Abstract
A yellow, Gram-stain-negative, aerobic, non-spore-forming, motile, and rod-shaped bacterial strain designated M6T was isolated from fully weathered granitic soil. The strain showing the highest 16S rRNA gene sequence similarity to M6T was Sandaracinobacteroides hominis SZY PN-1T (96.3%), the only species in the genus Sandaracinobacteroides. The average nucleotide identity and digital DNA-DNA hybridization value between these two strains were 72.6% and 18.0% respectively. Growth was inhibited by NaCl (≥0.1% (w/v)). Strain M6T contained C18:1ω7c (33.8%), C14:0 2-OH (16.6%), summed feature 3 (15.8%), and C16:0 (12.6%) as the major fatty acids. The polar lipids profile consisted of phosphatidylglycerol, phosphatidylethanolamine, an unidentified glycolipid, four unidentified phospholipids, and four unidentified lipids. The genome of strain M6T was 3.4 Mb with 67.7% GC content. Further genomic analysis revealed a biosynthetic gene cluster for zeaxanthin, the production of which was verified by a high-resolution mass spectrum. The existence of multiple genes for aromatic ring-hydroxylating dioxygenases implies the potential ability for organic pollution controlling. The morphological, physiological, chemotaxonomic, and phylogenetic analysis clearly distinguished this strain from its phylogenetic neighbors, thus strain M6T represents a novel species of the genus Sandaracinobacteroides, for which the name Sandaracinobacteroides saxicola sp. nov. is proposed. The type of strain is M6T (=CGMCC 1.19164T=NBRC 115420T).
1. Introduction
The genus Sandaracinobacteroides, a member of the family Sphingosinicellaceae, order Sphingomonadales, and class Alphaproteobacteria was proposed in 2021 [1]. A similar genus Sandaracinobacter with closely phylogenetic distance had been described in 1997 [2], and subsequently amended in 2020 [3], however, this genus is not a validly published name according to the List of Prokaryotic Names with Standing in Nomenclature (www.bacterio.net (accessed on 19 July 2022)) [4].
At the time of writing, the genus Sandaracinobacteroides includes only one species, the S. hominis SZY PN-1T originated from human skin [1]. The genus Sandaracinobacter contains 2 species, the S. sibiricus RB16-17 T and S. neustonicus PAMC 28131T. They originated from freshwater and sea surface microlayers respectively [2,3]. All three species were Gram-stain-negative, strictly aerobic, or facultatively anaerobic. Their colonies were yellow-orange or yellow due to the presence of carotenoid pigments. Summed feature 8 (C18:1ω6c and/or C18:1ω7c) and summed feature 3 (C16:1ω6c and/or C16:1ω7c) had been discovered as their major cellular fatty acids. Phosphatidylglycerol and phosphatidylethanolamine were the major known polar lipids.
The majority of bacteria in nature have not been cultivated in the laboratory yet [5]. They are likely to possess novel biosynthetic pathways and unknown biochemical characteristics and therefore could provide potential applications in biotechnology, agriculture, and bioremediation [5]. Rock surfaces are challenging habitats for microbes due to the rapid changes in the intensity of radiation, temperature, water supply, and nutrient availability [6]. Aimed at investigating the unexplored bacterial lineages from the fully weathered granitic rack, a soil sample was collected from a rocky mountain in South China. In this study, we aimed to report the phenotypic, genetic, and chemotaxonomic features of the strain M6T to characterize it as a novel species in the genus Sandaracinobacteroides, with an emphasis on its ability to produce zeaxanthin, a coloring additive in the food industry and an essential micronutrient for humans. In addition, the sequences of the six aromatic ring-hydroxylating dioxygenases encoded by its genome were analyzed in detail in order to evaluate its potential ability to degrade organic pollutants.
2. Materials and Methods
2.1. Isolation and Maintenance of the Organisms
In 2019, the strain M6T was isolated from a soil sample collected on a rocky mountain in Changsha, Hunan province, South China (28.46° N, 113.18° E). For isolation, 5 g dried soil was taken in 250 mL Erlenmeyer flasks containing 45 mL of sterile 0.25% Ringer’s solution (2.25 g NaCl, 0.105 g KCl, 0.045 g CaCl2, 0.05 g NaHCO3 L−1) and agitated on a rotary shaker at 30 °C for 30 min. Subsequently, the suspension was serially diluted up to 10−5 times. An aliquot of 0.2 mL of each of these dilutions was spread SSE/HD agar [7]. After six days of aerobic incubation at 30 °C, one yellow colony was transferred, purified, and designated as M6T. The isolates were sub-cultivated routinely on R2A (Reasoner’s 2A) agar or modified RO (rich organic) medium (1 g yeast extract, 1 g Bacto peptone, 1 g sodium acetate, 0.3 g KCl, 0.5 g MgSO4∙7H2O, 0.05 g CaCl2∙2H2O, 0.3 g NH4Cl, 0.3 g K2HPO4, 20 μg vitamin B12, 15 g Bacto agar L−1) [8,9], and preserved in a glycerol solution (20%, v/v) at −80 °C. When compared with the sequences in the NCBI and EzBioCloud database, nearly half of the strains isolated from this soil sample showed the highest 16S rRNA gene sequence similarity below 98.7%, the threshold proposed for differentiating two species [10]. At that time, the M6T strain showed the highest similarity to the available sequence of Sandaracinobacter sibiricus (Table S1) and its sequenced genome was related to this genus (NCBI Reference Sequence: NZ_CP059851.1). In this study, we compared the M6T strain with reference type strain S. neustonicus PAMCS 28131T and the recently described Sandaracinobacteroides hominis SZY PN-1T, purchased from the Japan collection of microorganisms and the Japanese national biologic resource center respectively, while S. sibiricus RB16-17 T was unavailable from any public culture collections [11].
