Abstract
The protection of wetlands is a cornerstone in the conservation of pond-breeding amphibians. Because protected wetlands are rarely natural areas, but are often man-made, at least in Europe, it is important that they are well managed to fulfill their intended function. Appropriate management requires knowledge of the ecology of the species, particularly habitat requirements. Here, we combine species monitoring data and habitat mapping data in an analysis where our goal was to describe the factors that determine the occupancy of amphibian species in federally protected amphibian breeding sites. As expected, every species had its own habitat requirements, often a combination of both a terrestrial and aquatic habitat (i.e., landscape complementation). In most species, occupancy was strongly positively affected with the amount of aquatic habitat, but predicted occupancy probabilities were low because the amount of aquatic habitat was low in most sites. The area or proportion of ruderal vegetation also had positive effects on multiple species, while other types of terrestrial habitat (e.g., meadows) led to low occupancy probabilities. The total area of the protected breeding sites was never included in a final model and connectivity was important only for one species (Triturus cristatus). The latter finding implies that the quality of the landscape between breeding sizes is more important than distance per se, while the former implies that the area of some specific habitats within breeding sites is crucial for high occupancies. Thus, increasing the amount of aquatic habitats and likewise terrestrial habitats within protected areas would make them more likely to achieve their conservation objectives. Our study is an example of how the joint analysis of monitoring data and habitat data (based on mapping in the field) can lead to evidence-based suggestions on how to improve conservation practice.
Keywords:
amphibian; monitoring; protected area; occupancy; habitat; temporary pond; terrestrial habitat 1. Introduction
Freshwater ecosystems such as geographically isolated wetlands and ponds are home to a large number of species [1,2]. Yet, the decline in wetlands, ponds, and the associated species outpaces the loss of biodiversity in marine and terrestrial ecosystems [3]. Halting this decline was dubbed as the “ultimate conservation frontier” [4]. It is thus imperative that conservation scientists provide solutions on how to master the freshwater biodiversity crisis [5,6,7]. Numerous reviews offer research priorities for freshwater biodiversity conservation. A common suggestion is the preservation of freshwater species and habitats through the creation of dedicated nature reserves [8,9,10]. However, freshwater biodiversity is often insufficiently represented in networks of protected areas [11,12]. While nature reserves are well-known to be beneficial for wildlife, they often do not fulfil their intended function because existing reserves are not well maintained [13]. For example, the authors of [14] quantified the decline in amphibians in North America. Most study sites in [14] were located in protected areas such as national parks or other government-owned land, yet declines were stronger than the declines reported by the authors of [15], who used data from a variety of habitats, both protected and unprotected. Thus, a better understanding of the qualitative and quantitative requirements of a species regarding ecological resources (e.g., food and breeding sites) could help to manage nature reserves in a better way. Importantly, research that directly informs conservation practice was a research priority identified by conservation practitioners [16].
Amphibians are a prime example of the decline in freshwater biodiversity [17]. Declines in amphibians of considerable magnitude have been observed for decades and are caused by a large number of stressors, including habitat loss and deterioration, pollution, invasive species, diseases, and climate change [14,15,17]. While there are many causes that contribute to global amphibian declines, every population experiences its own local combination of stressors [14]. This suggests that it is important to look for local causes for both population declines and the determinants of population persistence [7,18].
Here, we studied the relationship between the characteristics of the aquatic and terrestrial habitat in federally protected amphibian breeding sites of national importance (the highest degree of protection that a Swiss nature reserve can attain; [19]) and the occupancy of amphibians in Switzerland. We studied pond-breeding amphibians, which require both a suitable terrestrial and aquatic habitat (landscape complementation; [20,21,22]). The species that we studied are known to vary in their preferences for aquatic and terrestrial habitats [23,24,25]. Most amphibian breeding sites in Switzerland are man-made and require habitat management, or else succession of both the aquatic and terrestrial habitat will lead to a reduction in habitat quality or even habitat loss [19,26]. Reduced habitat quality may have contributed to the declines in both rare and common species in Switzerland [27,28]. Describing the relationship between the quality of the habitat and distribution and abundance of threatened species is thus essential in order to restore, manage, upgrade, or create habitats suitable for the target species [10,22,29].
We combined data from a large-scale amphibian monitoring programme with data from a habitat mapping programme to determine the relationship between amphibian occupancy and the structure of the aquatic and terrestrial habitat. Specifically, we quantified the relationship between the amount and type of aquatic habitats such as pond surface area or hydroperiod and species presence/absence. We also tested which types of terrestrial habitat, which was only mapped within the nature reserves, had positive or negative effects on occupancy. Even though the wider landscape is often used by amphibians, we did not include it in this analysis as the effects are often weak in our study area (the exception was connectivity; [30,31,32,33]).
Given that species have different preferences, we expected species-specific responses to the quality and quantity of aquatic and terrestrial habitats (e.g., [23,24,25]). While we expected that all species would respond favourably to a larger number of ponds within reserves, we expected that preferences for the type of pond (i.e., temporary vs. permanent) would depend on the species’ position along the pond hydroperiod gradient [34,35]. Given different preferences for the terrestrial habitat [23,24,36,37,38], we expected species-specific responses to variation in the structure of the terrestrial habitat.
2. Materials and Methods
2.1. The Amphibian Monitoring Programme
We used data collected by the Swiss national monitoring programme “Monitoring the Effectiveness of Habitat Conservation in Switzerland” (“Wirkungskontrolle Biotopschutz Schweiz” (WBS); see https://biotopschutz.wsl.ch/en/index.html (accessed on 2 October 2022); [26,33,39,40]). We used data from a subset of the sites for which habitat mapping data were available (n = 113).
Sites are visited according to a rotating panel design [41], such that each of the 258 sites is visited once every six years (43 sites visited every year). For our analyses, we used data collected from 2011 to 2016. For every site, we had data from one year (for example, the site shown in Figure 1 was surveyed in 2011). Five additional sites were surveyed in 2020 to increase the sample size in the Swiss biogeographic region Jura (the total n was thus 118; for a map, see Appendix A). Survey protocols for the monitoring programme include four visits during the breeding season (March–June). Alpine sites were visited only twice. The survey time window spans four months in order to include the breeding seasons of all amphibians in the assemblage. Repeated visits to a site allow imperfect detection to be accounted for when estimating site occupancy and breeding probabilities [42]. Site visits were limited to one hour. If sites were large and could not be completely surveyed within this time, surveyors searched for the amphibians in the parts of the sites that the surveyors judged to be most suitable for amphibians. At each visit, the goal was to detect all pond-breeding amphibian species and all life history stages (eggs, larvae, adults, and calling males) that are present (i.e., the two Salamandra species were not the targets of the survey). The methods included visual encounter surveys, aural surveys, and dip-netting [43], and have been approved by the Swiss Federal Office for the Environment and the Swiss Federal Food Safety and Veterinary Office [44]. Permits for field work were obtained from the cantonal conservation authorities.
