomicrobiol. J. 2017, 34, 811–822. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high-and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, R713–R715. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T.; Novigatsky, A.N. Phase distribution of elements in ferromanganese nodules of the Kara Sea. Dokl. Earth Sci. 2016, 471, 1199–1203. (In Russian) [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Z.; González, F.J.; Hein, J.R.; Zheng, X.; Li, G.; Luo, Y.; Mo, A.; Tian, Y.; Wang, S. Composition and genesis of ferromanganese deposits from the northern South China Sea. J. Asian Earth Sci. 2017, 138, 110–128. [Google Scholar] [CrossRef]
- Edgington, D.N.; Callender, E. Minor element geochemistry of Lake Michigan ferromanganese nodules. Earth Planet. Sci. Lett. 1970, 8, 97–100. [Google Scholar] [CrossRef]
- Moore, W.S.; Dean, W.E.; Krishnaswami, S.; Borole, D.V. Growth rates of manganese nodules in Oneida Lake, New York. Earth Planet. Sci. Lett. 1980, 46, 191–200. [Google Scholar] [CrossRef]
- Och, L.M.; Müller, B.; Voegelin, A.; Ulrich, A.; Göttlicher, J.; Steiniger, R.; Mangold, S.; Vologina, E.G.; Sturm, M. New insights into the formation and burial of Fe/Mn accumulations in Lake Baikal sediments. Chem. Geol. 2012, 330–331, 244–259. [Google Scholar] [CrossRef]
- Liu, Q.; Huo, Y.Y.; Wu, Y.H.; Bai, Y.; Yuan, Y.; Chen, M.; Xu, D.; Wang, J.; Wang, C.S.; Xu, X.W. Bacterial community on a Guyot in the Northwest Pacific Ocean influenced by physical dynamics and environmental variables. J. Geophys. Res. Biogeosci. 2019, 124, 2883–2897. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Gong, J.-L.; Ren, J.-B.; Liu, Q.-S.; Zhang, J.; Chou, Y.-M. An interdependent relationship between microbial ecosystems and ferromanganese nodules from the Western Pacific Ocean. Sediment. Geol. 2020, 398, 105588. [Google Scholar] [CrossRef]
- Nitahara, S.; Kato, S.; Usui, A.; Urabe, T.; Suzuki, K.; Yamagishi, A. Archaeal and bacterial communities in deep- sea hydrogenetic ferromanganese crusts on old seamounts of the northwestern Pacific. PloS ONE 2017, 12, e0173071. [Google Scholar] [CrossRef]
- Granina, L.Z. Early Diagenesis in Bottom Sediments of Lake Baikal; Academic Publishing House Geo: Novosibirsk, Russia, 2008; 159p. [Google Scholar]
- Nealson, K.H. The manganese-oxidizing bacteria. Prokaryotes 2006, 5, 222–231. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.L.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planetary. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Strakhovenko, V.; Shkol’nikc, S.I.; Danilenkoa, I.V. Ferromanganese nodules of freshwater reservoirs of Ol’khon Island (Baikal) and the Kulunda Plain (West Siberia). Russ. Geol. Geophys. 2018, 59, 123–134. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Kulichevskaya, I.S.; Serkebaeva, Y.M.; Mityaeva, M.A.; Sorokin, V.V.; Suzina, N.E.; Rijpstra, W.I.; Damsté, J.S. Bryocella elongata gen. nov., sp. nov., a member of subdivision 1 of the Acidobacteria isolated from a methanotrophic enrichment culture, and emended description of Edaphobacter aggregans Koch et al. 2008. Int. J. Syst. Evol. Microbiol. 2012, 62, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, V.M.; Dubinina, G.A.; Kuznetsov, S.I. Ecology of Aquatic Organisms; Nauka: Moscow, Russia, 1977; 289p. [Google Scholar]