2.2. Phenotypic and Biochemical Characteristics
Cell size and morphology of strain M6T grown in R2A for five days at 30 °C were studied by Hitachi SU8010 cold field scanning electron microscopy. The Gram reaction was determined by the standard Gram staining method. The motility of cells was performed by observing the growth spread of cells in test tubes containing semi-solid modified RO agar (0.3% w/v). Growth on modified RO and R2A agar at different temperatures (4, 10, 15, 20, 25, 30, 37, 42 °C) was observed. The pH range (pH 5.0–10.0 at intervals of 0.5 pH unit) for growth and tolerance to different NaCl concentration (0, 0.1, 0.2, 0.3, 0.4, 0.5, 1.0, 2.0, 3.0 and 5.0%, w/v) was assessed by using R2A and modified RO medium after incubation for one week. The pH of the media was adjusted with 10 mM MES (pH 5.0–6.0), 10 mM Tris (pH 7.0–9.0) or sodium carbonate/sodium bicarbonate (pH 10.0). Tests for hydrolysis of casein, starch, chitin, and Tweens (40, 60, 80) were performed using the methods described previously [12]. Catalase activity was examined by bubble production after application of 3% (v/v) H2O2 solution to the isolated colony and oxidase activity was accessed by using 1% (v/v) N, N, N′, N′,-tetramethyl-1, 4-phenylenediamine reagent [13]. Other physiological, biochemical, and enzymatic activities were conducted by using API 20NE, API ID32, API ZYM, and API 50CH test kit (bioMérieux) according to the manufacturer’s instructions. Cells grown on the modified RO agar plates at 30 °C for three days were employed for API tests.
For determining the presence of bacteriochlorophyll a and carotenoid pigment, cells were harvested and extracted with an acetone-methanol mixture. The supernatant was detected for absorbance at different wavelengths on a spectrophotometer (Pgeneral, T6) [11]. High-resolution mass spectrometry (HRMS) was carried out on an Agilent 6545 Quadrupole Time of Flight (Q-TOF) high-resolution mass spectrometer equipped with a reverse phase C18 column (Agilent, Eclipse Plus, 1.8 μm 50 × 2.1 mm), running in positive ionization mode with a resolution of 30,000. The flow rate was set at 0.3 mL/min with a mobile phase of H2O/ACN each containing 0.1% of formic acid. The ACN percentage gradually increased from 5% to 95% in 12 min. The injection volume was 2 μL. Voges-Proskauer (VP) reaction was tested as previously described [12] with Escherichia coli and Enterobacter aerogenes as negative and positive control respectively. H2S production assay was performed using triple sugar iron agar.
2.3. Chemotaxonomic Analysis
The polar lipids were analyzed using freeze-dried cells as described by Minnikin et al., 1984 [14]. Fatty acid methyl esters were prepared according to the standard MIDI protocol (Sherlock Microbial Identification System, version 6.0B) and identified by using the MIDI with the TSBA database version 6.1. Isoprenoid quinones were analyzed by using reversed-phase HPLC as described by Shin, et al. 1996 [15].
2.4. The 16S rRNA Gene Sequencing and Phylogenetic Analysis
The 16S rRNA gene of strain M6T was amplified by PCR using forward primer 27F and reverse primer 1492R [16] and sequenced with an Applied Biosystem 3730XL DNA analyzer. The closest phylogenetic neighbors of this sequence were identified by using BLASTN search program at the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 19 July 2022)) and the EZBioCloud server [17]. The 16S rRNA gene sequence of the strain M6T was subjected to multiple alignments with the sequences of the closely related bacteria by using CLUSTAL Ω [18]. Gaps at the 5′ and 3′ ends were deleted using the software package BioEdit. Phylogenetic trees were reconstructed by using three different methods, the neighbor-joining method [19], the maximum-likelihood algorithm [20], and the minimum-evolution method [21] with the MEGA7 program [22]. During the phylogenetic analysis, evolutionary distances were calculated using Kimura’s two-parameter model [23], and bootstrap values were calculated based on 1000 replications [24].