2.2. Habitat Mapping
The sites include a wide variety of habitats such as natural ponds, pond clusters, or wetlands, as well as man-made sites such as gravel pits or quarries. All sites are federally protected amphibian breeding sites of national importance. The perimeter of the protected areas includes the aquatic habitats plus the immediate adjacent terrestrial habitat (core areas), as well as the surrounding terrestrial habitat (a buffer zone; e.g., forests, meadows, and agricultural fields). We mapped both the core area and the buffer zone.
Habitat mapping was carried out in the field from March to August in 2017, 2018, and 2020 when amphibians were active and included both the aquatic and terrestrial habitat within the protected sites. Mapping was performed on colour orthophotos obtained from the Swiss Federal Office of Topography swisstopo. The habitat typology was based on the standard definition of natural habitats in Switzerland (the second level of [45]; the list of habitat types is also available online (in German and French): https://www.infoflora.ch/de/lebensraeume/vollst%C3%A4ndige-auflistung/vollst%C3%A4ndige-auflistung-typoch.html (accessed on 16 September 2022)). Only habitat elements larger than 100 m2 were mapped (~10 × 10 m minimum size), except for temporary or permanent water bodies. Flooded meadows in which amphibians regularly reproduce were considered water bodies. The description of water bodies was based on the average water level during the breeding season (April to July) and was described in detail in a separate field sheet (the document was written in German and is available from the authors upon reasonable request). Habitats were delineated on the orthophotos in the field and later digitized using a GIS system. From the GIS, we computed areas of different habitat types. Table 1 shows the list of habitat types mapped in the field and Figure 1 shows an example.

Table 1.
Variables used in occupancy models. The habitat variables are based on an established classification of habitats in Switzerland [45]. For an example of a habitat map, see Figure 1. In the modelling, habitat types were used either as areas (in hectares) or as proportions (area of habitat type divided by total area of the site). Sites were protected amphibian breeding sites of national importance.
Table 1.
Variables used in occupancy models. The habitat variables are based on an established classification of habitats in Switzerland [45]. For an example of a habitat map, see Figure 1. In the modelling, habitat types were used either as areas (in hectares) or as proportions (area of habitat type divided by total area of the site). Sites were protected amphibian breeding sites of national importance.
| Variable Name | Meaning | Range |
|---|---|---|
| Past occurrence | Past population presence or absence (based on the records in the national database) | 0–1 |
| Connectivity | Connectivity, based on [30] (but modified) | 0–0.53 |
| Altitude | Altitude of the site [m] | 330–1460 |
| Total area of site | Total surface area of the site [ha] | 0.14–290.1 |
| Water | Area of water bodies within site (lentic and lotic) [ha] | 0–28.0 |
| Number of ponds | Number of ponds within site | 0–53 |
| Number of temporary ponds | Number of temporary ponds within site | 0–30 |
| Lentic aquatic habitat | Area of lentic aquatic habitats within site (a subset of “water”) [ha] | 0–27.2 |
| Built | Area of built area within site (e.g., surface of landfills, buildings, and roads) [ha] | 0–15.7 |
| Wetland | Area of wetlands within site (surface of artificial shores, reed beds, marshland, wet meadows, and bogs) [ha] | 0–90.9 |
| Meadow | Area of meadows within site (surface of meadows, and pastures) [ha] | 0–34.9 |
| Shrubs and tall forbs | Area of shrubs and tall herbs within site [ha] | 0–20.4 |
| Forest | Area of forests within site (including plantations) [ha] | 0–227.5 |
| Field | Area of agricultural fields within sites (surface of cultivation of woody and herbaceous plants) [ha] | 0–65.5 |
| Ruderal | Area of pioneer vegetation in man-made disturbed areas [ha] | 0–23.4 |
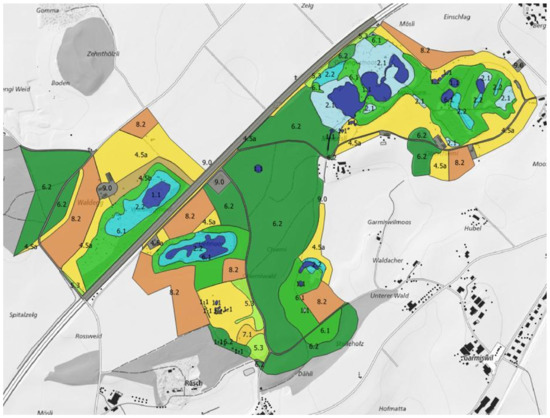
Figure 1.
Example of a habitat map. It shows the protected amphibian breeding site of national importance “Düdingermoos” in western Switzerland. Blue colours indicate ponds (dark blue) and surrounding wetland vegetation (light blue). Green colours are forests (dark green) and shrubs (light green), yellow colours are meadows and pastures, and brown colours are arable lands. Grey is human infrastructure (e.g., roads). Numbers denote habitat types following [45]. The mapped area (in colour in this figure) is the total area of the protected site (cf. Table 1). Areas that are not coloured (different types of grey) represent the non-protected landscape surrounding the amphibian breeding sites; dark grey is used for forests. The site has a west–east extent of 1.65 km. © swisstopo.
2.3. Connectivity
Amphibian patch occupancy often depends on connectivity to neighbouring populations. We thus calculated the connectivity index proposed by [30], which is an extension of the measure of spatial autocorrelation proposed by [46]. The connectivity of site i, ci, is calculated as
where dij is the distance (in km) between amphibian breeding sites i and j. In contrast to [30], we used the distance to all neighbouring amphibian breeding sites rather than only distances to neighbouring amphibian breeding sites where the focal species occurs. The calculation was based on the amphibian presence records maintained in the national database of info fauna karch (more than 360,000 observations as of June 2022; [19]).
