Comparing Genetic and Field-Based Estimates of Population Connectivity in Marbled Salamanders, Ambystoma opacum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Study Species
2.2. Genotyping Individuals
2.3. Calculating Genetic Diversity and Population Differentiation
2.4. Estimating Field Movement and Gene Flow
2.5. Least-Cost Path Analysis
3. Results
3.1. Genetic Diversity and Population Differentiation
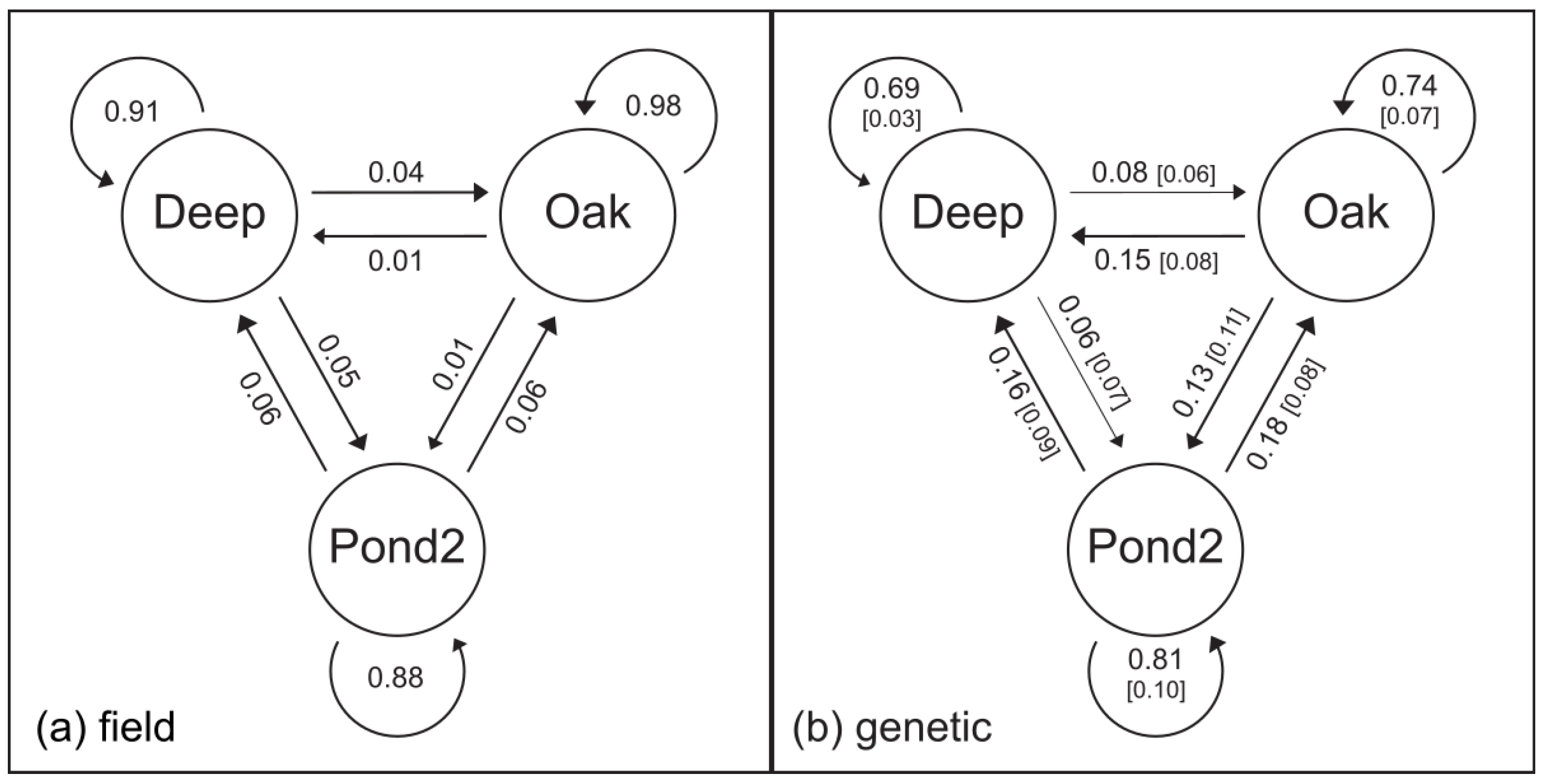
3.2. Estimates of Gene Flow and Migration
3.3. Least-Cost Movement Routes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cayuela, H.; Rougemont, Q.; Prunier, J.G.; Moore, J.S.; Clobert, J.; Besnard, A.; Bernatchez, L. Demographic and genetic approaches to study dispersal in wild animal populations: A methodological review. Mol. Ecol. 2018, 27, 3976–4010. [Google Scholar] [CrossRef]
- Fahrig, L.; Merriam, G. Habitat patch connectivity and population survival: Ecological archives e066-008. Ecology 1985, 66, 1762–1768. [Google Scholar] [CrossRef]
- Lowe, W.H.; Allendorf, F.W. What can genetics tell us about population connectivity? Mol. Ecol. 2010, 19, 3038–3051. [Google Scholar] [CrossRef]
- Wang, I.J.; Shaffer, H.B. Population genetic and field-ecological analyses return similar estimates of dispersal over space and time in an endangered amphibian. Evol. Appl. 2017, 10, 630–639. [Google Scholar] [CrossRef]
- Andreasen, A.M.; Stewart, K.M.; Longland, W.S.; Beckmann, J.P.; Forister, M.L. Identification of source-sink dynamics in mountain lions of the G reat B asin. Mol. Ecol. 2012, 21, 5689–5701. [Google Scholar] [CrossRef] [Green Version]
- Kool, J.T.; Moilanen, A.; Treml, E.A. Population connectivity: Recent advances and new perspectives. Landsc. Ecol. 2013, 28, 165–185. [Google Scholar] [CrossRef]
- Cushman, S.A.; Landguth, E.L.; Flather, C.H. Evaluating population connectivity for species of conservation concern in the American Great Plains. Biodivers. Conserv. 2013, 22, 2583–2605. [Google Scholar] [CrossRef] [Green Version]
