Understory Vegetation Dynamics in Non-Native Douglas Fir Forests after Management Abandonment—A Case Study in Two Strict Forest Reserves in Southwest Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
| Strict Forest Reserve (SFR) | Grünberg (GB-DF) | Eselskopf (EK-DF) | Adelsberg (AB-NAT) |
|---|---|---|---|
| Geographic location | 49°20’8’’ N 7°58’12’’ E | 50°2’51’’ N 6°37’11’’ E | 49°3’32’’ N 7°30’24’’ E |
| Forest ecoregion (and subregion) | Pfälzerwald (Middle Pfälzerwald) | Nordwesteifel (Islek and Oesling) | Pfälzerwald (Southern Pfälzerwald, Wasgau) |
| Reserve total size | 64 ha | 30 ha | 192 ha |
| Year of establishment | 2001 | 2002 | 1976 |
| Geology | Bunter (Trifels subdivision) | Lower Devonian argillite | Bunter (Rehberg subdivision) |
| Soil type | Dystric Cambisol | Spodic Cambisol | Dystric Cambisol |
| Nutrient status | Oligotrophic | Mesotrophic | Oligotrophic |
| Elevation | 210–420 m a. s. l. | 310–440 m a. s. l. | 245–399 m a. s. l. |
| Potential natural vegetation | Acidic beech forests (Luzulo-Fagetum) | Acidic to mesotrophic beech forests (Luzulo-Fagetum to Galio-Fagetum [52]) | Acidic beech forests (Luzulo-Fagetum) |
| Mean annual air temperature (subregion) | 8.4 °C | 7.6 °C | 8.8 °C |
| Mean annual precipitation (subregion) | 933 mm | 928 mm | 926 mm |
| Tree species composition of core area (>7 cm diameter at breast height (DBH)) | ~73% Pseudotsuga menziesii, 17% Fagus sylvatica, 7% Pinus sylvestris, 3% Picea abies (Year 2004) | ~95% Pseudotsuga menziesii, 4% Picea abies, others (Year 2006) | 54% Quercus petraea, 30% Tilia cordata, 14% Fagus sylvatica, 2% Carpinus betulus (Year 2006) |
| Years of vegetation surveys (core area) | 2005 and 2017 | 2005 and 2017 | 2000 and 2016 |
| Number of permanent subplots (400 m2) surveyed in the core area | 13 | 14 | 29 |
2.2. Data Sampling and Analysis
3. Results
3.1. Changes in Vegetation Structure, Species Richness and Environmental Conditions
3.2. Changes in Field Layer Species Composition
3.3. Tree Species Dynamics in the Three SFRs
4. Discussion
4.1. Vascular Plant Species Richness Declines in All Strict Forest Reserves
4.2. Contrasting Patterns in Community Composition and Homogenization among the Three Reserves
4.3. Douglas Fir Regeneration Is Decreasing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pötzelsberger, E.; Spiecker, H.; Neophytou, C.; Mohren, F.; Gazda, A.; Hasenauer, H. Growing non-native trees in European forests brings benefits and opportunities but also has its risks and limits. Curr. For. Rep. 2020, 6, 339–353. [Google Scholar] [CrossRef]
- Senf, C.; Seidl, R. Mapping the forest disturbance regimes of Europe. Nat. Sustain. 2021, 4, 63–70. [Google Scholar] [CrossRef]
- Jandl, R.; Spathelf, P.; Bolte, A.; Prescott, C.E. Forest adaptation to climate change—Is non-management an option? Ann. For. Sci. 2019, 76, 48. [Google Scholar] [CrossRef]
- Kamp, J.; Trappe, J.; Dübbers, L.; Funke, S. Impacts of windstorm-induced forest loss and variable reforestation on bird communities. For. Ecol. Manag. 2020, 478, 118504. [Google Scholar] [CrossRef]
- Hazarika, R.; Bolte, A.; Bednarova, D.; Gaviria, J.; Kanzian, M.; Kowalczyk, J.; Lackner, M.; Lstibůrek, M.; Longauer, R.; Nagy, L.; et al. Multi-actor perspectives on afforestation and reforestation strategies in Central Europe under climate change. Ann. For. Sci. 2021, 78, 60. [Google Scholar] [CrossRef]
- Brus, R.; Pötzelsberger, E.; Lapin, K.; Brundu, G.; Orazio, C.; Straigyte, L.; Hasenauer, H. Extent, distribution and origin of non-native forest tree species in Europe. Scand. J. For. Res. 2019, 34, 533–544. [Google Scholar] [CrossRef]
- Spellmann, H. Ertragskundliche Aspekte des Fremdländeranbaus. Allg. Forst Jagdztg. 1994, 165, 27–34. [Google Scholar]
- Hermann, R.K.; Lavender, D.P. Douglas-fir planted forests. New For. 1999, 17, 53–70. [Google Scholar] [CrossRef]
- Lévesque, M.; Rigling, A.; Bugmann, H.; Weber, P.; Brang, P. Growth response of five co-occurring conifers to drought across a wide climatic gradient in Central Europe. Agric. For. Meteorol. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- Vor, T.; Spellmann, H.; Bolte, A.; Ammer, C. Potentiale und Risiken eingeführter Baumarten—Baumartenportraits mit naturschutzfachlicher Bewertung. Göttinger Forstwiss. 2015, 7, 187–217. [Google Scholar]
- Vitali, V.; Büntgen, U.; Bauhus, J. Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob. Chang. Biol. 2017, 23, 5108–5119. [Google Scholar] [CrossRef] [PubMed]