2.4. Statistical Analyses
We used single-season occupancy models to analyse the data [42]. Our goal was to test for the effects of explanatory variables on the site occupancy of the species while accounting for imperfect detection [27]. Models were fitted to the data using the “unmarked” package in R [47,48]. Detection probability was modelled as constant in the occupancy models. In the first step of the analysis, we fitted models to the data of each species. Each model included one single habitat variable as the explanatory variable (derived from the habitat mapping, Table 1). Correlations among variables are shown in Appendix B. We did this because, for many of the habitat variables and species, we had no specific expectation as to how they may affect amphibian occupancy. In addition to the habitat variable, these models included two additional variables that were thought a priori to be important (e.g., [30,33]). The first variable was connectivity to neighbouring amphibian breeding sites and the second one was whether the species was known to occur at the site in the past (mid-1990s; based on [49]). Systematic surveys were conducted in almost all of the Swiss cantons, such that a comprehensive atlas of the distributions of the Swiss amphibians could be published in 1988; this database has been maintained and updated ever since [19]. Past presences and absences are thus reliable. The latter variable describes whether the species occurred in the past and differentiates between sites that were suitable in the past from those that were not suitable, or not occupied. By doing so, we can account for some fundamental differences between sites that are not accounted for in our modelling. Furthermore, because, in a world without local extirpations, the occupancy probability should be 1 in sites where the species occurred in the past, we can use this variable to quantify how local extinctions depend on habitat attributes.
The resulting models were ranked using AIC [50,51]. In the second step of the analysis, we built models with multiple habitat variables. These models were based on the AIC scores of the models in the first step, but also on the confidence interval of parameter estimates themselves (e.g., a model/explanatory variable could be excluded if the model had a low AIC value, but the 95% confidence interval included zero; [52]). We also used previous knowledge on the ecology of the species to guide model building (e.g., known preference for some habitats, such as the preference for temporary ponds over permanent ponds) and sometimes replaced similar variables (e.g., area covered by ruderal vegetation with the proportion of the area covered by ruderal vegetation). We started this process with models that included all variables that seemed important (based on step one) and reduced model complexity in a stepwise fashion based on AIC and the 95% confidence intervals of the parameter estimates. In some cases, the final model included explanatory variables where the confidence interval overlaps zero. We kept these variables in the model when AIC scores suggested that a model with the variable was a better model than a model without it. Parameter estimates and figures are derived from the final model from the second step (i.e., for most species models with multiple explanatory variables). Apart from a few exceptions, the figures show the effects of the variables where the 95% confidence interval does not include zero.
Data of the 118 sites are used for the analyses, except for two species (Hyla intermedia and Triturus carnifex), for which only 34 of the 118 sites were used. These two species only occur in southern Switzerland [53]. Therefore, data from northern Switzerland were discarded for these two species. Data of frogs from the genus Pelophylax were pooled because these frogs form a hybridogenetic species complex and because there are several invasive species present in Switzerland. The species are morphologically difficult to distinguish, particularly when they are not captured, as was the case in our amphibian survey [54,55].
3. Results
The median number of detections was three for most species (if the species was detected at least once) and two for a few species (Hyla arborea, Hyla intermedia, and Triturus carnifex).
For eight of the thirteen species, variables describing the aquatic habitat were included in the best models. The most important one was the number of temporary ponds (four species), followed by the proportion of wetland habitat within the protected area (three species). For ten out of the thirteen species, one or two variables describing the terrestrial habitat were included in the best models. Ruderal vegetation was the most important (four species), either as the area or the proportion. For many species, high proportions or large areas of some types of terrestrial habitats decreased occupancy (e.g., meadows). Past occurrence was important for all but one species (the very widespread common frog Rana temporaria). Connectivity was important for two species and the total area of the protected area was never included in the best models. Some explanatory variables always had positive effects (e.g., the number of temporary ponds), but there was no explanatory variable that always had negative effects on occupancy (if we only look at variables that were important for multiple species). Several variables had both positive and negative effects (e.g., the area of forest or the proportion of wetland vegetation). For each species, explanatory variables included in the final model can be found in Table 2 and Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12.

Table 2.
Parameter estimates of the final models. The coefficients are the logit-scale estimates for the relationship between occupancy and the explanatory variable. See text for additional explanation and Table 1 for explanation of explanatory variables. CI: confidence interval.
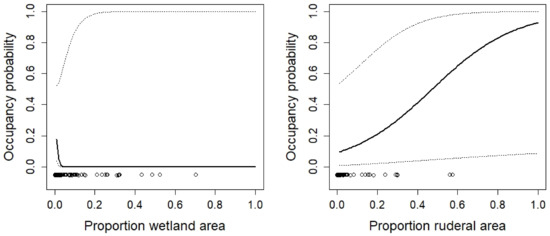
Figure 2.
The effects of the proportion of the area within a site covered with wetland vegetation and ruderal vegetation within a site on the occupancy probability of Alytes obstetricans. The dotted lines show the 95% confidence interval. Circles indicate observed values of the explanatory variable. Other explanatory variables in the model were fixed either at their median or the mean values, depending on the skewness of the distribution. The relationship is for sites where the species was known to occur in the past (i.e., the occupancy probability can be interpreted as a persistence probability).
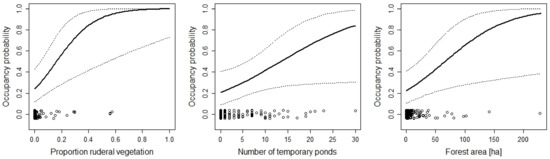
Figure 3.
The effect of the number of temporary ponds, the percentage of the area within a site covered with ruderal vegetation within a site, and the area of forest on the occupancy probability of Bombina variegata. For additional explanations, see the caption of Figure 2.
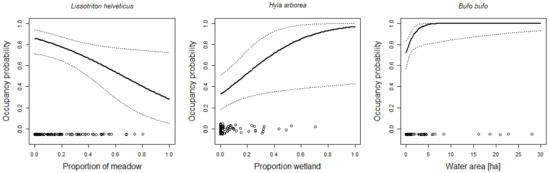
Figure 4.
The effects of habitat characteristics on the occupancy probability of Lissotriton helveticus, Hyla arborea, and Bufo bufo. For additional explanations, see the caption of Figure 2.
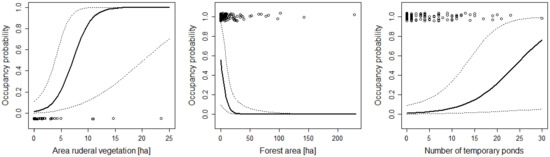
Figure 5.
The effect of the area of ruderal vegetation, number of temporary ponds, and forest area on the occupancy probability of Epidalea calamita. For additional explanations, see the caption of Figure 2.
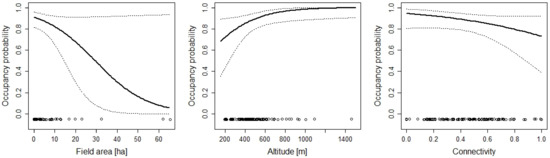
Figure 6.