2.5. Complete Genome Sequencing and Phylogenomic Analysis
Genomic DNA was extracted by a standard phenol-chloroform method and further purified by AMPure XP beads (Beckman Coulter, Brea, CA, USA) and then quantified and quality controlled using a Qubit 2.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), NanoDrop software and agarose gel electrophoreses [25]. High-molecular-weight DNA was isolated using a BluePinppin system (Sage Science, Beverly, MA, USA). Approximately 1.5 μg of genomic DNA was used for library construction using a one-dimensional (1D) ligation sequencing kit (SQK-LSK109 kit; Oxford Nanopore, Oxford, UK). No size selection or shearing was applied. The library was loaded into an R9.4 flow cell for the PromethION platform (PromethION flow cells, FLO-PRO002; Oxford Nanopore). Nanopore quality control was achieved with a threshold value (Q) of 7. Illumina sequencing was performed on the NovaSeq PE150 instrument at the Wuhan Benagen Co. Ltd. (Wuhan, China) Low-quality bases (quality value, ≤30, account for only 9.0%), and were removed. De novo assembly in combination with the Illumina short reads and ONT long reads was conducted using SPAdes 3.10.0, Unicycler 0.4.8., Racon, Miniasm and Pilon1.22.
Digital DNA-DNA hybridization (dDDH) values were determined by using the genome-to-genome distance calculator (GGDC 2.1) at http://ggdc.dsmz.de (accessed on 2 July 2022) [26]. The average nucleotide identity (ANI) was calculated using OrthoANI with default parameters on the website [27]. For phylogenetic analysis of core proteome, the extraction of the core proteome from the genomic sequence was automatically executed based on the M1CR0B1AL1Z3R pipeline (https://github.com/orenavram/microbializer (accessed on 2 July 2022)) with the default parameters as described by [28]. A phylogenomic tree based on a bacterial core gene set was reconstructed with the genome sequences of the isolate and its closely related species, using an automated multi-locus species tree (autoMLST) pipeline (https://automlst.ziemertlab.com/ (accessed on 7 July 2022)) [29]. Conserved proteins shared between strain pairs were estimated based on the percentage of conserved proteins (POCP), which were calculated as the algorithm [(C1 + C2)/(T1 + T2)] × 100% [30]. The average amino acid identity (AAI) was obtained from the website http://enve-omics.ce.gatech.edu/aai/ (accessed on 5 September 2022) [31].
3. Results and Discussion
3.1. Phylogenetic Placement and Phylogenomics
An almost complete 16S rRNA gene sequence of strain M6T (1407 nucleotides) was deposited in GeneBank under accession number ON876070. Sequence analysis by BLASTN and on the EzBioCloud database revealed the highest similarity of 96.3% to S. hominis SZY PN-1T, followed by S. sibiricus RB16-17T and S. neustonicus PAMC 28131T with a similarity of 95.5% and 95.1%, respectively, all below the proposed threshold 98.7% for differentiating two species [10] but above the minimum identity value that guarantees the circumscription of a single genus [32]. Other closely related genera were Sphingomonas (≤94.6%), Sandarakinorhabdus (≤93.8), and Polymorphobacter (≤93.8%). The phylogenetic analyses based on 16S rRNA gene sequences demonstrated that strain M6T formed a distinct lineage in a stable clade with the two Sandaracinobacter species and the S. hominis in the maximum-likelihood phylogenetic tree (Figure 1), and this relationship was also supported by the neighbor-joining and minimum-evolution trees (Figures S1 and S2).
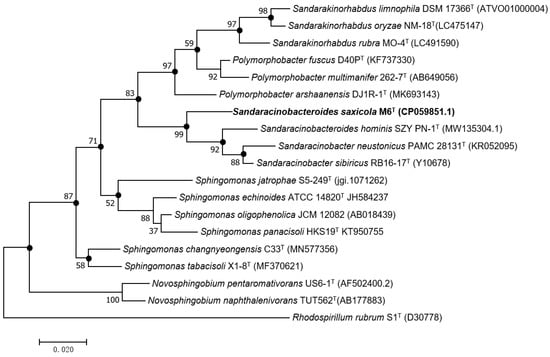
Figure 1.
Maximum-likelihood phylogenetic tree based on 16S rRNA gene sequence of strain M6T and other related species. Bootstrap values (expressed as a percentage of 1000 replications) above 50% are shown at the branch points. Filled circles at nodes, denotes branches recovered using the neighbor-joining and minimum-evolution methods. Rhodospirillum rubrum S1T was used as an out group. Bar 0.02 substitutions per nucleotide position (S. saxicola, see Section 3.6).
To further prove that strain M6T should be placed in the genus Sandaracinobacteroides other than establishing a new genus, we calculated the POCP (percentage of conserved proteins) and AAI values between M6T and its phylogenetic relatives. As summarized in Table 1, they were all above the established cut-off values for genus delineation of 50% and 60% respectively [30,31].

Table 1.
The 16S rRNA gene similarity, POCP, AAI, ANI, and dDDH values between M6T and its closely related strains available with genome sequence.
