Global Biodiversity of the Family Lecithoceridae (Gelechioidea) with a Brief Historical Review
Abstract
1. Introduction
2. Material and Methods
3. Synopsis of the Family Lecithoceridae
3.1. General
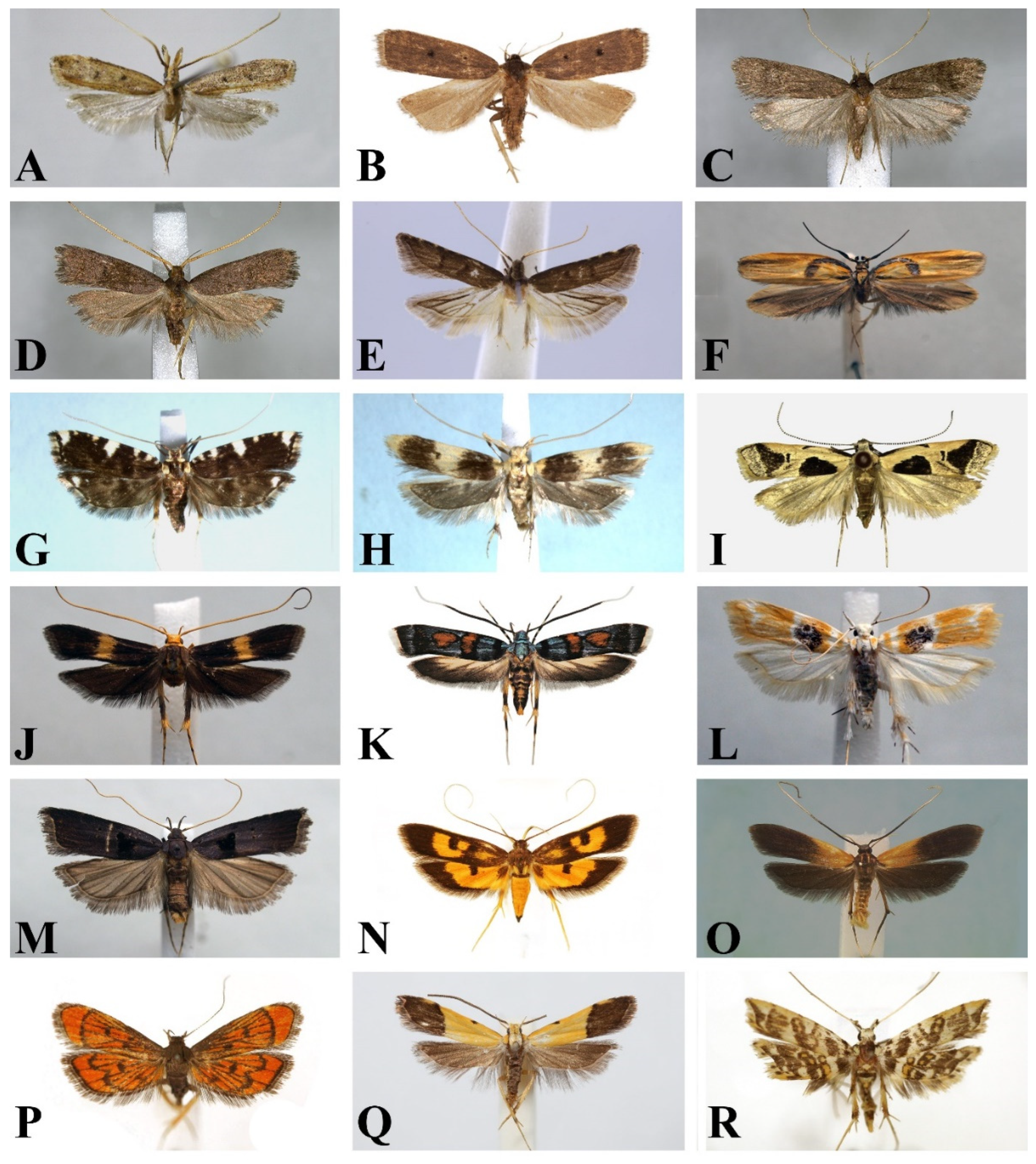
3.2. Morphology
3.3. Division of Subfamilies of the Family
3.4. Biology
3.5. Phylogeny
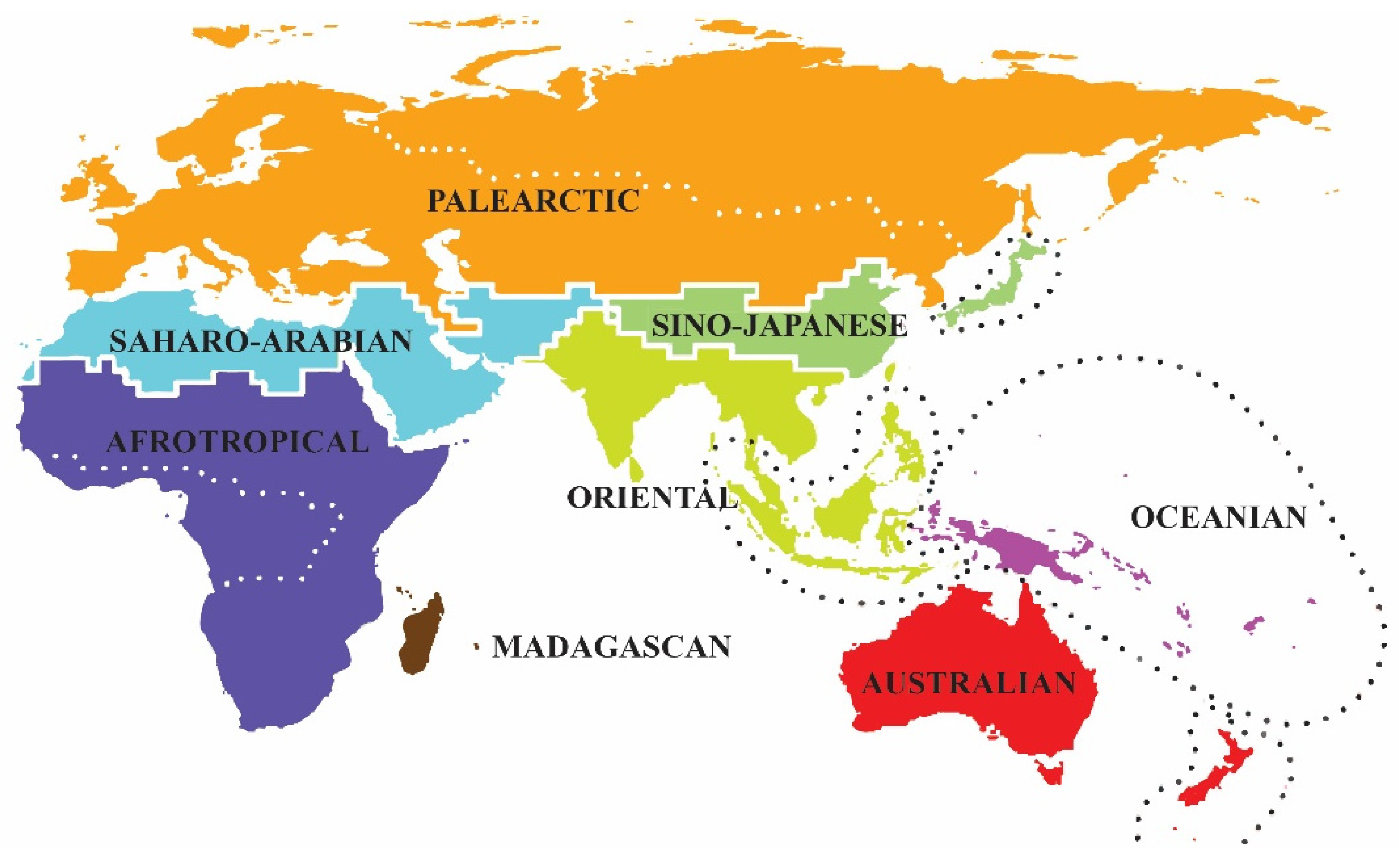
3.6. Zoogeographical Remarks
| OR | PA | AF + MA | AU | OC | Subtotal | |
|---|---|---|---|---|---|---|
| Ceuthomadarinae | - | 8 | - | - | 8 | |
| Lecithocerinae | 581 | 26 | 68 | 28 | 89 | 792 |
| Torodorinae | 389 | 30 | 120 | 2 | 3 | 544 |
| Crocanthinae | 3 | - | - | 17 | 71 | 91 |
| Total | 973 | 64 | 188 | 47 | 163 | 1435 |
| (67.8%) | (4.5%) | (13.1%) | (3.3%) | (11.3%) | (100%) |
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MGCL | McGuire Center of Lepidoptera and Biodiversity: University of Florida: Gainesville, USA. |
| MfN | Zoologisches Museum für Naturkunde, Humboldt-Universität, Berlin, Germany. |
| MNHN | Museum national d’Historie naturelle, Paris, France. |
| NHMO | The Natural History Museum, University of Oslo, Oslo, Norway. |
| NHMUK | The Natural History Museum, London, UK. |
| NKU | Nankai University: Tienjin, China. |
| RMNH (=NCB) | Rijksmuseum van Natuurlijke Historie, Leiden, The Netherlands. |
| ZMUC (=ZMCD) | Zoological Museum, University of Copenhagen, Denmark. |
| USNM | US National Museum of Natural History, Wash. D.C., USA. |
References
- Walker, F. List of the Specimens of the Lepidopterous Insects in the Collection of the British Museum; British Museum: London, UK, 1864; Volumes 29–30, pp. 563–835, 837–1044. [Google Scholar]
- Walker, F. List of the Specimens of Lepidopterous Insects in the Collection of the British Museum; British Museum: London, UK, 1886; Volume 35, pp. 1535–2040. [Google Scholar]
- Walsingham, T.G. On the Tortricidae, Tineidae and Pterophoridae of South Africa. Trans. Ent. Soc. Lond. 1881, 1881, 219–288. [Google Scholar] [CrossRef][Green Version]
- Lower, O. New Australian Lepidoptera. Trans. Royal Soc. South Aust. 1896, 20, 52–170. [Google Scholar] [CrossRef]
- Lower, O. Description of new Australian Lepidoptera. Trans. Royal Soc. South Aust. 1897, 21, 50–60. [Google Scholar]
- Meyrick, E. On a collection of Lepidoptera from Upper Burma. Trans. R. Ent. Soc. Lond. 1894, 42, 16–19. [Google Scholar]