The effect of the area of fields, altitude, and connectivity on the occupancy probability of Ichthyosaura alpestris. For additional explanations, see the caption of Figure 2.
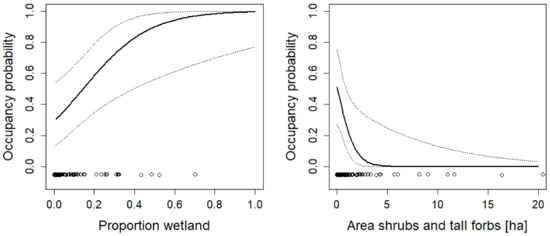
Figure 7.
The effect of the proportion of wetlands and the area of shrubs and tall forbs on the occupancy probability of Lissotriton vulgaris. For additional explanations, see the caption of Figure 2.
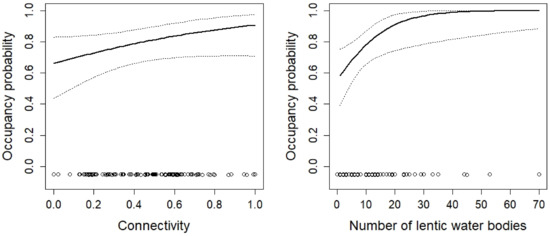
Figure 8.
The effect of connectivity and the number of lentic water bodies on the occupancy probability of Pelophylax frogs. For additional explanations, see the caption of Figure 2.
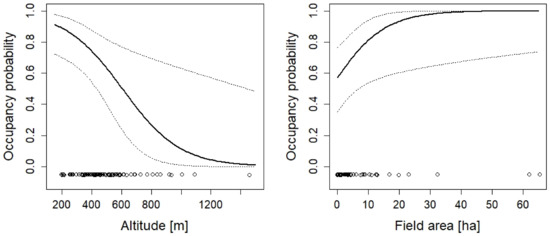
Figure 9.
The effect of altitude and the area of fields on the occupancy probability of Rana dalmatina. For additional explanations, see the caption of Figure 2. The effect of connectivity is not shown because the confidence intervals widely overlap zero.
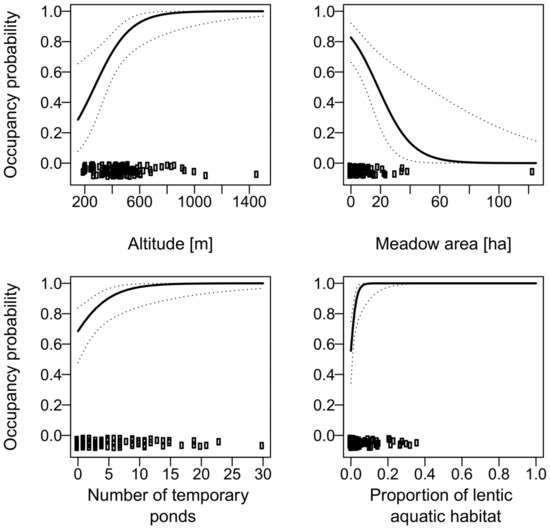
Figure 10.
The effect of altitude, number of temporary ponds, proportion of freshwater habitat, and the area of meadows on the occupancy probability of Rana temporaria. For additional explanations, see the caption of Figure 2, but note that the explanatory variable “past presence” was not included in the final model.
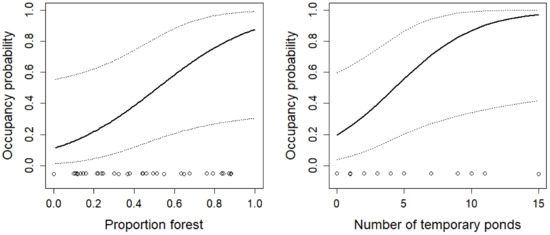
Figure 11.
The effect of the proportion of forest and the number of temporary ponds on the occupancy probability of Triturus carnifex. For additional explanations, see the caption of Figure 2.
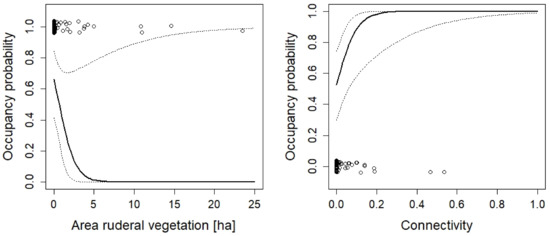
Figure 12.
The effect of connectivity and the area of ruderal vegetation on the occupancy probability of Triturus cristatus. For additional explanations, see the caption of Figure 2.
4. Discussion
The results show that every species had its own habitat requirements and that, for most species, landscape complementation, i.e., a combination of both aquatic and terrestrial habitat characteristics, was important [21,22,23]. Even though it is well known that amphibian occurrence can depend on a variety of factors (e.g., chemical, abiotic, and biotic characteristics of ponds such as macrophyte cover, pH, and the occurrence of predators, as well as microhabitats in the terrestrial habitat (shelter); e.g., [22,25,56,57,58,59,60]), the relatively simple habitat mapping provided valuable data on the suitability of the sites for amphibians and may inform the management of the sites. This insight is further corroborated by studies [61,62] on the newt Triturus cristatus, which showed that a habitat suitability index that can easily be measured in the field predicts occupancy, abundance, and demography well. Nevertheless, for some species, more detailed habitat data are required to predict occupancy and, therefore, ways to manage habitats for increased suitability. For example, there was no good model for the treefrog Hyla intermedia (in the sense that all confidence intervals were wide and overlapped zero). Other habitat data and probably also data from more sites are necessary for this species. Below, we focus on habitat characteristics that can be modified by managers of the protected areas (e.g., construction of new ponds).
While many variables had positive or negative effects on patch occupancy, a few never or rarely had an effect. Some of those “no-effect” variables are worth discussing. The total size (area) of the protected area was never included in models that described the data best, although area ranged between 0.1 and 290 hectares. This supports the view that small patches can be valuable for biodiversity conservation [63]. However, the species occupancy–patch area relationship generally appears to be weak in amphibians [64,65], which may explain why the results showed no effect of patch area in this study. Furthermore, it should be kept in mind that the nature reserves mostly cover the breeding site and not the terrestrial habitat. Thus, the size of the patch used by the amphibian populations may have been underestimated, at least for those species where the terrestrial summer habitat is far away from the pond [66,67]. Nevertheless, the absence of a patch size effect is good news for amphibian conservation because it may support the empirical result that small patches can be very valuable for conservation [68]. However, whereas total patch size did not matter, the areas of some habitat types had an effect on occupancy. For example, occupancy of the natterjack toad (Epidalea calamita) increased with a larger area of ruderal vegetation, but was negatively affected by the area of forests (Figure 5). Thus, area can be important, but it is not the area of the patch in total, but rather of some specific habitat. This makes sense given the fact that the protected areas that we studied are mosaics of different habitat types.