In order to establish a more specific taxonomic position at the species level, genome comparison was performed between M6T and its phylogenetic relatives first using ANI (Average Nucleotide Identity) and dDDH (digital DNA-DNA Hybridization) value calculation (Table 1). The levels of dDDH between M6T and its phylogenetic relatives were far below the threshold value of 70% for assigning strains to the same genomic species. The ANI values were also under the proposed cut-off ANI values of 95–96% for demarcating bacterial species [33]. Moreover, maximum-likelihood phylogenetic trees based on either the core proteome or the 80 core genes from the autoMLST analysis both revealed a separate lineage for strain M6T (Figure 2 and Figure S3). These consistent results indicated that strain M6T represented a new member of the genus Sandaracinobacteroides.
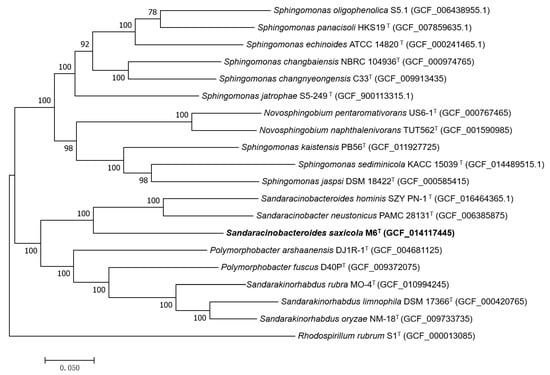
Figure 2.
Maximum-likelihood phylogenetic tree based on the core genome indicating the phylogenetic positions of strains M6T with the related species. The scale bar indicates 0.020 substitutions per nucleotide position. Rhodospirillum rubrum S1T (GCF_000013085) was used as an out group. GenBank accession numbers are listed for each sequence in parentheses.
In spite that Sandaracinobacteroides hominis SZY PN-1T and Sandaracinobacter neustonics PAMC 28131T were placed in two genera, the values based on genome and proteome comparison between these two strains (Table 1) were actually above the threshold limits for delineation of the bacterial genus.
Venn diagram generated from the genome comparative studies of S. saxicola M6T, S. hominis SZY PN-1T, and S. neustonicus PAMC 28131T using the OrthoVenn2 tool further demonstrated that the three species form a total of 2718 clusters, of which 1071 orthologous clusters (at least contains two species) and 1647 are single-copy gene clusters. The S. saxicola M6T contains 50 unique clusters, some of which are involved in cellular aromatic compound or heterocycle metabolic process (Figure 3). Overall genome sequence identity between these three strains is shown in Figure 4.
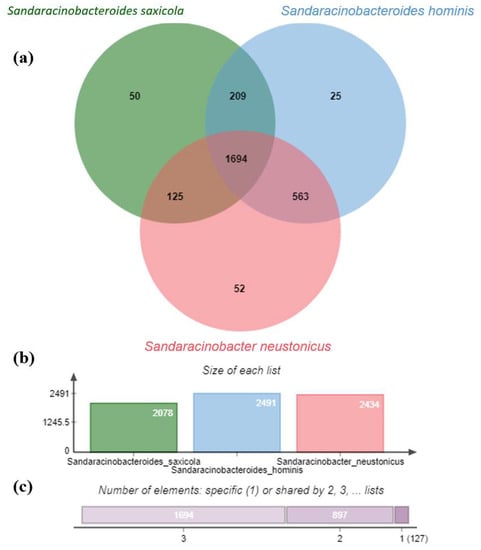
Figure 3.
Venn diagram and the bar plots generated by Orthovenn2 represent the distribution of shared and unique gene clusters amongst S. saxicola M6T, S. hominis SZY PN-1T, and S. neustonicus PAMC 28131T. (a) The Venn diagram represents the distribution of core ortholog clusters, shared clusters, and unique clusters in all three species. (b) The bar plot represents the cumulative ortholog clusters found in each species. (c) The bar plot illustrates the cumulative core, shared, and unique clusters in all the three species, where label 1 on the horizontal scale shows the cumulative number of unique clusters (127) for all the three species, while label 2 shows the total number of clusters shared by two species (897) and so on.
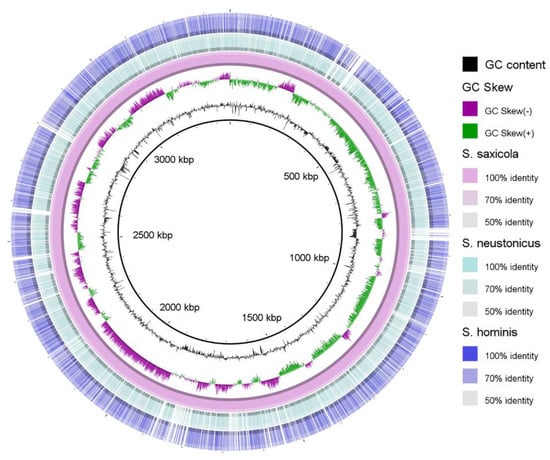
Figure 4.