- Meyrick, E. Descriptions of Indian Microlepidoptera. J. Bombay Nat. Hist. Soc. 1906, 17, 138–155; 403–417. [Google Scholar]
- Meyrick, E. Descriptions of Indian Microlepidoptera. J. Bombay Nat. Hist. Soc. 1911, 20, 707–715; 721–722. [Google Scholar]
- Meyrick, E. Descriptions of Indian Microlepidoptera. J. Bombay Nat. Hist. Soc. 1914, 22, 773. [Google Scholar]
- Meyrick, E. Lepidoptera Heterocera. Family Gelechiidae. In Genera Insectorum 184; Wytsman, P., Ed.; Philogène Wytsman: Bruxelles, Belgium, 1925; p. 290. [Google Scholar]
- Meyrick, E. Exotic Microlepidoptera. Marlbgh. Wilts 1932, 4, 203–205. [Google Scholar]
- Meyrick, E. Exotic Microlepidoptera. Marlbgh. Wilts 1934, 4, 453, 513–514. [Google Scholar]
- Meyrick, E. Exotic Microlepidoptera. Marlbgh. Wilts 1935, 4, 563–566, 591. [Google Scholar]
- Gozmány, L. Lecithoceridae. In Microlepidoptera Palaearctica Vol. 5; Amsel, H.G., Gregor, F., Reisser, H., Eds.; Verlag Greorg Fromme and Co.: Wien, Australia, 1978; p. 306. [Google Scholar]
- Wang, Y.Q.; Park, K.-T.; Wang, S. Taxonomic review of the Genus Deltoplastis Meyrick (Lepidoptera: Lecithoceridae) in China and Indochina, with a world catalogue of the genus. Zootaxa 2015, 4057, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S. The Lecithoceridae (Lepidoptera) of China with descriptions of new taxa. Sinozoologia 1994, 11, 123–154. [Google Scholar]
- Wu, C.S. Genus Philharmonia Gozmany from China (Lepidoptera: Lecithoceridae). Ent. Sinica 1994, 1, 214–216. [Google Scholar] [CrossRef]
- Wu, C.S. Lepidoptera, Lecithoceridae. Fauna Sinica, Insecta. Vol.7; Science Press: Beijing, China, 1997; p. 302. [Google Scholar]
- Wu, C.S.; Liu, Y.Q. A new genus and four new species of Lecithocerinae from China (Lepidoptera: Lecithoceridae). Sinozoologia 1993, 10, 347–354. [Google Scholar]
- Wu, C.S.; Liu, Y.Q. A study of the Chinese Torodora Meyrick, 1894 and descriptions of new species (Lepidoptera: Lecithoceridae). Sinozoologia 1994, 11, 155–173. [Google Scholar]
- Yu, S.A.; Zhu, Y.M.; Wang, S.X. Eighteen new species and fifteen new records of the genus Torodora Meyrick (Lepidoptera: Lecithoceridae) from China. Zootaxa 2022, 5133, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-T. A new subfamily Crocanthinae based on the genus Crocanthes Meyrick and its related genera, with a world catalog of the subfamily (Lepidoptera, Lecithoceridae). J. Asia-Pacific Biod. 2015, 8, 251–286. [Google Scholar] [CrossRef]