- Schueler, S.; George, J.-P.; Karanitsch-Ackerl, S.; Mayer, K.; Klumpp, R.T.; Grabner, M. Evolvability of drought response in four native and non-native conifers: Opportunities for forest and genetic resource management in Europe. Front. Plant Sci. 2021, 12, 648312. [Google Scholar] [CrossRef] [PubMed]
- Höltermann, A.; Nehring, S.; Herberg, A.; Krug, A. Die Douglasie aus Sicht des Bundesamtes für Naturschutz. AFZ-Der Wald 2016, 71, 34–37. [Google Scholar]
- Krüger, U. Gefährdet die Douglasie die Biodiversität wirklich nicht?—Fragen zu den Folgen forstlichen Handelns. Nat. Landsch. 2017, 49, 110–111. [Google Scholar]
- Vor, T.; Nehring, S.; Bolte, A.; Höltermann, A. Assessment of invasive tree species in nature conservation and forestry—Contradictions and coherence. In Introduced Tree Species in European Forests: Opportunities and Challenges; Krumm, F., Vitkova, L., Eds.; European Forest Institute: Freiburg, Germany, 2016; pp. 148–157. [Google Scholar]
- Broncano, M.J.; Vilà, M.; Boada, M. Evidence of Pseudotsuga menziesii naturalization in montane Mediterranean forests. For. Ecol. Manag. 2005, 211, 257–263. [Google Scholar] [CrossRef]
- Fagúndez, J. Heathlands confronting global change: Drivers of biodiversity loss from past to future scenarios. Ann. Bot. 2013, 111, 151–172. [Google Scholar] [CrossRef]
- Bindewald, A.; Miocic, S.; Wedler, A.; Bauhus, J. Forest inventory-based assessments of the invasion risk of Pseudotsuga menziesii (Mirb.) Franco and Quercus rubra L. in Germany. Eur. J. For. Res. 2021, 140, 883–899. [Google Scholar] [CrossRef]
- Knoerzer, D. Zur Naturverjüngung der Douglasie im Schwarzwald. Inventur und Analyse von Umwelt- und Konkurrenzfaktoren Sowie eine Naturschutzfachliche Bewertung; Dissertationes Botanicae 306; Gebrüder Borntraeger Verlagsbuchhandlung: Stuttgart, Germany, 1999; pp. 1–283. [Google Scholar]
- Lange, F.; Ammer, C.; Leitinger, G.; Seliger, A.; Zerbe, S. Is Douglas fir (Pseudotsuga menziesii [Mirbel] Franco) invasive in Central Europe? A case study from south-west Germany. Front. For. Glob. Chang. 2022, 5, 844580. [Google Scholar] [CrossRef]
- Frei, E.R.; Moser, B.; Wohlgemuth, T. Competitive ability of natural Douglas fir regeneration in central European close-to-nature forests. For. Ecol. Manag. 2022, 503, 119767. [Google Scholar] [CrossRef]
- Ammer, C.; Bolte, A.; Herberg, A.; Höltermann, A.; Krüß, A.; Krug, A.; Nehring, S.; Schmidt, O.; Spellmann, H.; Vor, T. Empfehlungen für den Anbau eingeführter Waldbaumarten—Gemeinsames Papier von Forstwissenschaft und Naturschutz. Nat. Landsch. 2016, 48, 168–172. [Google Scholar]
- Brundu, G.; Richardson, D.M. Planted forests and invasive alien trees in Europe—A code for managing existing and future plantings to mitigate the risk of negative impacts from invasions. NeoBiota 2016, 30, 5–47. [Google Scholar] [CrossRef]
- Brundu, G.; Pauchard, A.; Pyšek, P.; Pergl, J.; Bindewald, A.M.; Brunori, A.; Canavan, S.; Campagnaro, T.; Celesti-Grapow, L.; de Sá Dechoum, M.; et al. Global guidelines for the sustainable use of non-native trees to prevent tree invasions and mitigate their negative impacts. NeoBiota 2020, 61, 65–116. [Google Scholar] [CrossRef]
- Thomas, F.M.; Rzepecki, A.; Werner, W. Non-native Douglas fir (Pseudotsuga menziesii) in Central Europe: Ecology, performance and nature conservation. For. Ecol. Manag. 2022, 506, 119956. [Google Scholar] [CrossRef]
- Frank, D.; Finckh, M. Impact of Douglas-fir plantations on vegetation and soil in south-central Chile. Rev. Chil. Hist. Nat. 1997, 70, 191–211. (In Spanish) [Google Scholar]
- Richardson, D.M.; Rejmánek, M. Conifers as invasive aliens: A global survey and predictive framework. Divers. Distrib. 2004, 10, 321–331. [Google Scholar] [CrossRef]
- Orellana, I.A.; Raffaele, E. The spread of the exotic conifer Pseudotsuga menziesii in Austrocedrus chilensis forests and shrublands in northwestern Patagonia, Argentina. N. Z. J. For. Sci. 2010, 40, 199–209. [Google Scholar]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of Douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. For. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef]
- Tschopp, T.; Holderegger, R.; Bollmann, K. Auswirkungen der Douglasie auf die Waldbiodiversität. Schweiz. Z. Forstwes. 2015, 166, 9–15. [Google Scholar] [CrossRef]
- Wohlgemuth, T.; Moser, B.; Pötzelsberger, E.; Rigling, A.; Gossner, M.M. Über die Invasivität der Douglasie und ihre Auswirkungen auf Boden und Biodiversität. Schweiz. Z. Forstwes. 2021, 172, 118–127. [Google Scholar] [CrossRef]
- Gossner, M. Insektenwelten—Die Douglasie im Vergleich mit der Fichte. LWF Wissen 2008, 59, 70–73. [Google Scholar]