The importance of patch size is often analysed in combination with patch isolation. Here, it is noteworthy that connectivity, the inverse of isolation, only mattered for one species, the crested newt (Triturus cristatus). This result matches well with what we know about the dispersal ecology of crested newts. This species is known to disperse a lot, but dispersal distances are generally less than 500 m [62,69]. Connectivity was also included in the best model for four other species, but an interpretation of this result is difficult because of the wide confidence intervals, which included zero. Overall, however, the absence of strong and positive effects of connectivity for most species contrasts with previous studies on the same species in Swiss landscapes where connectivity was found to be important [30,33,64]. One explanation may be that connectivity per se (essentially a weighted sum of distances to neighbouring ponds) does not matter, but rather the quality of the matrix habitat between the patches [65,70,71].
Even though the protected areas mostly cover the aquatic habitat, the terrestrial habitat within the protected habitat mattered for many species. The results corroborated what was known about the ecology of the species (e.g., the importance of ruderal vegetation for species such as Epidalea calamita, Bombina variegate, and Alytes obstetricans). From a conservation perspective, the most important result is that most species would require much larger areas or proportions of the preferred habitat type. For example, the area of ruderal vegetation was important for Epidalea calamita, a species with ongoing declines in Switzerland [26,72]. Yet, the area of ruderal vegetation was less than five hectares in most sites and the predicted occupancy probability for those sites was very low (less 0.2). Larger areas would be necessary to substantially increase occupancy probabilities (Figure 5). Similar patterns can be observed for the proportion of ruderal vegetation for Bombina variegata and Alytes obstetricans (Figure 2 and Figure 3): much higher proportions would be necessary to increase occupancy probabilities. This is a clear result for conservation agencies, conservation NGOs, and land owners who manage the protected areas. Existing areas of ruderal vegetation should be maintained at least in their current state. This means that site management should prevent succession. In sites where there is not much ruderal vegetation, the amount of it should be increased, e.g., through the conversion of habitat types that have a low value for biodiversity to ruderal vegetation.
Unsurprisingly, the aquatic habitat was important for the amphibians. With the exception of Alytes obstetricans, the occupancy probability of all species increased with an increasing amount of aquatic habitat (e.g., the number of temporary ponds or the proportion of wetland within the protected area). For only a few species (e.g., Rana temporaria, Pelophylax spp.), there was sufficient aquatic habitat to guarantee high occupancy probabilities (Figure 8 and Figure 10). For other species, such as the red-listed species Epidalea calamita and Bombina variegata, there was a clear need for more temporary ponds (Figure 2 and Figure 5). Unfortunately, most sites had far fewer temporary ponds than the number that would be necessary to lead to high occupancy probabilities. This result has clear implications for habitat management. To increase the suitability of the sites for the species, it is necessary to increase the number of temporary ponds. The techniques to create such ponds are well known and can be readily used by conservation practitioners [73,74,75]. A study conducted in England [76] showed that pond density within sites correlated with the abundance of Epidalea calamita. Thus, increasing the number of temporary ponds is likely to increase occupancy and abundance. In fact, most species were positively affected by the amount of aquatic habitat (number of ponds, number of temporary ponds, area of lentic and lotic water bodies, or area of lentic water bodies). This suggests that increasing the amount aquatic habitat will make the sites more attractive for most species. The number of temporary ponds was an important predictor of site occupancy for the toad Bombina variegata (Figure 3). A recent study on this species [77] may suggest the underlying demographic mechanism. In a man-made habitat, population persistence depends heavily on recruitment, while recruitment is low in natural habitats. The majority of the sites included in this study are man-made, suggesting that recruitment may be of great importance for population persistence. High numbers of recruits are more likely when there are many temporary ponds because tadpole survivorship is higher in temporary ponds (compared to permanent ponds; [35,78,79]).
The results show clearly that both the terrestrial and aquatic habitat determine amphibian occupancy in federally protected amphibian breeding sites. Yet, not all species have the same habitat requirements. We thus conclude that the best strategy to preserve diverse amphibian communities is to manage the sites in such a way that a large amount of diverse types of aquatic habitat is available, including temporary ponds. Such a management strategy could make sure that many amphibian species can breed successfully in those wetlands [80,81,82] and may lead to the recovery of amphibian communities [72].
Author Contributions
Conceptualization, O.S. and B.R.S.; Data curation, J.P., P.R., U.T. and B.R.S.; Formal analysis, O.S. and B.R.S.; Funding acquisition, J.P., P.R., U.T., A.B. and B.R.S.; Investigation, O.S., J.P., P.R., U.T. and B.R.S.; Methodology, O.S., J.P., P.R. and U.T.; Project administration, B.R.S.; Resources, J.P., P.R., U.T. and A.B.; Supervision, J.P., P.R., U.T., A.B. and B.R.S.; Visualization, J.P. and B.R.S.; Writing—original draft, O.S. and B.R.S.; Writing—review and editing, O.S., J.P., P.R., U.T., A.B. and B.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
The monitoring programme and the habitat mapping projects were supported through grants from the Swiss Federal Office for the Environment (contracts 00.5149.PZ/R242-1 677 and A200.0001/00.5062.PZ/4E5606796).
Institutional Review Board Statement
Ethical review and approval was not required. Capture permits were issued by the cantonal conservation authorities.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We thank the field surveyors for collecting the amphibian data, E. Rey for calculating the connectivity values, and the reviewers for comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The map shows the study sites, i.e., all 118 amphibian breeding sites of national importance, that were included in the study (open circles; circles may overlap when sites are close to each other). All sites are protected areas. The map shows the elevation of the study area in colour, ranging from green (low elevations) to red and white (high elevations). The elevation scale on the right is in meters. The x- and y-axis show the Swiss coordinate system (which is in km × 1000; e.g., the distance between 250,000 and 200,000 on the y-axis is 50 km).
Figure A1.
Map of Switzerland and the study sites. See text for further explanations.