- Park, K.-T. The Subfamily Crocanthinae (Lepidoptera, Lecithoceridae) of the World; Lap Lambert Academic Publishing: Saarbrucken, UK, 2017; p. 134. [Google Scholar]
- Park, K.-T.; Cho, S.W.; Koo, J.M. The Subfamily Torodorinae (Lepidoptera: Lecithoceridae) of the World; National Institute of Biological Resources: Incheon, Korea, 2022; p. 584. [Google Scholar]
- Ghesquiere, J. Lépidoptères, Microlépidoptères. In Catalogues Raisonnes de la Faune Entomologique du Congo; Annales du Musée du Congo (Belge). Série III; Botanique: Tervueren, Belgium, 1940; p. 120. [Google Scholar]
- Komai, F.; Yoshiyasu, Y.; Nasu, Y.; Saito, T. A Guide to the Lepidoptera of Japan; Tokai University Press: Tokyo, Japan, 2011; p. 1308. [Google Scholar]
- Diakonoff, A. Gelechiidae. Microlepidoptera of New Guinea. Results of the third archbold expedition. Part IV. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen. AFD; Naturrrkunde Tweede Reeks, Deel L, No. 1; North- Holland Publishing: Amsterdam, The Netherlands, 1954; p. 185. [Google Scholar]
- Diakonoff, A. Microlepidoptera of Philippine Islands; United States National Museum: Washington, DC, USA, 1967; Volume 257, pp. 125–147. [Google Scholar]
- Janse, A.J.T. Gelechiidae. In The Moths of South Africa; Janse, A.J.T., Ed.; Transvaal Museum: Pretoria, South Africa, 1954; Volume 5, pp. 332–349, 362–363, 366–370, 377–384. [Google Scholar]
- Clarke, J.F.G. Catalogue of the type Specimens of Microlepidoptera in the British Museum (Natural History) Described by Edward Meyrick; British Museum (Natural History): London, UK, 1965; p. 255. [Google Scholar]
- Park, K.-T.; Koo, J.M.; Minet, J. Review of the Malagasy lecithocerid species described by Pierre Viette and deposited in MNHN (Paris), with new generic combinations and descriptions of a new subfamily and genus of Momphidae (Lepidoptera: Gelechioidea). Zootaxa 2020, 4845, 151–190. [Google Scholar] [CrossRef]
- Common, I.F.B. Family Lecithoceridae. Check List of the Lepidoptera of Australia. In Monographs on Australian Lepidoptera; Nielsen, E.S., Edwards, E.D., Rangsi, T.V., Eds.; CSIRO Publishing: Canbera, Australia, 1996; Volume 4, pp. 116–117. [Google Scholar]