- Ziesche, T.M.; Roth, M. Influence of environmental parameters on small-scale distribution of soil-dwelling spiders in forests: What makes the difference, tree species or microhabitat? For. Ecol. Manag. 2008, 255, 738–752. [Google Scholar] [CrossRef]
- Budde, S.; Schmidt, W.; Weckesser, M. Impact of the admixture of European beech (Fagus sylvatica L.) on plant species diversity and naturalness of conifer stands in Lower Saxony. Waldökol. Landsch. Nat. 2011, 11, 49–61. [Google Scholar]
- Buée, M.; Maurice, J.-P.; Zeller, B.; Andrianarisoa, S.; Ranger, J.; Courtecuisse, R.; Marcais, B.; Le Tacon, F. Influence of tree species on richness and diversity of epigeous fungal communities in a French temperate forest stand. Fungal Ecol. 2011, 4, 22–31. [Google Scholar] [CrossRef]
- Schuldt, A.; Scherer-Lorenzen, M. Non-native tree species (Pseudotsuga menziesii) strongly decreases predator biomass and abundance in mixed-species plantations of tree diversity experiment. For. Ecol. Manag. 2014, 327, 10–17. [Google Scholar] [CrossRef]
- Gossner, M.; Utschick, H. Douglas fir stands deprive overwintering bird species of food resource. NeoBiota 2004, 3, 105–121. [Google Scholar]
- Gossner, M.M.; Wende, B.; Levick, S.; Schall, P.; Floren, A.; Linsenmair, K.E.; Steffan-Dewenter, I.; Schulze, E.-D.; Weisser, W.W. Deadwood enrichment in European forests—Which tree species should be used to promote saproxylic beetle diversity? Biol. Conserv. 2016, 201, 92–102. [Google Scholar] [CrossRef]
- Kriegel, P.; Matevski, D.; Schuldt, A. Monoculture and mixture-planting of non-native Douglas fir alters species composition, but promotes the diversity of ground beetles in a temperate forest system. Biodivers. Conserv. 2021, 30, 1479–1499. [Google Scholar] [CrossRef]
- Podrázský, V.; Martiník, A.; Matejka, K.; Viewegh, J. Effects of Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) on understorey layer species diversity in managed forests. J. For. Sci. 2014, 60, 263–271. [Google Scholar] [CrossRef]
- Heinrichs, S.; Ammer, C.; Mund, M.; Boch, S.; Budde, S.; Fischer, M.; Müller, J.; Schöning, I.; Schulze, E.-D.; Schmidt, W.; et al. Landscape-scale mixtures of tree species are more effective than stand-scale mixtures for biodiversity of vascular plants, bryophytes and lichens. Forests 2019, 10, 73. [Google Scholar] [CrossRef]
- Kostić, O.; Jarić, S.; Gajić, G.; Pavlović, D.; Marković, M.; Mitrović, M.; Pavlović, P. The effects of Douglas fir monoculture on stand characteristics in a zone of montane beech forest. Arch. Biol. Sci. 2016, 68, 753–766. [Google Scholar] [CrossRef]
- Eberhard, B.; Hasenauer, H. Modeling regeneration of Douglas fir forests in Central Europe. Austrian J. For. Sci. 2018, 135, 33–51. [Google Scholar]
- Wolf, G.; Bohn, U. Naturwaldreservate in der Bundesrepublik Deutschland und Vorschläge zu einer bundesweiten Grunddatenerfassung. Schriftenr. Veg. 1991, 21, 9–19. [Google Scholar]
- Parviainen, J.; Bücking, W.; Vandekerkhove, K.; Schuck, A.; Päivinen, R. Strict forest reserves in Europe: Efforts to enhance biodiversity and research on forests left for free development in Europe (EU-COST-Action E4). Forestry 2000, 73, 107–118. [Google Scholar] [CrossRef]
- Endres, U.; Förster, B. Die Douglasie in Naturwaldreservaten—Passt das zusammen? Vorkommen der Douglasie in bayerischen Naturwaldreservaten. LWF Aktuell 2013, 93, 37–139. [Google Scholar]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef]
- Neff, F.; Brändle, M.; Ambarli, D.; Ammer, C.; Bauhus, J.; Boch, S.; Hölzel, N.; Klaus, V.H.; Kleinebecker, T.; Prati, D.; et al. Changes in plant-herbivore network structure and robustness along land-use intensity gradients in grasslands and forests. Sci. Adv. 2021, 7, eabf3985. [Google Scholar] [CrossRef]
- BMEL (Bundesministerium für Ernährung und Landwirtschaft) Bundeswaldinventur. 2022. Available online: https://www.bundeswaldinventur.de/ (accessed on 4 March 2022).
- Gauer, J.; Aldinger, E. Waldökologische Naturräume Deutschlands—Forstliche Wuchsgebiete und Wuchsbezirke. Mitt. Ver. Forstl. Standortskde. Forstpflanzenz. 2005, 43, 1–324. [Google Scholar]
- Vor, T.; Schmidt, W. Auswirkungen des Douglasienanbaus auf die Vegetation der Naturwaldreservate “Eselskopf” (Nordwesteifel) und “Grünberg” (Pfälzer Wald). Forstarchiv 2006, 77, 169–178. [Google Scholar]
- BLE (Bundesanstalt für Landwirtschaft und Ernährung). Naturwaldreservate—Urwälder von Morgen (Datenbank zu Naturwaldreservaten in Deutschland). 2022. Available online: https://fgrdeu.genres.de/naturwaldreservate/ (accessed on 4 March 2022).