- Sommers, M.; Dollhopf, M.; Douglas, S. Freshwater ferromanganese stromatolites from Lake Vermilion, Minnesota: Microbialculturing and environmental scanning electron microscopy investigations. Geomicrobiol. J. 2002, 19, 407–427. [Google Scholar] [CrossRef]
- Hirano, S.; Ito, Y.; Tanaka, S.; Nagaoka, T.; Oyama, T. Microbial community composition in iron deposits and manganese crusts formed in riverine environments around the Aso area in Japan. Res. Microbiol. 2020, 171, 271–280. [Google Scholar] [CrossRef]
- Dittrich, M.; Moreau, L.; Gordon, J.; Quazi, S.; Palermo, C.; Fulthorpe, R.; Katsev, S.; Bollmann, J.; Chesnyuk, A. Geomicrobiology of iron layers in the sediment of Lake Superior. Aquat. Geochem. 2015, 21, 123–140. [Google Scholar] [CrossRef]
- Hauck, S.; Benz, M.; Brune, A.; Schink, B. Ferrous iron oxidation by denitrifying bacteria in profundal sediments of a deep lake (Lake Constance). FEMS Microbiol. Ecol. 2001, 37, 127–134. [Google Scholar] [CrossRef]
- Huo, Y.; Cheng, H.; Anton, F.; Wang, C.; Jiang, X.; Pan, J.; Wu, M.; Xu, X. Ecological functions of uncultured microorganisms in the cobalt-rich ferromanganese crust of a seamount in the central Pacific are elucidated by fosmid sequencing. Acta Oceanol. Sin. 2015, 34, 92–113. [Google Scholar] [CrossRef]
- Nitahara, S.; Kato, S.; Urabe, T.; Usui, A.; Yamagishi, A. Molecular characterization of the microbial community in hydrogenetic ferromanganese crusts of the Takuyo-Daigo Seamount, northwest Pacific. FEMS Microbiol. Lett. 2011, 321, 121–129. [Google Scholar] [CrossRef]
- Granina, L.; Karabanov, E.; Callender, E. Relics of oxidised ferromanganese formations in the bottom sediments of Lake Baikal. IPPCCE Newsl. 1993, 7, 32–39. [Google Scholar]
- Dubinina, G.A. Study of the ecology of iron bacteria in fresh water basins. Izv. Acad. Sci. USSR. Ser. Biol. 1976, 4, 575–592. (In Russian) [Google Scholar]
- Zakharova, Y.R.; Parfenova, V.V.; Granina, L.Z.; Kravchenko, O.S.; Zemskaya, T.I. Distribution of iron and manganeseoxidizing bacteria in the bottom sediments of Lake Baikal. Inland Water Biol. 2010, 3, 313–321. [Google Scholar] [CrossRef]
- Zemskaya, T.I.; Lomakina, A.V.; Mamaeva, E.V.; Zakharenko, A.S.; Likhoshvai, A.V.; Galachyants, Y.P.; Miller, B. Composition of microbial communities in sediments from southern Baikal containing Fe/Mn concretions. Microbiology 2018, 87, 382–392. (In Russian) [Google Scholar] [CrossRef]
- Bukharov, A.A.; Fialkov, V.A. The Geologic Structure of the Baikal Bottom; Nauka: Moscow, Russia, 1996. [Google Scholar]
- Manceau, A.; Kersten, M.; Marcus, M.A.; Geoffroy, N.; Granina, L. Ba and Ni speciation in a nodule of binary Mn oxide phase composition from Lake Baikal. Geoch. Cosmochim. Acta 2007, 71, 1967–1981. [Google Scholar] [CrossRef]
- Amirzhanov, B.J.; Pampoura, V.D.; Piskunova, L.F.; Karabanov, E.V. Geochemical Types of Ferromanganese Nodules of Lake Baikal. Dokl. Akad. Nauk 1992, 326, 530–534. (In Russian) [Google Scholar]
- Bukharov, A.A.; Murashko, D.N.; Fialkov, V.A. Iron-manganese nodules on the underwater slope of the Ushkany Islands (Lake Baikal). Geol. Ore Depos. 1992, 34, 80–91. [Google Scholar]