- DeMaynadier, P.G.; Hunter, M.L., Jr. The relationship between forest management and amphibian ecology: A review of the North American literature. Environ. Rev. 1995, 3, 230–261. [Google Scholar] [CrossRef]
- Beebee, T.J.; Griffiths, R.A. The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 2005, 125, 271–285. [Google Scholar] [CrossRef]
- Collins, J.P.; Storfer, A. Global amphibian declines: Sorting the hypotheses. Divers. Distrib. 2003, 9, 89–98. [Google Scholar] [CrossRef]
- Belasen, A.M.; Bletz, M.C.; Leite, D.d.S.; Toledo, L.F.; James, T.Y. Long-term habitat fragmentation is associated with reduced MHC IIB diversity and increased infections in amphibian hosts. Front. Ecol. Evol. 2019, 6, 236. [Google Scholar] [CrossRef] [Green Version]
- Petranka, J.W. Salamanders of the United States and Canada Smithsonian; Smithsonian Books: Washington, DC, USA, 1998. [Google Scholar]
- Vos, C.C.; Chardon, J. Effects of habitat fragmentation and road density on the distribution pattern of the moor frog Rana arvalis. J. Appl. Ecol. 1998, 35, 44–56. [Google Scholar] [CrossRef]
- Wang, I.J.; Savage, W.K.; Bradley Shaffer, H. Landscape genetics and least-cost path analysis reveal unexpected dispersal routes in the California tiger salamander (Ambystoma californiense). Mol. Ecol. 2009, 18, 1365–1374. [Google Scholar] [CrossRef]
- Croshaw, D.A.; Schable, N.A.; Peters, M.B.; Glenn, T.C. Isolation and characterization of microsatellite DNA loci from Ambystoma salamanders. Conserv. Genet. 2005, 6, 473–479. [Google Scholar] [CrossRef]
- Broquet, T.; Petit, E.J. Molecular estimation of dispersal for ecology and population genetics. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 193–216. [Google Scholar] [CrossRef]
- Fedy, B.; Martin, K.; Ritland, C.; Young, J. Genetic and ecological data provide incongruent interpretations of population structure and dispersal in naturally subdivided populations of white-tailed ptarmigan (Lagopus leucura). Mol. Ecol. 2008, 17, 1905–1917. [Google Scholar] [CrossRef]
- Cowen, R.K.; Paris, C.B.; Srinivasan, A. Scaling of connectivity in marine populations. Science 2006, 311, 522–527. [Google Scholar] [CrossRef] [Green Version]