- Balcar, P. Waldstrukturen im grenzüberschreitenden Naturwaldreservat Adelsberg-Lutzelhardt. Ann. Sci. Rés. Bios. Trans. Vosges Nord-Pfälzerwald 2008, 14, 27–45. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W. Zeigerwerte von Pflanzen in Mitteleuropa, 3rd ed.; Scripta Geobot. 18; Verlag Erich Goltze GmbH & Co KG: Göttingen, Germany, 2001; pp. 1–262. [Google Scholar]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Bartelheimer, M.; Poschlod, P. Functional characterizations of Ellenberg indicator values—A review on ecophysiological determinants. Funct. Ecol. 2016, 30, 506–516. [Google Scholar] [CrossRef]
- Van der Veken, S.; Bossuyt, B.; Hermy, M. Climate gradients explain changes in plant community composition of the forest understorey. Belg. J. Bot. 2004, 137, 55–69. [Google Scholar] [CrossRef]
- Simmel, J.; Ahrens, M.; Poschlod, P. Ellenberg N values of bryophytes in Central Europe. J. Veg. Sci. 2021, 32, e12957. [Google Scholar] [CrossRef]
- Schmidt, M.; Kriebitzsch, W.-U.; Ewald, J. Waldartenliste der Farn- und Blütenpflanzen, Moose und Flechten Deutschlands. BfN-Skr. 2011, 299, 1–111. [Google Scholar]
- Oberdorfer, E. Pflanzensoziologische Exkursionsflora, 8th ed.; Ulmer: Stuttgart, Germany, 2001. [Google Scholar]
- Nebel, M.; Philippi, G. (Eds.) Die Moose Baden-Württembergs, Band 1–3; Ulmer: Stuttgart, Germany, 2000; Volume 2001, p. 2005. [Google Scholar]
- Schmidt, M.; Bedarff, U.; Meyer, P. Einfluss von Störungen auf die Vegetation von Buchenwäldern. AFZ-Der Wald 2018, 73, 20–22. [Google Scholar]
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5.4. 2021. Available online: https://CRAN.R-project.org/package=betapart (accessed on 17 September 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, R.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 17 September 2022).
- Fischer, C.; Parth, A.; Schmidt, W. Vegetationsdynamik in Buchen-Naturwäldern: Ein Vergleich aus Süd-Niedersachsen. Hercynia-Okol. Umw. Mitteleur. 2009, 42, 45–68. [Google Scholar]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Does biodiversity differ between managed and unmanaged forests? A meta-analysis on species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Heinrichs, S.; Schulte, U.; Schmidt, W. Veränderung der Buchenwaldvegetation durch Klimawandel? Ergebnisse aus Naturwaldzellen in Nordrhein-Westfalen. Forstarchiv 2011, 82, 48–61. [Google Scholar]
- Leuschner, C. Mechanismen der Konkurrenzüberlegenheit der Buche. Ber. D. Reinh.-Tüxen-Ges. 1998, 10, 5–18. [Google Scholar]
- Mölder, A.; Streit, M.; Schmidt, W. When beech strikes back: How strict nature conservation reduces herb-layer diversity and productivity in Central European deciduous forests. For. Ecol. Manag. 2014, 319, 51–61. [Google Scholar] [CrossRef]
- Forrester, D.; Ammer, C.; Annighöfer, P.; Barbeito, I.; Bielak, K.; Bravo-Oviedo, A.; Coll, L.; del Río, M.; Drössler, L.; Heym, M.; et al. Effects of crown architecture and stand structure on light absorption in mixed and monospecific Fagus sylvatica and Pinus sylvestris forests along a productivity and climate gradient through Europe. J. Ecol. 2018, 106, 746–760. [Google Scholar] [CrossRef]