Appendix B
The figure shows the results of the correlation analysis. Variables are explained in Table 1 in the main text. Variables ending with “.a” are areas (e.g., total.a = total area of site), whereas those ending with “.p” are proportions (e.g., water.p is the proportion of the area of the site covered with lentic or lotic water bodies, i.e., water.p = water.a/total.a). Colours indicate the strength of the correlation. Darker tones are used for stronger correlations. Blue indicates positive correlations, whereas red colours indicate negative correlations. The variable “past occurrence” was not included in the correlation analysis because it is different for every species.
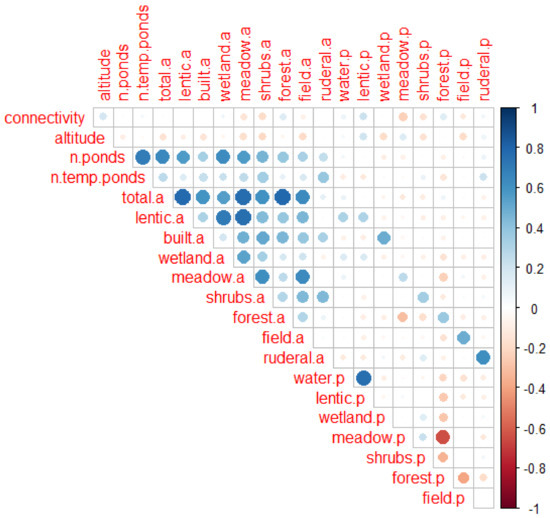
Figure A2.
Results of the correlation analysis. See text for further explanations.
References
- Céréghino, R.; Biggs, J.; Oertli, B.; Declerck, S. The ecology of European ponds: Defining the characteristics of a neglected freshwater habitat. Hydrobiologia 2008, 597, 1–6. [Google Scholar] [CrossRef]
- Davies, B.; Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Sear, D.; Bray, S.; Maund, S. Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agric. Ecosyst. Environ. 2008, 125, 1–8. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Soulé, M.E. What is conservation biology? BioScience 1985, 35, 727–734. [Google Scholar]
- Arlettaz, R.; Schaub, M.; Fournier, J.; Reichlin, T.S.; Sierro, A.; Watson, J.E.M.; Braunisch, V. From publications to public actions: When conservation biologists bridge the gap between research and implementation. BioScience 2010, 60, 835–842. [Google Scholar] [CrossRef]
- Grant, E.H.C.; Muths, E.; Schmidt, B.R.; Petrovan, S.O. Amphibian conservation in the Anthropocene. Biol. Conserv. 2019, 236, 543–547. [Google Scholar] [CrossRef]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef]
- Flitcroft, R.; Cooperman, M.S.; Harrison, I.J.; Juffe-Bignoli, D.; Boon, P.J. Theory and practice to conserve freshwater biodiversity in the Anthropocene. Aquat. Conserv. 2019, 29, 1013–1021. [Google Scholar] [CrossRef]
- Harper, M.; Mejbel, H.S.; Longert, D.; Abell, R.; Beard, T.D.; Bennett, J.R.; Carlson, S.M.; Darwall, W.; Dell, A.; Domisch, S.; et al. Twenty-five essential research questions to inform the protection and restoration of freshwater biodiversity. Aquat. Conserv. 2021, 9, 2632–2653. [Google Scholar] [CrossRef]
- Leverington, F.; Costa, K.L.; Pavese, H.; Lisle, A.; Hockings, M. A global analysis of protected area management effectiveness. Environ. Manag. 2010, 46, 685–698. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Dudley, N.; Segan, D.B.; Hockings, M. The performance and potential of protected areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef]
- Wauchope, H.S.; Jones, J.G.P.; Geldmann, J.; Simmons, B.I.; Amano, T.; Blanco, D.E.; Fuller, R.A.; Johnston, A.; Langendoen, T.; Mundkur, T.; et al. Protected areas have a mixed impact on waterbirds, but management helps. Nature 2022, 605, 103–107. [Google Scholar] [CrossRef]
- Grant, E.H.C.; Miller, D.A.W.; Schmidt, B.R.; Adams, M.J.; Amburgey, S.M.; Chambert, T.; Cruickshank, S.S.; Fisher, R.N.; Green, D.M.; Hossack, B.R.; et al. Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci. Rep. 2016, 6, 25625. [Google Scholar] [CrossRef]
- Houlahan, J.E.; Findlay, C.S.; Schmidt, B.R.; Meyer, A.H.; Kuzmin, S.L. Quantitative evidence for global amphibian declines. Nature 2000, 404, 752–755. [Google Scholar] [CrossRef]
- Braunisch, V.; Home, R.; Pellet, J.; Arlettaz, R. Conservation science relevant to action: A research agenda identified and prioritized by practitioners. Biol. Conserv. 2012, 153, 201–210. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Smalling, K.L.; Eagles-Smith, C.A.; Katz, R.A.; Grant, E.H.C. Managing the trifecta of disease, climate, and contaminants: Searching for robust choices under multiple sources of uncertainty. Biol. Conserv. 2019, 236, 153–161. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Zumbach, S. Amphibian conservation in Switzerland. In Amphibian Biology: Volume 11, Status of Conservation and Decline of Amphibians, Eastern Hemisphere, Part 5, Northern Europe; Heatwole, H., Wilkinson, J.W., Eds.; Pelagic Publishing: Exeter, UK, 2019; pp. 46–51. [Google Scholar]
- Dunning, J.B.; Danielson, B.J.; Pulliam, H.R. Ecological processes that affect populations in complex landscapes. Oikos 1992, 65, 169–175. [Google Scholar] [CrossRef]
- Pope, S.E.; Fahrig, L.; Merriam, H.G. Landscape complementation and metapopulation effects on leopard frog populations. Ecology 2000, 81, 2498–2508. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Arlettaz, R.; Schaub, M.; Lüscher, B.; Kröpfli, M. Benefits and limits of comparative effectiveness studies in evidence-based conservation. Biol. Conserv. 2019, 236, 115–123. [Google Scholar] [CrossRef]