- LaRue, M.A.; Nielsen, C.K. Modelling potential dispersal corridors for cougars in midwestern North America using least-cost path methods. Ecol. Model. 2008, 212, 372–381. [Google Scholar] [CrossRef]
- Coulon, A.; Cosson, J.; Angibault, J.; Cargnelutti, B.; Galan, M.; Morellet, N.; Petit, E.; Aulagnier, S.; Hewison, A. Landscape connectivity influences gene flow in a roe deer population inhabiting a fragmented landscape: An individual–based approach. Mol. Ecol. 2004, 13, 2841–2850. [Google Scholar] [CrossRef]
- Etherington, T.R. Least-cost modelling and landscape ecology: Concepts, applications, and opportunities. Curr. Landsc. Ecol. Rep. 2016, 1, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Stevens, V.M.; Verkenne, C.; Vandewoestijne, S.; Wesselingh, R.A.; Baguette, M. Gene flow and functional connectivity in the natterjack toad. Mol. Ecol. 2006, 15, 2333–2344. [Google Scholar] [CrossRef]
- Cushman, S.A.; McKelvey, K.S.; Hayden, J.; Schwartz, M.K. Gene flow in complex landscapes: Testing multiple hypotheses with causal modeling. Am. Nat. 2006, 168, 486–499. [Google Scholar] [CrossRef] [Green Version]
- Driezen, K.; Adriaensen, F.; Rondinini, C.; Doncaster, C.P.; Matthysen, E. Evaluating least-cost model predictions with empirical dispersal data: A case-study using radiotracking data of hedgehogs (Erinaceus europaeus). Ecol. Model. 2007, 209, 314–322. [Google Scholar] [CrossRef]
- Epps, C.W.; Wehausen, J.D.; Bleich, V.C.; Torres, S.G.; Brashares, J.S. Optimizing dispersal and corridor models using landscape genetics. J. Appl. Ecol. 2007, 44, 714–724. [Google Scholar]
- Buhlmann, K.A.; Mitchell, J.C.; Smith, L.R. Descriptive ecology of the Shenandoah Valley sinkhole pond system in Virginia. Banisteria 1999, 13, 23–51. [Google Scholar]
- Cartwright, J.M.; Wolfe, W.J. Insular Ecosystems of the Southeastern United States—A Regional Synthesis to Support Biodiversity Conservation in a Changing Climate; U.S. Geological Survey: Reston, VA, USA, 2016; pp. 2330–7102.