- Thurm, E.A.; Pretzsch, H. Improved productivity and modified tree morphology of mixed versus pure stands of European beech (Fagus sylvatica) and Douglas-fir (Pseudotsuga menziesii) with increasing precipitation and age. Ann. For. Sci. 2016, 73, 1047–1061. [Google Scholar] [CrossRef]
- Jucker, T.; Bouriaud, O.; Coomes, D.A. Crown plasticity enables trees to optimize canopy packing in mixed-species forests. Funct. Ecol. 2015, 29, 1078–1086. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Cavard, X.; Langanière, J.; Reich, P.B.; Bergeron, Y.; Paré, D.; Yuan, Z. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 101, 210–219. [Google Scholar] [CrossRef]
- Lwila, A.; Mund, M.; Ammer, C.; Glatthorn, J. Site conditions more than species identity drive fine root biomass, morphology and spatial distribution in temperate pure and mixed forests. For. Ecol. Manag. 2021, 499, 119581. [Google Scholar] [CrossRef]
- Hendriks, C.M.A.; Bianchi, F.J.J.A. Root density and root biomass in pure and mixed forest stands of Douglas-fir and beech. Neth. J. Agric. 1995, 11, 321–331. [Google Scholar] [CrossRef]
- Mölder, A.; Bernhardt-Römermann, M.; Schmidt, W. Herb-layer diversity in deciduous forests: Raised by tree richness or beaten by beech? For. Ecol. Manag. 2008, 256, 272–281. [Google Scholar] [CrossRef]
- Augusto, L.; Dupouey, J.-L.; Ranger, J. Effects of tree species on understory vegetation and environmental conditions in temperate forests. Ann. For. Sci. 2003, 60, 823–831. [Google Scholar] [CrossRef]
- Bailey, J.D.; Mayrsohn, C.; Doescher, P.S.; Pierre, E.S.; Tappeiner, J.C. Understory vegetation in old and young Douglas-fir forests of western Oregon. For. Ecol. Manag. 1998, 112, 289–302. [Google Scholar] [CrossRef]
- He, F.; Duncan, R.P. Density-dependent effects on tree survival in an old-growth Douglas fir forest. J. Ecol. 2000, 88, 676–688. [Google Scholar] [CrossRef]
- Oliver, C.D. Forest development in North America following major disturbances. For. Ecol. Manag. 1980, 3, 153–168. [Google Scholar] [CrossRef]
- Friedel, A.; Oheimb, G.v.; Dengler, J.; Härdtle, W. Species diversity and species composition of epiphytic bryophytes and lichens—A comparison of managed and unmanaged beech forests in NE Germany. Feddes Repert. 2006, 117, 172–185. [Google Scholar] [CrossRef]
- Raabe, S.; Müller, J.; Manthey, M.; Dürhammer, O.; Teuber, U.; Göttlein, A.; Förster, B.; Brandl, R.; Bässler, C. Drivers of bryophyte diversity allow implications for forest management with a focus on climate change. For. Ecol. Manag. 2010, 260, 1956–1964. [Google Scholar] [CrossRef]
- Márialigeti, S.; Németh, B.; Tinya, F.; Ódor, P. The effects of stand structure on ground-floor bryophyte assemblages in temperate mixed forests. Biodivers. Conserv. 2009, 18, 2223–2241. [Google Scholar] [CrossRef]
- Heinrichs, S.; Dölle, M.; Balcar, P.; Schmidt, W. NWR Adelsberg-Lutzelhardt: Keine Chance für die Eiche. AFZ-Der Wald 2018, 73, 29–32. [Google Scholar]
- Müller, J.; Boch, S.; Prati, D.; Socher, S.A.; Pommer, U.; Hessenmöller, D.; Schall, P.; Schulze, E.-D.; Fischer, M. Effects of forest management on bryophyte species richness in Central European forests. For. Ecol. Manag. 2019, 432, 850–859. [Google Scholar] [CrossRef]