- Takhteev, V.V.; Bukharov, A.A.; Proviz, V.I.; Sitnikova, T.Y.; Galkin, A.N. A peculiarity of bottom fauna within unusual geological environment of the northern slope of Bolshoi Ushkany Island (Lake Baikal). In Studies of the Fauna in Waters Bodies of Eastern Siberia; Takhtaeev, V.V., Ed.; Irkutsk University: Irkutsk, Russia, 2001; pp. 3–8. [Google Scholar]
- Wetzel, R.G.; Likens, G.E. Limnological Analyses; Springer: New York, NY, USA, 1991; 391p. [Google Scholar] [CrossRef]
- Baram, G.I.; Vereshchagin, A.L.; Golobokova, L.P. Microcolumn highperformance liquid chromatography with UV detection for the determination of anions in environmental materials. J. Anal. Chem. 1999, 54, 854–857. [Google Scholar]
- McLennan, S.M. Rare Earth Elements in Sedimentary Rocks: Influence of Provenance and Sedimentary Process. Rev. Mineral. 1989, 21, 169–200. [Google Scholar]
- Konstantinova, N.; Hein, J.R.; Gartman, A.; Mizell, K.; Barrules, P.; Cherkashov, G.; Mikhailik, P.; Khanchuk, A. Mineral phase-element associations based on sequential leaching of ferromanganese crusts, Amerasia Basin Arctic Ocean. Minerals 2018, 8, 460. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochim. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase associations and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Mikhailik, P.E.; Mikhailik, E.V.; Zarubina, N.V.; Blokhin, M.G. Distribution of rare-earth elements and yttrium in hydrothermal sedimentary ferromanganese crusts of the Sea of Japan (from phase analysis results). Russ. Geol. Geophys. 2017, 58, 1530–1542. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Cherkashev, G.; Lein, A.; Shilov, V.; Maksimov, F.; Arslanov, K.; Stepanova, T.; Baranova, N.; Chernov, S.; Tarasenko, D. 230Th/U dating of massive sulfides from the Logatchev and Rainbow hydrothermal fields (Mid-Atlantic Ridge). Geochronometria 2006, 25, 51–56. [Google Scholar]
- Bol’shakov, A.M.; Egorov, A.V. On the application of phase equilibrium degassing for gasometric research in water areas. Okeanologiya 1987, 37, 861–862. (In Russian) [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1987. [Google Scholar]
- Sahm, K.; John, P.; Nacke, H.; Wemheuer, B.; Grote, R.; Daniel, R.; Antranikian, G. High abundance of heterotrophic prokaryotes in hydrothermal springs of the Azores as revealed by a network of 16S rRNA gene-based methods. Extremophiles 2013, 17, 649–662. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High throughput Sequence Data. 2020. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 February 2022).
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Opensource, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 4 April 2022).
- Mizell, K.; Hein, J.R.; Au, M.; Gartman, A. Estimates of metals contained in abyssal manganese nodules and ferromanganese crusts in the global ocean based on regional variations and genetic types of nodules. In Perspectives on Deep-Sea Mining; Sharma, R., Ed.; Springer: Cham, Switzerland, 2022; pp. 53–80. [Google Scholar] [CrossRef]