- Le Marchand, S. Les Tineina Gelechiidae. Revue Fr. Lépidopt. 1947, 11, 145–163. [Google Scholar]
- Clarke, J.F.G. Catalogue of the Type Specimens of Microlepidoptera in the British Museum (Natural History) Described by Edward Meyrick; British Museum (Natural History): London, UK, 1955; Volume 1, p. 322. [Google Scholar]
- Hodges, R.W. Gelechoidea, Cosmopterigidae. In The Moths of America North of Mexico. Fasc. 6.1. E.W.; Dominick, R.B., Ferguson, D., Franclemont, J., Hodges, R.W., Munroe, E., Eds.; Classy Limited and The Wedge Entomological Research Foundation: London, UK, 1978; p. 166. [Google Scholar]
- Common, I.F.B. Superfamily Gelechoidea. In Moths of Australia; Common, I.B.F., Ed.; Melbourne University Press: Melbourne, Australia, 1990; pp. 217–266. [Google Scholar]
- Karsholt, O.; Razowski, J. The Lepidoptera of Europe, a Distributional Check List; Karsholt, O., Razowski, J., Eds.; Apollo Books: Stenstrup, Denmark, 1996; p. 380. [Google Scholar]
- Nielsen, E.S.; Edwards, E.D.; Rangsi, T.V. Monograph on Australian Lepidoptera. Vol. 4. Checklist of the Lepidopter of Australia; CSIRO Div. Ent.: Canbera, Australia, 1996; p. 529. [Google Scholar]
- De Prins, J.; De Prins, W. Afromoths, Online Database of Afrotropical Moth Species (Lepidoptera). 2022. Available online: http://www.afromoths.net (accessed on 1 March 2022).
- Sattler, K. A catalogue of the Family-group and genus-group names of Gelechiidae, Holcopogonidae, Lecithoceridae and Symmocidae (Lepidoptera). Bull. Br. Mus. Nat. Hist. Entom. 1973, 28, 153–282. [Google Scholar]
- Kaila, L. Phylogeny of the superfamily Gelechioidea (Lepidoptera: Ditrysia): An exemplar approach. Cladistics 2004, 20, 303–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, H.H. Phylogeny of the superfamily Gelechioidea (Lepidoptera: Obtectomera), with an exploratory application on geometric morphometrics. Zool. Scripta 2020, 49, 307–328. [Google Scholar] [CrossRef]
- Heikkilä, M.; Mutanen, M.; Kekonen, M.; Kaila, L. Morphology reinforces proposed molecular phylogenetic affinities: A revised classification for Gelechioidea (Lepidoptera). Cladistics 2014, 30, 563–589. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.C.; Regier, J.C.; Mitter, C.; Adamski, D.; Landry, J.F.; Heikkila, M.M.; Park, K.-T.; Harrison, T.; Mitter, K.; Zwick, A.; et al. Phylogeny and feeding trait evolution of the mega-diverse Gelechioidea (Lepidoptera: Obtectomera): New insight from 19 nuclear genes. Syst. Ent. 2015, 41, 112–132. [Google Scholar] [CrossRef]