- Van Buskirk, J. Local and landscape influence on amphibian occurrence and abundance. Ecology 2005, 86, 1936–1947. [Google Scholar] [CrossRef]
- Denoel, M.; Ficetola, G.F. Conservation of newt guilds in an agricultural landscape of Belgium: The importance of aquatic and terrestrial habitats. Aquat. Conserv. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Indermaur, L.; Schaub, M.; Jokela, J.; Tockner, K.; Schmidt, B.R. Differential response to abiotic conditions and predation risk rather than competition avoidance determine breeding site selection by anurans. Ecography 2010, 33, 887–895. [Google Scholar] [CrossRef]
- Bergamini, A.; Ginzler, C.; Schmidt, B.R.; Bedolla, A.; Boch, S.; Ecker, K.; Graf, U.; Küchler, H.; Küchler, M.; Dosch, O.; et al. Zustand und Entwicklung der Biotope von nationaler Bedeutung: Resultate 2011–2017 der Wirkungskontrolle Biotopschutz Schweiz. WSL Ber. 2019, 85, 1–104. [Google Scholar]
- Cruickshank, S.S.; Ozgul, A.; Zumbach, S.; Schmidt, B.R. Quantifying population declines based on presence-only records for Red List assessments. Conserv. Biol. 2016, 30, 1112–1121. [Google Scholar] [CrossRef]
- Petrovan, S.O.; Schmidt, B.R. Volunteer conservation action data reveals large-scale and long-term negative population trends of a widespread amphibian, the Common toad (Bufo bufo). PLoS ONE 2016, 11, e0161943. [Google Scholar] [CrossRef]
- Adams, M.J.; Muths, E. Conservation research across scales in a national program: How to be relevant to local management yet general at the same time. Biol. Conserv. 2019, 236, 100–106. [Google Scholar] [CrossRef]
- Zanini, F.; Pellet, J.; Schmidt, B.R. The transferability of distribution models across regions: An amphibian case study. Divers. Distrib. 2009, 15, 469–480. [Google Scholar] [CrossRef]
- Van Buskirk, J. Permeability of the landscape matrix between amphibian breeding sites. Ecol. Evol. 2012, 2, 3160–3167. [Google Scholar] [CrossRef]
- Luqman, H.; Muller, R.; Vaupel, A.; Brodbeck, S.; Bolliger, J.; Gugerli, F. No distinct barrier effects of highways and a wide river on the genetic structure of the Alpine newt (Ichthyosaura alpestris) in densely settled landscapes. Conserv. Genet. 2018, 19, 673–685. [Google Scholar] [CrossRef]
- Cruickshank, S.S.; Schmidt, B.R.; Ginzler, C.; Bergamini, A. Local habitat measures derived from aerial pictures are not a strong predictor of amphibian occurrence and abundance. Basic Appl. Ecol. 2020, 45, 51–61. [Google Scholar] [CrossRef]
- Wellborn, G.A.; Skelly, D.K.; Werner, E.E. Mechanisms creating community structure across a freshwater habitat gradient. Annu. Rev. Ecol. Syst. 1996, 27, 337–363. [Google Scholar] [CrossRef]
- Van Buskirk, J. Habitat partitioning in European and North American pond-breeding frogs and toads. Divers. Distrib. 2003, 9, 399–410. [Google Scholar] [CrossRef]
- Indermaur, L.; Winzeler, T.; Schmidt, B.R.; Tockner, K.; Schaub, M. Differential resource selection within shared habitat types across spatial scales in sympatric toads. Ecology 2009, 90, 3430–3444. [Google Scholar] [CrossRef]
- Denton, J.S.; Hitchings, S.P.; Beebee, T.J.C.; Gent, A. A recovery program for the natterjack toad (Bufo calamita) in Britain. Conserv. Biol. 1997, 11, 1329–1338. [Google Scholar] [CrossRef]
- Jehle, R.; Arntzen, J.W. Post-breeding migrations of newts (Triturus cristatus and T. marmoratus) with contrasting ecological requirements. J. Zool. 2000, 251, 297–306. [Google Scholar] [CrossRef]
- BAFU. Monitoring und Wirkungskontrolle Biodiversität: Übersicht zu Nationalen Programmen und Anknüpfungspunkten; Bundesamt für Umwelt: Bern, Switzerland, 2020. [Google Scholar]
- Cruickshank, S.S.; Bergamini, A.; Schmidt, B.R. Estimation of breeding probability can make monitoring data more revealing: A case study of amphibians. Ecol. Appl. 2021, 31, e02357. [Google Scholar] [CrossRef]
- McDonald, T.L. Review of environmental monitoring methods: Survey designs. Environ. Monit. Assess. 2003, 85, 277–292. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. (Ed.) Amphibian Ecology and Conservation: A Handbook of Techniques; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Gerner, T. Fang, Markierung und Beprobung von Freilebenden Wildtieren: Vollzugshilfe zur Überwachung der Bestände und bei Erfolgskontrollen; Bundesamt für Umwelt: Bern, Switzerland, 2018. [Google Scholar]
- Delarze, R.; Gonseth, Y.; Eggenberg, S.; Vust, M. Lebensräume der Schweiz; Ott Verlag: Bern, Switzerland, 2015. [Google Scholar]
- Augustin, N.H.; Mugglestone, M.A.; Buckland, S.T. An autologistic model for the spatial distribution of wildlife. J. Appl. Ecol. 1996, 33, 339–347. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R. unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 2 October 2022).