- Petrov, L.L.; Kornakov, Y.N.; Persikova, L.A.; Anchutina, E.A. Reference samples of Lake Baikal bottom sediments—An essential part of regional collection of reference samples. Int. J. Environ. Anal. Chem. 1999, 74, 275–288. [Google Scholar] [CrossRef]
- Zhmodik, S.M.; Verkhovtseva, N.V.; Soloboeva, E.V.; Mironov, A.G.; Nemirovskaya, N.A.; Ilić, R.; Khlystov, O.M.; Titov, A.T. The study of distribution and forms of uranium occurrences in Lake Baikal sediments by the SSNTD method. Radiat. Meas. 2005, 40, 532–538. [Google Scholar] [CrossRef]
- Och, L.M.; Müller, B.; März, C.; Wichser, A.; Vologina, E.G.; Sturm, M. Elevated uranium concentrations in Lake Baikal sediments: Burial and early diagenesis. Chem. Geol. 2016, 441, 92–105. [Google Scholar] [CrossRef]
- Coates, J.D.; Ellis, D.J.; Gaw, C.V.; Lovley, D.R. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int. J. Syst. Bacteriol. 1999, 49, 1615–1622. [Google Scholar] [CrossRef]
- Costas, A.M.G.; Liu, Z.; Tomsho, L.P.; Schuster, S.C.; Ward, D.M.; Bryant, D.A. Complete genome of Candidatus Chloracidobacterium thermophilum, a chlorophyll-based photoheterotroph from the phylum Acidobacteria. Environ. Microbiol. 2012, 14, 177–190. [Google Scholar] [CrossRef]
- Woodcroft, B.J.; Singleton, C.M.; Boyd, J.A.; Evans, P.N.; Emerson, J.B.; Zayed, A.A.F.; Hoelzle, R.D.; Lamberton, T.O.; McCalley, C.K.; Hodgkins, S.B.; et al. Genome-centric view of carbon processing in thawing permafrost. Nature 2018, 560, 49–54. [Google Scholar] [CrossRef]
- Hao, L.; McIlroy, S.J.; Kirkegaard, R.H.; Karst, S.M.; Fernando, W.E.Y.; Aslan, H.; Meyer, R.L.; Albertsen, M.; Nielsen, P.H.; Dueholm, M.S. Novel prosthecate bacteria from the candidate phylum Acetothermia. ISME J. 2018, 12, 2225–2237. [Google Scholar] [CrossRef]
- Tang, Y.J.; Shan, Y.; Zhuang, W.-Q.; Zinder, S.H.; Keasling, J.D.; Alvarez-Cohen, L. Investigation of carbon metabolism in "Dehalococcoides ethenogenes" strain 195 by use of isotopomer and transcriptomic analyses. J. Bacteriol. 2009, 191, 5224–5231. [Google Scholar] [CrossRef]
- Cabello-Yeves, P.J.; Zemskaya, T.I.; Zakharenko, A.S.; Sakirko, M.V.; Ivanov, V.G.; Ghai, R.; Rodriguez-Valera, F. Microbiome of the deep Lake Baikal, a unique oxic bathypelagic habitat. Limnol. Oceanogr. 2020, 65, 1471–1488. [Google Scholar] [CrossRef]
- Becraft, E.D.; Woyke, T.; Jarett, J.; Ivanova, N.; Godoy-Vitorino, F.; Poulton, N. Rokubacteria: Genomic giants among the uncultured bacterial phyla. Front. Microbiol. 2017, 8, 2264. [Google Scholar] [CrossRef]
- Zemskaya, T.I.; Bukin, S.V.; Lomakina, A.V.; Pavlova, O.N. Microorganisms in the sediments of Lake Baikal, the deepest and oldest lake in the world. Microbiology 2021, 90, 286–303. (In Russian) [Google Scholar] [CrossRef]
- Lomakina, A.V.; Mamaeva, E.V.; Galachyants, Y.P.; Petrova, D.P.; Pogodaeva, T.V.; Shubenkova, O.V.; Khabuev, A.V.; Morozov, I.V.; Zemskaya, T.I. Diversity of Archaea in bottom sediments of the discharge areas with oil- and gas-bearing fluids in Lake Baikal. Geomicrobiol. J. 2018, 35, 50–63. [Google Scholar] [CrossRef]
- Walker, C.B.; Torre, J.R.; Klotz, M.G.; Urakawa, H.; Pinel, N.; Arp, D. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. USA 2010, 107, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Aylward, F.O.; Alyson, E.S. Heterotrophic Thaumarchaea with small genomes are widespread in the dark Ocean. mSystems 2020, 5, e00415–e00420. [Google Scholar] [CrossRef] [PubMed]