- Leitl, R. Artenvielfalt und Bestandesform am Beispiel der Bodenvegetation. Ber. Bayer Landesanst. Wald Forstwirtsch. 2001, 33, 9–13. [Google Scholar]
- Verstraeten, G.; Baeten, L.; De Frenne, P.; Vanhellemont, M.; Thomaes, A.; Boonen, W.; Muys, B.; Verheyen, K. Understorey vegetation shifts following the conversion of temperate deciduous forest to spruce plantation. For. Ecol. Manag. 2013, 289, 363–370. [Google Scholar] [CrossRef]
- Härdtle, W.; Oheimb, G.v.; WestphaL, C. The effects of light and soil conditions on the species richness of the ground vegetation of deciduous forests in northern Germany (Schleswig-Holstein). For. Ecol. Manag. 2003, 182, 327–338. [Google Scholar] [CrossRef]
- Keith, S.A.; Newton, A.C.; Morecroft, M.D.; Bealey, C.E.; Bullock, J.M. Taxonomic homogenization of woodland plant communities over 70 years. Proc. Biol. Sci. 2009, 276, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Montigny, L.E.D.; Smith, N.J. The effect of gap size in a group selection silvicultural system on the growth response of young, planted Douglas-fir. A sector plot analysis. Forestry 2017, 90, 426–435. [Google Scholar] [CrossRef]
- Mailly, D.; Kimmins, J.P. Growth of Pseudotsuga menziesii and Tsuga heterophylla seedlings along a light gradient: Resource allocation and morphological acclimation. Can. J. Bot. 1997, 75, 1424–1435. [Google Scholar] [CrossRef]
- Petriţan, I.C.; Lüpke, B.V.; Petriţan, A.M. Effects of root trenching of overstorey Norway spruce (Picea abies) on growth and biomass of underplanted beech (Fagus sylvatica) and Douglas fir (Pseudotsuga menziesii) sapling. Eur. J. For. Res. 2010, 130, 813–828. [Google Scholar] [CrossRef]
- Spies, T.A.; Franklin, J.F. The structure of natural young, mature, and old-growth Douglas-fir forests. In Wildlife and Vegetation of Unmanaged Douglas-fir Forests; Ruggiero, L.F., Aubry, K.B., Carey, A.B., Huff, M.H., Eds.; USDA For. Serv. Gen. Tech. Rep. PNW-GTR-285; US Department of Agriculture, Forest Service: Portland, OR, USA, 1991; pp. 91–110. [Google Scholar]
- Spies, T.A.; Franklin, J.F.; Klopsch, M. Canopy gaps in Douglas-fir forests of the Cascade Mountains. Can. J. For. Res. 1990, 20, 649–658. [Google Scholar] [CrossRef]
- Caccia, F.D.; Ballaré, C.L. Effects of tree cover, understory vegetation, and litter on regeneration of Douglas-fir (Pseudotsuga menziesii) in southwestern Argentina. Can. J. For. Res. 1998, 28, 683–692. [Google Scholar] [CrossRef]
- Vor, T. Bodenvegetation und Naturverjüngung in Douglasien-Altbeständen. Forstarchiv 2011, 82, 159–160. [Google Scholar] [CrossRef]
- Gill, R. A review of damage by mammals in north temperate forests: 1. Deer. Forestry 1992, 65, 145–169. [Google Scholar] [CrossRef]
- Nussbaumer, A.; Waldner, P.; Etzold, S.; Gessler, A.; Benham, S.; Thomsen, I.M.; Jørgensen, B.B.; Timmermann, V.; Verstraeten, A.; Sioen, G.; et al. Patterns of mast fruiting of common beech, sessile and common oak, Norway spruce and Scots pine in Central and Northern Europe. Forest Ecol. Manag. 2016, 363, 237–251. [Google Scholar] [CrossRef]