- Church, D.R.; Bailey, L.L.; Wilbur, H.M.; Green, J.H.; Hiby, L. Salamander Demography at Isolated Wetlands within Mature and Regenerating Forests. Diversity 2022, 14, 309. [Google Scholar]
- Bishop, S.C. Notes on Salamanders with Descriptions of Several New Forms; University of Michigan Press: Ann Arbor, MI, USA, 1941. [Google Scholar]
- Nobel, G.; Brady, M. Observations on the Life History of the Marbled salamander, Ambystoma opacum. Gravenhurst Zool. 1933, 11, 89–132. [Google Scholar] [CrossRef]
- Gamble, L.R.; McGarigal, K.; Compton, B.W. Fidelity and dispersal in the pond-breeding amphibian, Ambystoma opacum: Implications for spatio-temporal population dynamics and conservation. Biol. Conserv. 2007, 139, 247–257. [Google Scholar] [CrossRef]
- Raymond, M.; Rousset, F. An exact test for population differentiation. Evolution 1995, 49, 1280–1283. [Google Scholar] [CrossRef]
- Rousset, F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Brookfield, J. A simple new method for estimating null allele frequency from heterozygote deficiency. Mol. Ecol. 1996, 5, 453–455. [Google Scholar] [CrossRef]
- Meirmans, P.; Van Tienderen, P. GENOTYPE and GENODIVE: Two programs for the analysis of genetic diversity of asexual organisms. Mol. Ecol. Notes 2004, 4, 792–794. [Google Scholar] [CrossRef]
- Meirmans, P.G.; Hedrick, P.W. Assessing population structure: FST and related measures. Mol. Ecol. Resour. 2011, 11, 5–18. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, S120–S139. [Google Scholar] [CrossRef]
- Church, D.R.; Bailey, L.L.; Wilbur, H.M.; Kendall, W.L.; Hines, J.E. Iteroparity in the variable environment of the salamander Ambystoma tigrinum. Ecology 2007, 88, 891–903. [Google Scholar] [CrossRef]
- Bailey, L.L.; Kendall, W.L.; Church, D.R.; Wilbur, H.M. Estimating survival and breeding probabilities for pond-breeding amphibians: A modified robust design. Ecology 2004, 85, 2456–2466. [Google Scholar] [CrossRef]
- Bailey, L.L.; Kendall, W.L.; Church, D.R. Exploring extensions to multi-state models with multiple unobservable states. In Modeling Demographic Processes in Marked Populations; Thomson, D.L., Cooch, E.G., Conroy, M.J., Eds.; Environmental and Ecological Statistics Series; Springer Science+Business Media: New York, NY, USA, 2009; Volume 3, pp. 693–710. [Google Scholar]
- Wilson, G.A.; Rannala, B. Bayesian inference of recent migration rates using multilocus genotypes. Genetics 2003, 163, 1177–1191. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Faubet, P.; Waples, R.S.; Gaggiotti, O.E. Evaluating the performance of a multilocus Bayesian method for the estimation of migration rates. Mol. Ecol. 2007, 16, 1149–1166. [Google Scholar] [CrossRef] [Green Version]
- Meirmans, P.G. Nonconvergence in Bayesian estimation of migration rates. Mol. Ecol. Resour. 2014, 14, 726–733. [Google Scholar] [CrossRef]
- Trenham, P.C.; Koenig, W.D.; Shaffer, H.B. Spatially autocorrelated demography and interpond dispersal in the salamander Ambystoma californiense. Ecology 2001, 82, 3519–3530. [Google Scholar] [CrossRef] [Green Version]
- Church, D.R. Population Ecology of Ambystoma tigrinum (Caudata, Ambystomidae) and Occupancy Dynamics in an Appalachian Pond-breeding Amphibian Assemblage; University of Virginia: Charlottesville, VA, USA, 2004. [Google Scholar]
- Mitchell, J.C.; Buhlmann, K. Amphibians and reptiles of the Shenandoah Valley sinkhole pond system in Virginia. Banisteria 1999, 13, 129–142. [Google Scholar]
- Swartz, R.E.; Kocka, D.M. Wildlife Management Activities in Big Levels, Augusta County, Virginia: An Overview. Banisteria 1999, 13, 11–15. [Google Scholar]
- Johnston, B.; Frid, L. Clearcut logging restricts the movements of terrestrial Pacific giant salamanders (Dicamptodon tenebrosus Good). Can. J. Zool. 2002, 80, 2170–2177. [Google Scholar] [CrossRef]