- Northup, D.E.; Barns, S.M.; Yu, L.E.; Spilde, M.N.; Schelble, R.T.; Dano, K.E.; Crossey, L.J.; Connolly, C.A.; Boston, P.J.; Natvig, D.O.; et al. Diverse microbial communities inhabiting ferromanganese deposits in Lechuguilla and Spider Caves. Environ. Microbiol. 2003, 5, 1071–1086. [Google Scholar] [CrossRef]
- Kadnikov, V.V.; Mardanov, A.; Beletsky, A.V.; Shubenkova, O.V.; Pogodaeva, T.N.; Zemskaya, T.I.; Ravin, N.V.; Skryabin, K.G. Microbial community structure in methane hydrate-bearing sediments of freshwater Lake Baikal. FEMS Microbiol. Ecol. 2012, 79, 348–358. [Google Scholar] [CrossRef]
- Gebers, R.; Beese, M. Pedomicrobium americanum sp. nov. and Pedomicrobium australicum sp. nov. from aquatic habitats, Pedomicrobium gen. emend., and Pedomicrobium ferrugineum sp. emend. Int. J. Syst. Bacteriol. 1988, 38, 303–315. [Google Scholar] [CrossRef]
- Sujith, P.P.; Gonsalves, M.J.B.D.; Bhonsle, S.; Shaikh, S.; LokaBharathi, P.A. Bacterial activity in hydrogenetic ferromanganese crust from the Indian Ocean: A combined geochemical, experimental and pyrosequencing study. Environ. Earth Sci. 2017, 76, 191. [Google Scholar] [CrossRef]
- Sujith, P.P.; Gonsalves, M.J.B.D. Ferromanganese oxide deposits: Geochemical and microbiological perspectives of interactions of cobalt and nickel. Ore Geol. Rev. 2021, 139, 104458. [Google Scholar] [CrossRef]
- Yuling, L.; Boqing, T.; Yuanxinglu, L.; Ming, L.; Xiangdong, W.; Xiaoli, L.; Huihui, D. Inoculation of soil with cadmium-resistant bacterium Delftia sp. B9 reduces cadmium accumulation in rice (Oryza sativa L.) grains. Ecotoxicol. Environ. Saf. 2018, 163, 223–229. [Google Scholar] [CrossRef]
- Diels, L.; Geets, J.; Dejonghe, W.; Van Roy, S.; Vanbroekhoven, K.; Szewczyk, A.; Malina, G. Heavy metal immobilization in groundwater by in situ bioprecipitation: Comments and questions about efficiency and sustainability of the process. Proc. Annu. Int. Conf. Soils Sediments Water Energy 2010, 11, 7. Available online: https://scholarworks.umass.edu/soilsproceedings/vol11/iss1/7 (accessed on 1 February 2022).
- Daims, H.; Nielsen, J.L.; Nielsen, P.H.; Schleifer, K.H.; Wagner, M. In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl. Environ. Microbiol. 2001, 67, 5273–5284. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sunamura, M.; Yanagawa, K.; Ishibashi, J.; Miyoshi, Y.; Iino, T.; Suzuki, K.; Urabe, T. First cultivation and ecological investigation of a bacterium affiliated with the Candidate Phylum OP5 from Hot Springs. Appl. Environ. Microbiol. 2008, 74, 6223–6229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Dong, C.; Biao, S. Planiflum yunnanense sp nov., a thermophilic thermoactinomycete isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2007, 57, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Vuillemin, A.; Kerrigan, Z.; D’Hondt, S.; Orsi, W.D. Exploring the abundance, metabolic potential and gene expression of subseafloor Chloroflexi in million-year-old oxic and anoxic abyssal clay. FEMS Microbiol. Ecol. 2020, 96, fiaa223. [Google Scholar] [CrossRef]