- Borgula, A.; Fallot, P.; Ryser, J. Inventar der Amphibienlaichgebiete von Nationaler Bedeutung: Schlussbericht; Bundesamt für Umwelt, Wald und Landschaft: Bern, Switzerland, 1994. [Google Scholar]
- Franklin, A.B.; Shenk, T.M.; Anderson, D.R.; Burnham, K.P. Statistical model selection: An alternative to null hypothesis testing. In Modeling in Natural Resource Management; Shenk, T.M., Franklin, A.B., Eds.; Island Press: Washington, DC, USA, 2001; pp. 75–90. [Google Scholar]
- Hooten, M.; Cooch, E.G. Comparing Ecological Models. In Quantitative Analysis in Wildlife Science; Brennan, L.A., Tri, A.N., Marcot, B.C., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2019; pp. 63–76. [Google Scholar]
- Colegrave, N.; Ruxton, G.D. Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav. Ecol. 2003, 14, 446–450. [Google Scholar] [CrossRef]
- Meyer, A.; Zumbach, S.; Schmidt, B.; Monney, J.C. Auf Schlangenspuren und Krötenpfaden: Amphibien und Reptilien der Schweiz; Haupt: Bern, Switzerland, 2009. [Google Scholar]
- Schmidt, B.R. Are hybridogenetic frogs cyclical parthenogens? Trends Ecol. Evol. 1993, 8, 271–273. [Google Scholar] [CrossRef]
- Dubey, S.; Leuenberger, J.; Perrin, N. Multiple origins of invasive and ‘native’ water frogs (Pelophylax spp.) in Switzerland. Biol. J. Linn. Soc. 2014, 112, 442–449. [Google Scholar] [CrossRef]
- Stumpel, A.H.P.; van der Voet, H. Characterizing the suitability of new ponds for amphibians. Amphib. Reptil. 1998, 19, 125–142. [Google Scholar] [CrossRef]
- Oldham, R.S.; Keeble, J.; Swan, M.J.S.; Jeffcote, M. Evaluating the suitability of habitat for the great crested newt (Triturus cristatus). Herpetol. J. 2000, 10, 143–155. [Google Scholar]
- Denoel, M.; Lehmann, A. Multi-scale effect of landscape processes and habitat quality on newt abundance: Implications for conservation. Biol. Conserv. 2006, 130, 495–504. [Google Scholar] [CrossRef]
- Indermaur, L.; Schmidt, B.R. Quantitative recommendations for amphibian terrestrial habitat conservation derived from habitat selection behaviour. Ecol. Appl. 2011, 21, 2548–2554. [Google Scholar] [CrossRef][Green Version]
- Shulse, C.D.; Semlitsch, R.D.; Trauth, K.M.; Gardner, J.E. Testing wetland features to increase amphibian reproductive success and species richness for mitigation and restoration. Ecol. Appl. 2012, 22, 1675–1688. [Google Scholar] [CrossRef]
- Unglaub, B.; Steinfartz, S.; Kühne, D.; Haas, A.; Schmidt, B.R. The relationships between habitat suitability, population size and body condition in a pond-breeding amphibian. Basic Appl. Ecol. 2018, 27, 20–29. [Google Scholar] [CrossRef]
- Unglaub, B.; Cayuela, H.; Schmidt, B.R.; Preissler, K.; Glos, J.; Steinfartz, S. Context-dependent dispersal determines relatedness and genetic structure in a patchy amphibian population. Mol. Ecol. 2021, 30, 5009–5028. [Google Scholar] [CrossRef]
- Brown, J.H. Macroecology; University of Chicago Press: Chicago, IL, USA, 1995. [Google Scholar]
- Pellet, J.; Fleishman, E.; Dobkin, D.S.; Gander, A.; Murphy, D.D. An empirical evaluation of the area and isolation paradigm of metapopulation dynamics. Biol. Conserv. 2007, 136, 483–495. [Google Scholar] [CrossRef]
- Prugh, L.R.; Hodges, K.E.; Sinclair, A.R.E.; Brashares, J. Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. USA 2008, 105, 20770–20775. [Google Scholar] [CrossRef]
- Blab, J. Biologie, Ökologie und Schutz von Amphibien; Kilda-Verlag: Warendorf, Germany, 1986; Volume 18, 150p. [Google Scholar]
- Schmidt, B.R. Steps towards better amphibian conservation. Anim. Conserv. 2008, 11, 469–471. [Google Scholar] [CrossRef]
- Riva, F.; Fahrig, L. The disproportionately high value of small patches for biodiversity conservation. Conserv. Lett. 2022, 15, e12881. [Google Scholar] [CrossRef]
- Cayuela, H.; Schmidt, B.R.; Weinbach, A.; Besnard, A.; Joly, P. Multiple density-dependent processes shape the dynamics of a spatially structured amphibian population. J. Anim. Ecol. 2019, 88, 164–177. [Google Scholar] [CrossRef]
- Joly, P.; Miaud, C.; Lehmann, A.; Grolet, O. Habitat matrix effects on pond occupancy in newts. Conserv. Biol. 2001, 15, 239–248. [Google Scholar] [CrossRef]
- Churko, G.; Kienast, F.; Bolliger, J. A multispecies assessment to identify the functional connectivity of amphibians in a human-dominated landscape. ISPRS Int. J. Geo-Inf. 2020, 9, 287. [Google Scholar] [CrossRef]
- Moor, H.; Bergamini, A.; Vorburger, C.; Holderegger, R.; Bühler, C.; Egger, S.; Schmidt, B.R. Bending the curve: Simple but massive conservation action leads to landscape-scale recovery of amphibians. Proc. Natl. Acad. Sci. USA 2022, 119. in press. [Google Scholar] [CrossRef]
- Calhoun, A.J.K.; Arrigoni, J.; Brooks, R.P.; Hunter, M.L.; Richter, S.C. Creating successful vernal pools: A literature review and advice for practitioners. Wetlands 2014, 34, 1027–1038. [Google Scholar] [CrossRef]
- Pellet, J. Temporäre Gewässer für gefährdete Amphibien schaffen—Leitfaden für die Praxis. Beiträge zum Naturschutz in der Schweiz 2014, 35, 1–25. [Google Scholar]
- Schmidt, B.R.; Zumbach, S.; Tobler, U.; Lippuner, M. Amphibien brauchen temporäre Gewässer. Z. Feldherpetol. 2015, 22, 137–150. [Google Scholar]
- Beebee, T.J.C.; Denton, J.S.; Buckley, J. Factors affecting population densities of adult natterjack toads Bufo calamita in Britain. J. Appl. Ecol. 1996, 33, 263–268. [Google Scholar] [CrossRef]
- Cayuela, H.; Monod-Broca, B.; Lemaître, J.-F.; Besnard, A.; Gippet, J.M.W.; Schmidt, B.R.; Romano, A.; Hertach, T.; Angelini, C.; Canessa, S.; et al. Compensatory recruitment allows amphibian population persistence in anthropogenic habitats. Proc. Natl. Acad. Sci. USA 2022, 119, e2206805119. [Google Scholar] [CrossRef]
- Barandun, J.; Reyer, H.-U. Reproductive ecology of Bombina variegata: Development of eggs and larvae. J. Herpetol. 1997, 31, 107–110. [Google Scholar] [CrossRef]
- Laciak, M.; Zajac, T.; Adamski, P.; Bielanski, W.; Cmiel, A.; Laciak, T.; Lipinska, A. Small monsters: Insect predation limits reproduction of yellow-bellied toad Bombina variegata to ponds in their earliest successional stage. Aquat. Conserv. 2022, 32, 817–831. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Principles for management of aquatic-breeding amphibians. J. Wildl. Manag. 2000, 64, 615–631. [Google Scholar] [CrossRef]
- McCaffery, R.M.; Eby, L.A.; Maxell, B.A.; Corn, P.S. Breeding site heterogeneity reduces variability in frog recruitment and population dynamics. Biol. Conserv. 2014, 170, 169–176. [Google Scholar] [CrossRef]
- Stokes, D.L.; Messerman, A.F.; Cook, D.G.; Stemble, L.R.; Meisler, J.A.; Searcy, C.A. Saving all the pieces: An inadequate conservation strategy for an endangered amphibian in an urbanizing area. Biol. Conserv. 2021, 262, 109320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).