Overall genome sequence identity distribution between S. saxicola M6T, S. hominis SZY PN-1T, and S. neustonicus PAMC 28131T. Location in the reference genome is indicated by numeration on the inside of the ring. GC content in the reference genome is indicated by the black bar graphs between the genomic coordinates and the colored ring. The graphical view of the alignments was rendered using the BLAST Ring Image Generator (BRIG) [34].
3.2. Morphology and Metabolic Profile
Cells of M6T were Gram-negative, aerobic, long rod-shaped, with a size of 0.5–0.6 × 1.1–1.3 μm (Figure 5). Colonies grew in a circular, convex, opaque manner with detectable yellow pigmentation on the modified RO and R2A agar plates. Growth was observed at temperatures of 15–37 °C and pH 6.0–9.0, with optimal growth at 30 °C and pH 6.0–8.5. Strain M6 T was sensitive to NaCl, growth was inhibited by NaCl (≥0.1% (w/v)). Unlike the Sphingomonas oligophenolica JCM 12082T, a halo-sensitive soil bacterium with a 16S rDNA sequence similarity of 94.6% to M6T, this inhibition could not be recovered by 6 mM CaCl2 [35].
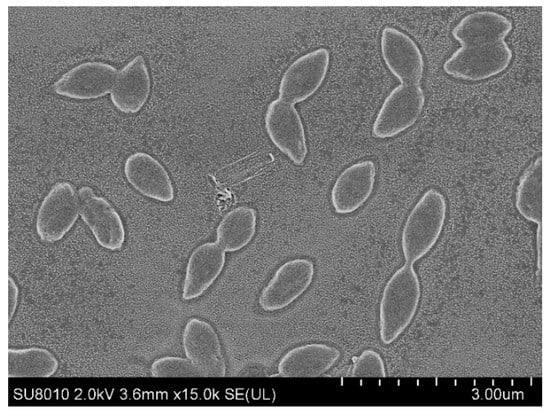
Figure 5.
Scanning electron microscopy image showing the cell morphology of strain M6T.
Though the two Sandaracinobacter species S. sibiricus RB16-17T and S. neustonicus PAMC 28131T were listed as invalid names, we still included them together with Sandaracinobacteroides hominis SZY PN-1T as reference strains for a comprehensive phenotypic, genomic and chemotaxonomic properties comparison. Moreover, our data based on phylogenomics analysis also implied that Sandaracinobacter neustonics PAMC 28131T and Sandaracinobacteroides hominis SZY PN-1T should be placed in a single genus. In contrast to S. sibiricus RB16-17T, strain M6T, S. hominis SZY PN-1T, and S. neustonicus PAMC 28131T all did not contain the bacteriochlorophyll a, a main feature of the genus Sandaracinobacter [11]. A major carotenoid peak of 424 nm was also not detected in these three strains (Figure S4).
According to the API tests and other biochemical assays, both strains M6T and S. hominis SZY PN-1T were positive for catalase and trypsin, and hydrolyzed starch and aesculin. Unlike S. hominis SZY PN-1T, strain M6T was negative for α-chymotrypsin, esterase (C4), and esterase lipase (C8), and consequently does not hydrolyze Tweens (40, 60, and 80), however, it was positive for valine arylamidase, cystine arylamidase, α-galactosidase, β-Galactosidase (Table 2). In short, the strain M6T maintained commonality within this genus in many respects, while also preserving many unique characteristics that could differentiate it from the related strains.

Table 2.
Phenotypic characteristics distinguishing strains M6T from other species of the genus Sandaracinobacteroides and Sandaracinobacter.
3.3. Chemotaxonomic Characteristics
Cellular fatty acids profiles of M6T, Sandaracinobacteroides hominis SZY PN-1T, and S. neustonicus PAMC 28131T are depicted in Table S2. The predominant fatty acids (relative account > 10%) of the novel isolate were C18:1ω7c, summed feature 3, C14:0 2-OH, and C16:0, whereas both Sandaracinobacteroides hominis SZY PN-1T and S. neustonicus PAMC 28131T contained summed feature 3, 8 and C17:1ω6c as the major fatty acids.
In consistent with S. hominis SZY PN-1T and S. neustonicus PAMC 28131T, phosphatidylglycerol (PG) and phosphatidylethanolamine (PE) have been determined as the predominant polar lipids of strain M6T. In comparing with S. hominis SZY PN-1T, which additionally included one diphosphatidylglycerol (DPG), two sphingoglycolipids (SGL), four unidentified glycolipids (GL), and seven unidentified lipids (L) as the major polar lipids, strain M6T contained four unidentified phospholipids (PL), four unidentified lipids (L) and an unidentified glycolipid (GL) (Figure S5).
3.4. The Biosynthesis of Zeaxanthin
The assembled genome sequence of strain M6T had a length of 3,364,212 bp and a GC content of 67.7% (Figure S6). Genome annotation by the NCBI Prokaryotic Genome Annotation Pipeline 4.12 (PGAP) predicted a total of 3375 genes with 3298 coding sequences and 49 RNA genes (three rRNAs, 43 tRNAs, and three noncoding RNAs).