- Lavender, D.P.; Hermann, R.K. Douglas-fir—The Genus Pseudotsuga; Oregon State University Press: Corvallis, OR, USA, 2014; pp. 1–352. [Google Scholar]
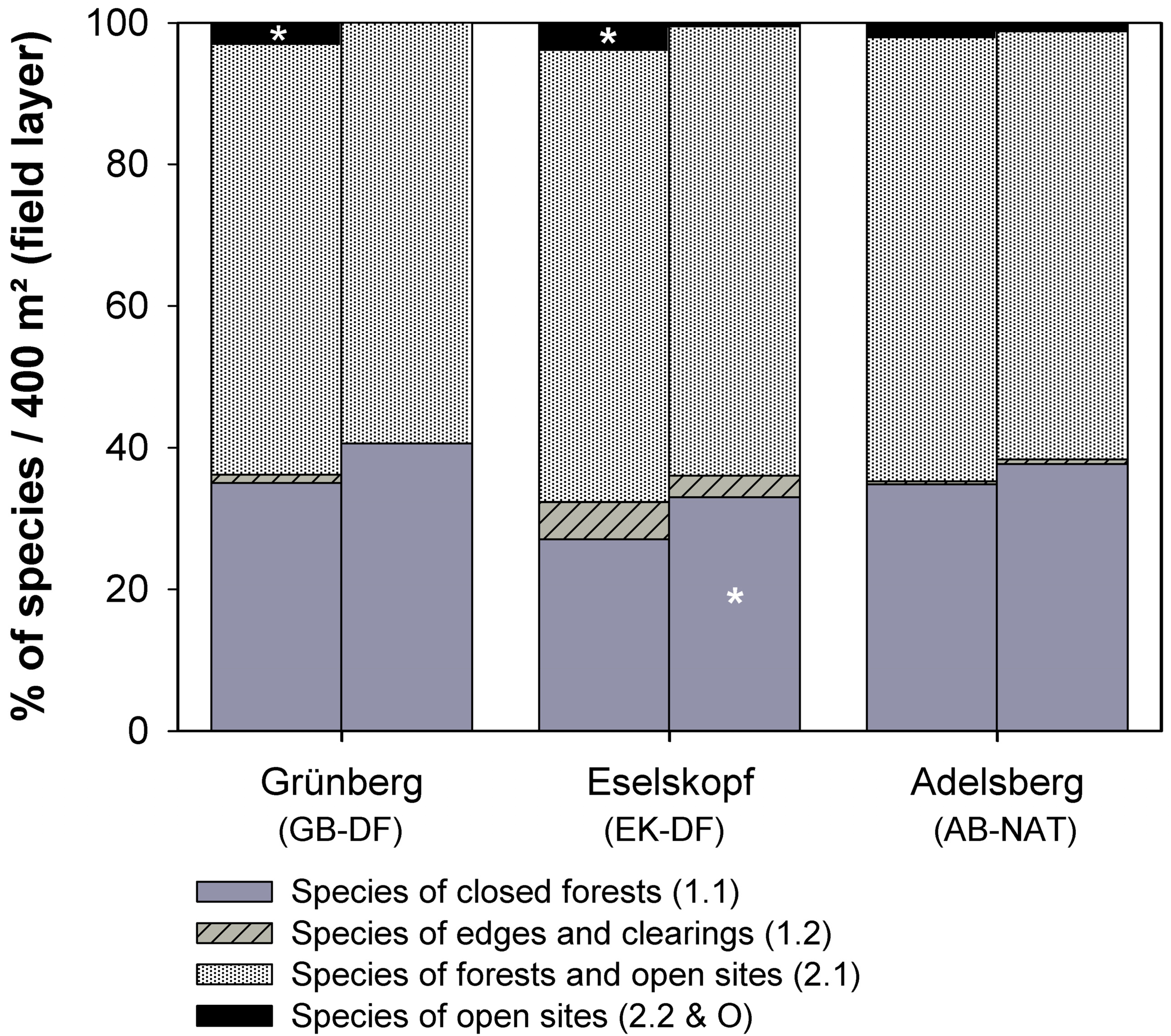


| Strict Forest Reserve (SFR) | Grünberg (GB-DF) | Eselskopf (EK-DF) | Adelsberg (AB-NAT) | |||
|---|---|---|---|---|---|---|
| Survey year | 2005 | 2017 | 2005 | 2017 | 2000 | 2016 |
| N | 13 | 13 | 14 | 14 | 29 | 29 |
| Cover value [%] | ||||||
| Tree layer | 84.4 (2.6) | 88.8 (1.5) | 75.2 (4.2) | 76.8 (3.7) | 71.9 (2.9) | 82.3 (1.9) |
| Shrub layer | 25.7 (3.4) | 20.5 (3.1) | 35.9 (5.4) | 15.4 (6.2) | 10.3 (1.6) | 13.9 (3.1) |
| Herb layer | 6.0 (1.3) | 1.9 (0.5) | 14.9 (2.8) | 5.8 (1.3) | 7.6 (2.1) | 11.1 (2.0) |
| Moss layer | 1.9 (0.4) | 2.6 (0.4) | 17.7 (4.5) | 25.3 (6.3) | 5.2 (1.0) | 0.9 (0.2) |
| Species richness/400 m2 | ||||||
| Tree layer | 2.9 (0.1) | 2.9 (0.2) | 3.2 (0.3) | 3.5 (0.4) | 3.0 (0.1) | 3.4 (0.3) |
| Shrub layer | 3.9 (0.3) | 3.4 (0.2) | 5.6 (0.5) | 4.6 (0.5) | 1.8 (0.2) | 2.6 (1.9) |
| Herb layer | 9.5 (1.5) | 2.2 (0.4) | 14.9 (1.7) | 8.4 (0.7) | 8.5 (0.7) | 7.5 (0.5) |
| Moss layer | 3.6 (0.6) | 4.8 (0.5) | 8.3 (0.9) | 9.1 (0.8) | 4.2 (0.3) | 2.5 (0.3) |
| Field layer (shrub + herb + moss) | 14.7 (2.0) | 8.8 (0.8) | 27.6 (2.2) | 21.7 (1.4) | 13.2 (0.7) | 10.5 (0.7) |
| Total species richness | ||||||
| Vascular plants | 27 | 9 | 48 | 34 | 39 | 28 |
| Bryophytes | 11 | 13 | 16 | 18 | 24 | 17 |
| EIVs 1 (Field layer) | ||||||
| Temperature | 3.8 (0.1) | 3.8 (0.1) | 4.4 (0.1) | 4.3 (0.1) | 4.8 (0.1) | 5.0 (0.1) |
| Continentality | 4.1 (0.1) | 4.3 (0.1) | 3.7 (0.1) | 3.7 (0.1) | 3.6 (0.1) | 3.3 (0.1) |
| Light | 4.7 (0.1) | 4.5 (0.0) | 5.0 (0.1) | 4.8 (0.1) | 4.8 (0.1) | 4.7 (0.1) |
| Moisture | 5.2 (0.1) | 5.4 (0.1) | 5.2 (0.0) | 5.1 (0.0) | 4.9 (0.0) | 5.1 (0.0) |
| Acidity | 3.7 (0.1) | 3.8 (0.2) | 4.2 (0.1) | 4.1 (0.1) | 4.1 (0.1) | 4.3 (0.2) |
| Nutrients (Nitrogen) | 4.3 (0.2) | 4.4 (0.1) | 5.0 (0.1) | 4.8 (0.1) | 4.5 (0.1) | 4.9 (0.1) |
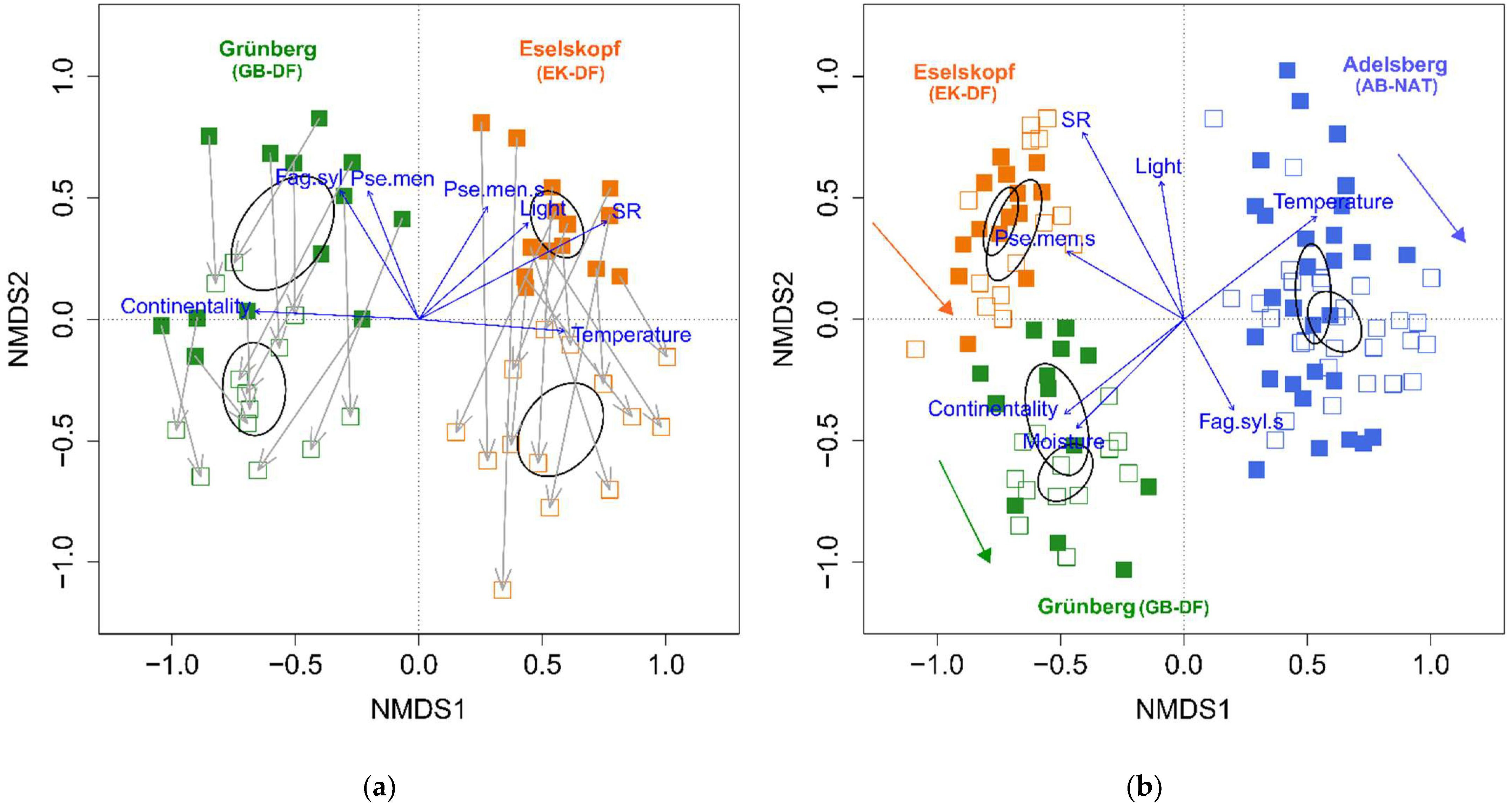
| First Survey | Second Survey | p-Value | |
|---|---|---|---|
| NMDS of Figure 4a | |||
| GB-DF NMDS 1 | −0.552 ± 0.085 | −0.668 ± 0.052 | 0.095 |
| GB-DF NMDS 2 | 0.355 ± 0.096 | −0.287 ± 0.078 | <0.001 |
| EK-DF NMDS 1 | 0.559 ± 0.044 | 0.573 ± 0.071 | 0.848 |
| EK-DF NMDS 2 | 0.391 ± 0.056 | −0.454 ± 0.079 | <0.001 |
| NMDS of Figure 4b | |||
| GB-DF NMDS1 | −0.514 ± 0.053 | −0.480 ± 0.045 | 0.593 |
| GB-DF NMDS2 | −0.412 ± 0.094 | −0.631 ± 0.048 | 0.005 |
| GB-DF NMDS1-NMDS2 | −0.101 ± 0.124 | 0.151 ± 0.054 | 0.034 |
| EK-DF NMDS1 | −0.741 ± 0.029 | −0.687 ± 0.046 | 0.152 |
| EK-DF NMDS2 | 0.404 ± 0.057 | 0.367 ± 0.085 | 0.613 |
| EK-DF NMDS1-NMDS2 | −1.145 ± 0.049 | −1.055 ± 0.068 | 0.235 |
| AB-NAT NMDS1 | 0.524 ± 0.030 | 0.611 ± 0.045 | 0.071 |
| AB-NAT NMDS2 | 0.105 ± 0.084 | −0.009 ± 0.051 | 0.095 |
| AB-NAT NMDS1-NMDS2 | 0.419 ± 0.092 | 0.620 ± 0.078 | 0.002 |
| Strict Forest Reserve (SFR) | Grünberg (GB-DF) | Eselskopf (EK-DF) | Adelsberg (AB-NAT) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 13 | 14 | 29 | |||||||||