- Wallace, A.R. The Geographical Distribution of Animals; with a Study of the Relations of Living and Extinct Faunas as Elu-Cidating the Past Changes of the Earth’s Surface; Harper & brothers: London, UK, 1876; Volume 1–2, pp. 503, 607. [Google Scholar]
- Holt, B.G.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-T.; Cho, S.; Bae, Y.S. Review of Lecithoceridae (Lepidoptera: Gelechioidea) in the Oceanian Region, with description of a new species and a checklist of the family. Zootaxa 2016, 4147, 143–161. [Google Scholar] [CrossRef]
- Park, K.-T.; De Prins, J.; De Prins, W. A checklist of Lecithoceridae (Lepidoptera: Gelechioidea) of the Afrotropical Region. J. Asia-Pacific Biod. 2021, 14, 355–370. [Google Scholar] [CrossRef]
- Cho, S.; Koo, J.M.; Agassiz, D.J.L. A new genus Parkiana Cho, gen. nov. (Lepidoptera, Lecithoceridae) from Madagascar, with descriptions of two new species. Anim. Syst. Evol. Divers. 2020, 36, 107–112. [Google Scholar]
- Kaila, L.; Mutanen, M.; Nyman, T. Phylogeny of the megadiverse Gelechioidea (Lepidoptera): Adaptations and determinants of success. Mol. Phylogenet. Evol. 2011, 61, 801–809. [Google Scholar] [CrossRef]

| Subfamily | No. of Genera | No. of Species (%) |
|---|---|---|
| Ceuthomadarinae | 1 | 8 (0.6%) |
| Lecithocerinae | 77 | 792 (55.2%) |
| Torodorinae | 45 | 544 (37.9%) |
| Crocanthinae | 6 | 91 (6.3%) |
| 129 | 1435 (100%) |
| Subfamily/Genus | Type Locality | Type Species |
|---|---|---|
| LECITHOCERINAE | ||
| Corymbus Park, 2019 | Kenya | C. deprinsi Park and Aarvik, 2019 |
| Crinellus Park, 2012 | PNG | C. eremicus Park, 2012 |
| Furcalis Park, 2018 | Cameroon | F. triodonta Park, 2018 |
| Halista Park, 2020 | Philippines | H. batillosa Park and Kim, 2020 |
| Hamatina Park, 2011 | Indonesia, Papua | H. hematoma (Diakonoff, 1954) |
| Lacuniola Park, 2012 | PNG | L. hadrorhacha Park, 2012 |
| Mireana Park, 2020 | Philippines | M. tawitawiensis Park and Kim |
| Neopectinimura Park, 2010 | PNG | N. neckeri Park, 2010 |
| Neotimyra Park, 2011 | PNG | N. senara Park, 2011 |