AntiSMASH [36] analysis of the genome detected a gene cluster for zeaxanthin biosynthesis. This gene cluster contains three genes located adjacent to each other. They encoded the phytoene synthase (RS11760), phytoene desaturase (RS11765), lycopene beta-cyclase (RS11770) respectively. The gene for beta-carotene hydroxylase (RS12330), the enzyme responsible for the last step in zeaxanthin biosynthesis was found upstream of the gene cluster (Figure 6a,b). The presence of zeaxanthin was subsequently confirmed by HRMS (high-resolution mass spectrum), in which a protonated molecular ion at m/z 568.4260 [M+H]+ (calcd. for C40H56O2, 568.4280, 3.5 ppm) was observed (Figure 6c).
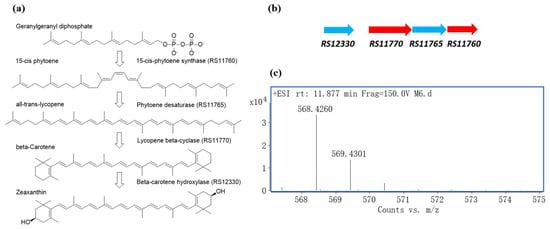
Figure 6.
Zeaxanthin production by M6T. (a) Zeaxanthin biosynthesis pathway. (b) Zeaxanthin biosynthesis gene cluster found in the genome of M6T. (c) HRMS chromatogram of zeaxanthin in the acetone-methanol extract of M6T.
3.5. Multiple Copies of Aromatic Ring-Hydroxylating Dioxygenases
According to the PGAP annotation, the genome of M6 also contained six genes encoding aromatic ring-hydroxylating dioxygenases (ARHD), the enzyme involved in the first and rate-limiting step of aerobic biodegradation of aromatic compounds [37]. Multiple sequence alignment demonstrated they all had a conserved Rieske [2Fe–2S] center and a C-terminal catalytic domain (Figure 7). Further analysis of Pfam indicated that these enzymes are homotrimers and are distantly related to the typical oxygenase [38].

Figure 7.
Amino acid sequence alignment of the six ARHDs showing the consensus pattern: C-x-H-R-[GAR]-x(7,8)-[GEKVI]-[NERAQ]-x(4,5)-C-x-[FY]-H, in which the 2 C’s and the 2 H’s are 2Fe-2S ligands [39]. Asterisks (*) indicate positions which have a single, fully conserved residue, a: (colon) indicates conservation between groups of strongly similar properties, a. (period) indicates conservation between groups of weakly similar properties.
Subsequently, ClassicRAST-based functional gene subsystem clustering analysis revealed 19 ORFs involved in the metabolism of aromatic compounds (Figure S7) [40]. Considering that the Sphingomonas oligophenolica JCM 12082T, a strain with a 16S rDNA sequence similarity of 94.6% to M6T, was reported to degrade phenolic acids at low concentrations [35], and it has only 10 ORFs classified into the class for the metabolism of aromatic compounds, we speculate that the strain M6T might be able to degrade some unusual aromatic compounds.
3.6. Description of Sandaracinobacteroides saxicola sp. nov.
sa.xi’co.la, L. neut. n. saxum rock, L. masc./fem. Suffix n. -cola inhabitant, N.L. masc./fem. n. (nominative in apposition) saxicola rock dweller.
Cells are Gram-negative, motile, and long rod-shaped with a width of 0.5–0.6 μm and a length of 1.1–1.3 μm. The colonies are round convex, opaque, and in yellow color. Growth is observed on R2A agar at 15–37 °C (optimum, 30 °C), and at pH 6.0–9.0 (optimum 6.0–8.5). Growth occurs in the absence of NaCl. Negative for VP reaction, H2S production, and oxidase activities. Positive for catalase activities. Xanthine, hypoxanthine and Tweens (40, 60 and 80) are not hydrolyzed, but casein and starch are hydrolyzed. According to the API ZYM test, strain M6T is positive for alkaline phosphatase, trypsin, acid phosphatase, naphthol-AS-BI-phosphohydrolase, α-galactosidase and α-glucosidase, β-glucosidase, leucine arylamidase, β-galactosidase, N-acetyl-β-glucosaminidase, valine arylamidase, cystine arylamidase, α-fucosidase, but negative for esterase (C4), esterase lipase (C8), lipase (C14), α-chymotrypsin, β-glucuronidase. In API 20NE test strips, this novel isolated was positive for aesculin hydrolysis and β-galactosidase, but negative for nitrate reduction to nitrite, nitrite reduction, urease, indole production, acidification of glucose, arginine dihydrolase, and gelatin hydrolysis. In the API 50CH test, acid was produced from aesculin, 5-ketogluconate, sucrose and maltose, DL-arabinose, starch, melezitose, but not from 2-ketogluconate, N-acetylglucosamine, D-adonitol, amygdalin, DL-arabitol, arbutin, cellobiose, dulcitol, erythritol, D-fructose, DL-fucose, D-galactose, gentiobiose, D-glucose, glycerol, glycogen, inositol, inulin, lactose, D-lyxose, D-mannitol, D-mannose, melibiose, methyl α-D-glucopyranoside, methyl α-D-mannopyranoside, methyl β-D-xylopyranoside, potassiumgluconate, raffinose, L-rhamnose, D-ribose, salicin, D-sorbitol, L-sorbose, D-tagatose, trehalose, turanose, xylitol or DL-xylose. Cell could assimilate sucrose, maltose, L-alanine, L-serine, glycogen, D-glucose, 3-hydroxybutyrate, L-proline, but not assimilate adipate, 3-hydroxybenzoate, acetate, N-acetylglucosamine, L-arabinose, citrate, L-fucose, caprate, L-histidine, inositol, itaconate, 2-ketogluconate, 5-ketogluconate, lactate, malonate, D-mannitol, D-melibiose, phenylacetate, propionate, L-rhamnose, D-ribose, salicin, D-sorbitol, suberate, gluconate, D-mannose, 4-hydroxybenzoate, malate or valerate (according to API 20NE and API ID 32GN test strips).