- Templeton, A.R.; Robertson, R.J.; Brisson, J.; Strasburg, J. Disrupting evolutionary processes: The effect of habitat fragmentation on collared lizards in the Missouri Ozarks. Proc. Natl. Acad. Sci. USA 2001, 98, 5426–5432. [Google Scholar] [CrossRef] [Green Version]
- Funk, W.C.; Blouin, M.S.; Corn, P.S.; Maxell, B.A.; Pilliod, D.S.; Amish, S.; Allendorf, F.W. Population structure of Columbia spotted frogs (Rana luteiventris) is strongly affected by the landscape. Mol. Ecol. 2005, 14, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Rothermel, B.B.; Semlitsch, R.D. An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conserv. Biol. 2002, 16, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Todd, B.D.; Luhring, T.M.; Rothermel, B.B.; Gibbons, J.W. Effects of forest removal on amphibian migrations: Implications for habitat and landscape connectivity. J. Appl. Ecol. 2009, 46, 554–561. [Google Scholar] [CrossRef]
- Rothley, K. Finding and filling the “cracks” in resistance surfaces for least-cost modeling. Ecol. Soc. 2005, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Pittman, S.; Semlitsch, R. Habitat type and distance to edge affect movement behavior of juvenile pond-breeding salamanders. J. Zool. 2013, 291, 154–162. [Google Scholar] [CrossRef]
- Whiteley, A.R.; McGarigal, K.; Schwartz, M.K. Pronounced differences in genetic structure despite overall ecological similarity for two Ambystoma salamanders in the same landscape. Conserv. Genet. 2014, 15, 573–591. [Google Scholar] [CrossRef]
- Purrenhage, J.; Niewiarowski, P.; Moore, F.G. Population structure of spotted salamanders (Ambystoma maculatum) in a fragmented landscape. Mol. Ecol. 2009, 18, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Spear, S.F.; Peterson, C.R.; Matocq, M.D.; Storfer, A. Landscape genetics of the blotched tiger salamander (Ambystoma tigrinum melanostictum). Mol. Ecol. 2005, 14, 2553–2564. [Google Scholar] [CrossRef] [PubMed]
- Storfer, A. Gene flow and endangered species translocations: A topic revisited. Biol. Conserv. 1999, 87, 173–180. [Google Scholar] [CrossRef]
- Zamudio, K.R.; Wieczorek, A.M. Fine-scale spatial genetic structure and dispersal among spotted salamander (Ambystoma maculatum) breeding populations. Mol. Ecol. 2007, 16, 257–274. [Google Scholar] [CrossRef]

| Population | N | Ne | Ho | Hs |
|---|---|---|---|---|
| Oak99 | 8.714 | 5.064 | 0.676 | 0.692 |
| Deep | 9.429 | 4.573 | 0.704 | 0.680 |
| Pond2 | 8.143 | 4.501 | 0.643 | 0.672 |
| Source of Variation | % Variation | F | p-Value |
|---|---|---|---|
| Within populations | 0.224 | – | – |
| Among populations | 0.008 | 0.008 | 0.181 |
| OAK | DEEP | POND2 | |
|---|---|---|---|
| OAK | 0.024 | 0.797 | 0.071 |
| DEEP | –0.002 | –0.035 | 0.108 |
| POND2 | 0.005 | 0.004 | 0.043 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posbic-Leydet, K.E.; Glennon, K.L.; Fisher, J.T.; Church, D.R.; Church, S.A. Comparing Genetic and Field-Based Estimates of Population Connectivity in Marbled Salamanders, Ambystoma opacum. Diversity 2022, 14, 524. https://doi.org/10.3390/d14070524
Posbic-Leydet KE, Glennon KL, Fisher JT, Church DR, Church SA. Comparing Genetic and Field-Based Estimates of Population Connectivity in Marbled Salamanders, Ambystoma opacum. Diversity. 2022; 14(7):524. https://doi.org/10.3390/d14070524
Chicago/Turabian StylePosbic-Leydet, Karine E., Kelsey L. Glennon, Jolene T. Fisher, Don R. Church, and Sheri A. Church. 2022. "Comparing Genetic and Field-Based Estimates of Population Connectivity in Marbled Salamanders, Ambystoma opacum" Diversity 14, no. 7: 524. https://doi.org/10.3390/d14070524
APA StylePosbic-Leydet, K. E., Glennon, K. L., Fisher, J. T., Church, D. R., & Church, S. A. (2022). Comparing Genetic and Field-Based Estimates of Population Connectivity in Marbled Salamanders, Ambystoma opacum. Diversity, 14(7), 524. https://doi.org/10.3390/d14070524