- Ward, L.M.; Idei, A.; Nakagawa, M.; Ueno, Y.; Fischer, W.W.; McGlynn, S.E. Geochemical and metagenomics characterization of Jinata Onsen, a Proterozoic-analog hot spring, reveals novel microbial diversity including iron-tolerant phototrophs and thermophilic lithotrophs. Microbes Environ. 2019, 34, 278–292. [Google Scholar] [CrossRef]
- Colman, S.M.; Nicholsm, D.R.; Badardinov, A.A.; Foster, D.S.; O’Toole, J.K.; Parolski, K.E. High-resolution seismic-reflection surveys of Lake Baikal, Siberia 1990–1992: Methods and examples. International Project on Paleolimnology and Late Cenozoic Climate. IPPCCE Newsl. 1993, 7, 43–48. [Google Scholar]
- Vologina, E.G.; Sturm, M.; Vorobyova, S.S.; Granina, L.Z. New results of high-resolution studies of surface sediments of Lake Baikal. Terra Nostra 2000, 9, 115–131. [Google Scholar]
- Cronan, D.S. Manganese Nodules. In Earth Systems and Environmental Sciences, 3rd ed.; Steele, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 5, pp. 607–614. [Google Scholar] [CrossRef]
- Han, X.; Schubert, C.J.; Fiskal, A.; Dubois, N.; Lever, M.A. Eutrophication as a driver of microbial community structure in lake sediments. Environ. Microbiol. 2020, 22, 3446–3462. [Google Scholar] [CrossRef]
- Wurzbacher, C.; Nilsson, R.H.; Rautio, M.; Peura, S. Poorly known microbial taxa dominate the microbiome of permafrost thaw ponds. ISME J. 2017, 11, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, F.; Mitsunobu, S.; Suzuki, K.; Hoshino, T.; Morono, Y.; Inagaki, F. Dense microbial community on a ferromanganese nodule from the ultra-oligotrophic South Pacific Gyre: Implications for biogeochemical cycles. Earth. Planet. Sci. Lett. 2016, 447, 10–20. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Wear, E.K.; Church, M.J.; Orcutt, B.N.; Shulse, C.N.; Lindh, M.V.; Smith, C.R. Bacterial and Archaeal communities in polymetallic nodules, sediments, and bottom waters of the abyssal clarion-clipperton zone: Emerging patterns and future monitoring considerations. Front. Mar. Sci. 2021, 8, 634803. [Google Scholar] [CrossRef]
- Torres, N.T.; Och, L.M.; Hauser, P.C.; Furrer, G.; Brandl, H.; Vologina, E.; Sturm, M.; Burgmann, H.; Beat Muller, B. Early diagenetic processes generate iron and manganese oxide layers in the sediments of Lake Baikal, Siberia. Environ. Sci. 2014, 16, 879–889. [Google Scholar] [CrossRef]
- Castelle, C.J.; Brown, C.T.; Anantharaman, K.; Probst, A.J.; Huang, R.H.; Banfield, J.F. Biosynthetic capacity, metabolic variety and unusual biology in the CPR and DPANN radiations. Nat. Rev. Microbiol. 2018, 16, 629–645. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gu, J.-D. Ecological distribution and potential roles of Woesearchaeota in anaerobic biogeochemical cycling unveiled by genomic analysis. Comput. Struct. Biotechnol. J. 2021, 19, 794–800. [Google Scholar] [CrossRef]
- Berg, J.S.; Jeґzeґque, D.; Duverger, A.; Lamy, D.; Laberty-Robert, C.; Miot, J. Microbial diversity involved in iron and cryptic sulfur cycling in the ferruginous, low-sulfate waters of Lake Pavin. PLoS ONE 2019, 14, e0212787. [Google Scholar] [CrossRef]