| Notiosus Park, 2018 | Cameroon | N. cupripennis Park, 2018 |
| Onnuria Park, 2011 | PNG | O. xanthochlora Park, 2011 |
| Paniculata Park, 2018 | Cameroon | P. weberi Park, 2018 |
| Pectinimura Park, 2008 | Philippines | P. montiatilis Park and Byun, 2008 |
| Scolizona Park, 2011 | Indonesia, Papua | S. rhinoceros (Diakonoff, 1954) |
| Strombiola Park, 2011 | PNG | S. papuana Park, 2011 |
| Sulciolus Park, 2012 | Indonesia, Papua | S. pachystoma (Diakonoff, 1954) |
| Thailepidonia Park, 2007 | Thailand | T. yoshiyasui Park, 2007 |
| Thrombialis Park, 2020 | Uganda | T. sylvestrana Park and Koo |
| TORODORINAE | ||
| Caveana Park, 2010 | Thailand | C. diemseoki Park, 2010 |
| Chrysonasma Park, 2008 | Philippines | C. cassiterota (Meyrick, 1923) |
| Notialis Park, 2009 | Philippines | N. sigmatis Park, 2009 |
| Lepidozonates Park, 2013 | Taiwan | L. viciniolus Park, 2013 |
| Heppernalis Park, 2013 | Indonesia, Sulawesi | H. decorella Park, 2013 |
| Parkiana Cho, 2020 | Madagascar | P. matutinalis Cho and Agassiz, 2020 |
| Spiniola Park, 2022 | Malawi | S. hanaro Park, 2021 |
| Thubdora Park, 2018 | Rep. Congo | T. acutalis Park, 2018 |
| Triviola Park, 2010 | Thailand | T. puiensis Park, 2010 |
| Viperinus Park, 2021 | Kenya | V. orbiosus Park, 2021 |
| Woonpaikia Park, 2010 | Thailand | W. villosa Park, 2010 |
| CROCANTHINAE | ||
| Hannara Park, 2013 | PNG | H. buoloensis Park, 2013 |
| Lamprista Park, 2013 | PNG | L. emmeli Park, 2013 |
| Pacificulla Park, 2013 | PNG | P. flaviagera Park, 2013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-T. Global Biodiversity of the Family Lecithoceridae (Gelechioidea) with a Brief Historical Review. Diversity 2022, 14, 822. https://doi.org/10.3390/d14100822
Park K-T. Global Biodiversity of the Family Lecithoceridae (Gelechioidea) with a Brief Historical Review. Diversity. 2022; 14(10):822. https://doi.org/10.3390/d14100822
Chicago/Turabian StylePark, Kyu-Tek. 2022. "Global Biodiversity of the Family Lecithoceridae (Gelechioidea) with a Brief Historical Review" Diversity 14, no. 10: 822. https://doi.org/10.3390/d14100822
APA StylePark, K.-T. (2022). Global Biodiversity of the Family Lecithoceridae (Gelechioidea) with a Brief Historical Review. Diversity, 14(10), 822. https://doi.org/10.3390/d14100822