Sandaracinobacteroides saxicola sp. nov. was deposited in the China General Microbiological Culture Collection Center (CGMCC 1.19164) and the NITE Biological Resource Center (NBRC 115420) in Japan.
4. Conclusions
The 16S rRNA gene sequence similarity, POCP, and AAI values between strain M6T and type strains in genus Sandaracinobacteroides, were all below the prescribed threshold for differentiating two species but above the established cut-off values for genus delineation [26,33]. Phylogenetic analysis based on 16S rRNA gene sequence and core proteome showed that strain M6T was close to species in genera Sandaracinobacteroides and Sandaracinobacter but had obvious genetic distance. Furthermore, the discrepancies in the physiological, biochemical, and chemotaxonomic characteristics also could clearly differentiate M6T, and from the closely related species. In conclusion, we suggest that strain M6T represents a novel species of the genus Sandaracinobacteroides, for which the name Sandaracinobacteroides saxicola sp. nov. is proposed.
In addition, we testified that zeaxanthin was one of the carotenoid pigments produced by M6T. In view of its application in the food and pharmaceutical industry, strain M6T could be an attractive candidate for the production of zeaxanthin. Moreover, according to the ARHD sequence analysis, strain M6T could also be used in controlling organic pollution when applied alone or in combination with other strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14100807/s1, Table S1: The 16S rRNA gene sequence analysis of the isolated strains. Table S2: Cellular fatty acid composition (%) of M6T, S. hominis SZY PN-1T, and S. neustonicus PAMC 28131T. Figure S1: Neighbor-Joining tree based on the 16S rRNA gene sequences of strain M6T and representatives of related taxa. Figure S2: Minimum-evolution tree based on the 16S rRNA gene sequences of strain M6T and representatives of related taxa. Figure S3: Maximum-likelihood phylogenetic tree based on the core proteome indicating the phylogenetic positions of strains M6T with the related species. Figure S4: Total pigment absorption spectra of strain M6T. Figure S5: Two-dimensional TLC plate image of total polar lipids of strain M6T sprayed with phosphomolybdic acid. Figure S6: Circular map of the chromosome of strain M6T. Figure S7: Subsystem category distribution from strain M6T, generated through ClassicRAST pipeline (default settings).
Author Contributions
Conceptualization, Y.T. and Q.L. (Qingshu Liu); formal analysis, Y.T., Q.L. (Qingshan Long) and C.Z.; investigation, Y.T. and C.Z.; writing—original draft preparation, Y.T. and C.Z.; writing—review and editing, Q.L. (Qingshu Liu), Z.G. and Y.T.; supervision, P.L., Z.G. and Q.L. (Qingshu Liu); funding acquisition, Y.T., Z.G. and Q.L. (Qingshan Long). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by State Key Laboratory of Microbial Technology Open Projects Fund (M2021-10), Hunan Provincial Natural Science Foundation (2021JJ40306), National Natural Science Foundation (32000047), The Science and Technology Project of Hunan Province (2021NK1040), and Hunan Provincial Science & Technology Department Project (2020NK2006).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The DDBJ/EMBL/GenBank accession number for the 16S rRNA gene sequence and the complete genome sequence of Sandaracinobacteroides sp. M6 are ON876070 and CP059851.1 respectively. The SRA (Sequence Read Archive) raw data is available under accession number SRR12366083. The Biosample and BioProject accession numbers are SAMN15676387 and PRJNA649658 respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qu, P.H.; Luo, H.M. Sandaracinobacteroides hominis gen. nov., sp. nov., isolated from human skin. Arch. Microbiol. 2021, 203, 5067–5074. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.; Stackebrandt, E. Reorganization of the genus Erythromicrobium: Description of “Erythromicrobium sibiricum” as Sandaracinobacter sibiricus gen. nov., sp. nov., and of “Erythromicrobium ursincola” as Erythromonas ursincola gen. nov., sp. nov. Int. J. Syst. Bacteriol. 1997, 47, 1172–1178. [Google Scholar] [CrossRef]
- Lee, I.; Jang, I.G. Sandaracinobacter neustonicus sp. nov., isolated from the sea surface microlayer in the southwestern pacific ocean, and emended description of the genus Sandaracinobacter. Int. J. Syst. Evol. Microbiol. 2020, 70, 4698–4703. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Sardà Carbasse, J. List of prokaryotic names with standing in nomenclature (LPSN) moves to the dsmz. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Overmann, J.; Abt, B. Presence and future of culturing bacteria. Annu. Rev. Microbiol. 2017, 71, 711–730. [Google Scholar] [CrossRef]