- Crowe, S.A.; Maresca, J.A.; Jones, C.; Sturm, A.; Henny, C.; Fowle, D.A.; Cox, R.P.; Delong, E.F.; Canfield, D.E. Deep-water anoxygenic photosythesis in a ferruginous chemocline. Geobiology 2014, 12, 322–339. [Google Scholar] [CrossRef]
- Hansel, C.M.; Lentini, C.J.; Tang, Y.; Johnston, D.T.; Wankel, S.D.; Jardine, P.M. Dominance of sulfur-fueled iron oxide reduction in low-sulfate freshwater sediments. ISME J. 2015, 9, 2400–2412. [Google Scholar] [CrossRef]
- Granina, L.Z.; Mats, V.D.; Phedorin, M.A. Iron-manganese formations in the Baikal region. Russ. Geol. Geophys. 2010, 51, 650–660. [Google Scholar] [CrossRef]
- Soyol-Erdene, T.-O.; Huh, Y. Rare earth element cycling in the pore waters of the Bering Sea Slope (IODP Exp. 323). Chem. Geol. 2013, 358, 75–89. [Google Scholar] [CrossRef]




| Samples | Study Layers (cm) | 238U dpm/g * | 234U dpm/g | 230Th dpm/g | 232Th dpm/g | 230Th/234U | 234U/238U | 230Th/232Th | Age, Kyr |
|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | 0.6–0.8 | 32.96 ± 0.59 | 38.54 ± 0.68 | 21.10 ± 0.52 | 1.27 ± 0.08 | 0.55 ± 0.02 | 1.17 ± 0.02 | 16.59 ± 1.05 | 88 ± 4 |
| 0.4–0.6 | 21.28 ± 0.35 | 24.58 ± 0.40 | 17.64 ± 0.47 | 1.52 ± 0.09 | 0.72 ± 0.02 | 1.16 ± 0.01 | 11.64 ± 0.67 | 131 ± 8 | |
| 0.2–0.4 | 28.67 ± 0.69 | 34.43 ± 0.80 | 24.07 ± 0.68 | 1.44 ± 0.11 | 0.70 ± 0.03 | 1.20 ± 0.02 | 16.68 ± 1.26 | 124 ± 8 | |
| 0.0–0.2 | 35.95 ± 0.73 | 42.52 ± 0.84 | 29.28 ± 0.65 | 1.28 ± 0.08 | 0.69 ± 0.02 | 1.18 ± 0.02 | 22.93 ± 1.41 | 121 ± 7 | |
| Sample 3 | 0.4–0.6 | 31.93 ± 0.94 | 47.44 ± 1.34 | 27.69 ± 0.81 | 2.10 ± 0.11 | 0.58 ± 0.02 | 1.49 ± 0.03 | 13.20 ± 0.63 | 90 ± 5 |
| 0.2–0.4 | 25.93 ± 0.64 | 39.32 ± 0.91 | 23.48 ± 0.60 | 2.05 ± 0.11 | 0.60 ± 0.02 | 1.52 ± 0.03 | 11.45 ± 0.56 | 92 ± 5 | |
| 0.0–0.2 | 24.50 ± 0.62 | 38.80 ± 0.91 | 23.84 ± 0.48 | 2.02 ± 0.09 | 0.61 ± 0.02 | 1.58 ± 0.03 | 11.79 ± 0.51 | 96 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemskaya, T.; Konstantinova, N.; Shubenkova, O.; Pogodaeva, T.; Ivanov, V.; Bukin, S.; Khabuev, A.; Khlystov, O.; Vilkin, G.; Lomakina, A. Microbial Communities of Ferromanganese Sedimentary Layers and Nodules of Lake Baikal (Bolshoy Ushkany Island). Diversity 2022, 14, 868. https://doi.org/10.3390/d14100868
Zemskaya T, Konstantinova N, Shubenkova O, Pogodaeva T, Ivanov V, Bukin S, Khabuev A, Khlystov O, Vilkin G, Lomakina A. Microbial Communities of Ferromanganese Sedimentary Layers and Nodules of Lake Baikal (Bolshoy Ushkany Island). Diversity. 2022; 14(10):868. https://doi.org/10.3390/d14100868
Chicago/Turabian StyleZemskaya, Tamara, Natalia Konstantinova, Olga Shubenkova, Tatyana Pogodaeva, Vyacheslav Ivanov, Sergei Bukin, Andrey Khabuev, Oleg Khlystov, Grigory Vilkin, and Anna Lomakina. 2022. "Microbial Communities of Ferromanganese Sedimentary Layers and Nodules of Lake Baikal (Bolshoy Ushkany Island)" Diversity 14, no. 10: 868. https://doi.org/10.3390/d14100868
APA StyleZemskaya, T., Konstantinova, N., Shubenkova, O., Pogodaeva, T., Ivanov, V., Bukin, S., Khabuev, A., Khlystov, O., Vilkin, G., & Lomakina, A. (2022). Microbial Communities of Ferromanganese Sedimentary Layers and Nodules of Lake Baikal (Bolshoy Ushkany Island). Diversity, 14(10), 868. https://doi.org/10.3390/d14100868