- Gueidan, C.; Villaseñor, C.R. A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud. Mycol. 2008, 61, 111–119. [Google Scholar] [CrossRef]
- Pascual, J.; Wüst, P.K. Novel isolates double the number of chemotrophic species and allow the first description of higher taxa in Acidobacteria subdivision 4. Syst. Appl. Microbiol. 2015, 38, 534–544. [Google Scholar] [CrossRef]
- Maltman, C.; Yurkov, V. The effect of tellurite on highly resistant freshwater aerobic anoxygenic phototrophs and their strategies for reduction. Microorganisms 2015, 3, 826–838. [Google Scholar] [CrossRef]
- Yurkov, V.V.; Krieger, S. Citromicrobium bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S. Towards a taxonomic coherence between average nucleotide identity and 16s rrna gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Yurkov, V.; Gorlenko, V. Erythrobacter sibiricus sp. nov., a new fresh-water aerobic bacterial species containing bacteriochlorophyll a. Microbiology 1990, 59, 85–89. [Google Scholar]
- Smibert, R.M.; Krieg, N.R. Phenotypic characterization. In Methods for General Molecular Bacteriology; Gerhadt, P., Murray, R.G.E., Eds.; American Society for Microbiology: Washington, DC, USA, 1994; pp. 607–654. [Google Scholar]
- Kovacs, N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature 1956, 178, 703. [Google Scholar] [CrossRef] [PubMed]
- Minnikin, D.E.; O’Donnell, A.G. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Shin, Y.K.; Lee, J.S. Isoprenoid quinone profiles of the Leclercia adecarboxylata KCTC 1036T. J. Microbiol. Biotechnol. 1996, 6, 68–69. [Google Scholar]
- Frank, J.A.; Reich, C.I. Critical evaluation of two primers commonly used for amplification of bacterial 16s rRNA genes. Appl. Env. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M. Introducing ezbiocloud: A taxonomically united database of 16s rrna gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal omega, accurate alignment of very large numbers of sequences. Methods Mol. Biol. 2014, 1079, 105–116. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Rzhetsky, A.; Nei, M. A simple method for estimating and testing minimum-evolution trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar]
- Kumar, S.; Stecher, G. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.J. A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. J. Mol. Evol. 1979, 16, 111–120. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limit on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; David, R. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Meier-Kolthoff, J.P.; Auch, A.F. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Avram, O.; Rapoport, D. M1CR0B1aAL1Z3R-a user-friendly web server for the analysis of large-scale microbial genomics data. Nucleic Acids Res. 2019, 47, W88–W92. [Google Scholar] [CrossRef]
- Alanjary, M.; Steinke, K. Automlst: An automated web server for generating multi-locus species trees highlighting natural product potential. Nucleic Acids Res. 2019, 47, W276–W282. [Google Scholar] [CrossRef]
- Qin, Q.L.; Xie, B.B. A proposed genus boundary for the prokaryotes based on genomic insights. J. Bacteriol. 2014, 196, 2210–2215. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Konstantinidis, K.T. Bypassing cultivation to identify bacterial species. Microbe 2014, 9, 111–118. [Google Scholar] [CrossRef]
- Yarza, P.; Richter, M. The all-species living tree project: A 16s rRNA-based phylogenetic tree of all sequenced type strains. Syst. Appl. Microbiol. 2008, 31, 241–250. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.F.; Petty, N.K. Blast ring image generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Hattori, R. Sphingomonas oligophenolica sp. nov., a halo- and organo-sensitive oligotrophic bacterium from paddy soil that degrades phenolic acids at low concentrations. Int. J. Syst. Evol. Microbiol. 2004, 54, 2185–2190. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S. Antismash 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, W. Darhd: A sequence database for aromatic ring-hydroxylating dioxygenase analysis and primer evaluation. J. Hazard Mater. 2022, 436, 129230. [Google Scholar] [CrossRef]
- Nojiri, H.; Ashikawa, Y. Structure of the terminal oxygenase component of angular dioxygenase, carbazole 1,9a-dioxygenase. J. Mol. Biol. 2005, 351, 355–370. [Google Scholar]
- Botelho, H.M.; Leal, S.S. Role of a novel disulfide bridge within the all-beta fold of soluble rieske proteins. J. Biol. Inorg. Chem. 2010, 15, 271–281. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J. Rasttk: A modular and extensible implementation of the rast algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).