| Year | 2005 | 2017 | 2005 | 2017 | 2001 | 2016 | ||||||
| F | mCV (SE) | F | mCv (SE) | F | mCv (SE) | F | mCv (SE) | F | mCv (SE) | F | mCv (SE) | |
| Tree layer (>5 m height) | ||||||||||||
| Increasing | ||||||||||||
| Fagus sylvatica | 100 | 50.1 (3.6) | 100 | 75.0 (5.6) | 50 | 1.3 (0.4) | 64 | 3.2 (1.1) | 72 | 17.0 (3.2) | 86 | 31.0 (4.1) |
| Carpinus betulus | 43 | 1.3 (0.5) | 57 | 2.4 (0.8) | 45 | 5.0 (1.6) | 66 | 9.4 (2.0) | ||||
| Quercus petraea | 21 | 0.4 (0.2) | 7 | 0.1 (0.1) | 86 | 31.5 (3.6) | 97 | 40.5 (4.7) | ||||
| Corylus avellana | 7 | 1.0 (1.0) | 50 | 1.5 (0.5) | ||||||||
| Decreasing | ||||||||||||
| Pseudotsuga menziesii | 100 | 48.6 (4.5) | 100 | 35.9 (4.0) | 100 | 67.6 (3.3) | 100 | 69.1 (3.3) | ||||
| No significant change | ||||||||||||
| Pinus sylvestris | 69 | 4.3 (1.5) | 54 | 4.8 (1.8) | ||||||||
| Picea abies | 23 | 0.7 (0.4) | 38 | 0.9 (0.4) | 29 | 1.8 (1.1) | 36 | 1.8 (1.4) | ||||
| Betula pendula | 21 | 0.4 (0.2) | 14 | 0.3 (0.2) | ||||||||
| Salix caprea | 21 | 0.4 (0.2) | 7 | 0.1 (0.1) | ||||||||
| Sorbus aucuparia | 21 | 0.4 (0.3) | 7 | 0.2 (0.2) | ||||||||
| Tilia cordata | 93 | 28.0 (3.6) | 93 | 29.0 (3.3) | ||||||||
| Shrub layer (≤5 m height) | ||||||||||||
| Increasing | ||||||||||||
| Fagus sylvatica | 100 | 5.6 (0.9) | 100 | 4.3 (1.0) | 79 | 2.0 ± 0.5 | 100 | 2.6 ± 0.5 | 72 | 6.8 ± 1.5 | 93 | 6.6 ± 1.2 |
| Picea abies | 100 | 7.7 (1.6) | 100 | 14.8 (3.2) | 64 | 2.3 ± 1.0 | 64 | 0.8 ± 0.2 | 3 | + | 7 | 0.1 ± 0.1 |
| Rubus fruticosus agg. | 23 | 0.2 (0.1) | 7 | + | 41 | 6.8 ± 2.8 | ||||||
| Tilia cordata | 66 | 1.3 ± 0.3 | 86 | 1.6 ± 0.4 | ||||||||
| Decreasing | ||||||||||||
| Pseudotsuga menziesii | 100 | 11.4 (1.9) | 92 | 1.2 (0.2) | 100 | 20.7 ± 3.4 | 71 | 4.6 ± 2.8 | ||||
| Pinus sylvestris | 54 | 0.7 (0.3) | ||||||||||
| Carpinus betulus | 86 | 7.0 ± 2.9 | 50 | 5.0 ± 3.1 | 34 | 2.2 ± 0.7 | 21 | 0.6 ± 0.3 | ||||
| Quercus robur | 21 | 0.2 ± 0.1 | ||||||||||
| No significant change | ||||||||||||
| Corylus avellana | 86 | 2.8 ± 0.6 | 79 | 1.5 ± 0.3 | ||||||||
| Quercus petraea | 43 | 0.6 ± 0.3 | 43 | 0.7 ± 0.3 | 7 | + | ||||||
| Acer pseudoplatanus | 21 | 0.1 ± 0.1 | 14 | 0.1 ± 0.0 | ||||||||
| Sorbus aria | 21 | 0.2 ± 0.1 | 7 | + | ||||||||
| Herb layer (≤0.5 m height) | ||||||||||||
| Increasing | ||||||||||||
| Quercus petraea | 14 | 0.1 (0.0) | 14 | + | 93 | 0.4 (0.0) | 100 | 1.9 (0.6) | ||||
| Tilia cordata | 66 | 0.2 (0.0) | 93 | 0.4 (0.0) | ||||||||
| Decreasing | ||||||||||||
| Rubus idaeus | 77 | 0.8 ± 0.2 | 36 | 0.2 ± 0.1 | ||||||||
| Fagus sylvatica | 69 | 0.4 ± 0.1 | 31 | 0.1 ± 0.1 | 21 | + | 86 | 0.6 ± 0.1 | 66 | 0.6 ± 0.1 | ||
| Pseudotsuga menziesii | 69 | 0.4 ± 0.1 | 29 | + | ||||||||
| Cytisus scoparius | 31 | 0.1 ± 0.1 | 21 | + | 3 | + | ||||||
| Sambucus racemosa | 71 | 0.2 ± 0.1 | ||||||||||
| Contrary development | ||||||||||||
| Rubus fruticosus agg. | 69 | 1.5 ± 0.8 | 46 | 1.1 ± 0.5 | 100 | 3.2 ± 0.5 | 93 | 1.6 ± 0.5 | 38 | 1.7 ± 1.4 | 48 | 3.6 ± 1.2 |
| No significant change | ||||||||||||
| Picea abies | 85 | 0.9 ± 0.3 | 100 | 0.6 ± 0.1 | 14 | + | 7 | + | ||||
| Carpinus betulus | 29 | 0.2 ± 0.1 | 14 | + | 38 | 0.1 ± 0.0 | 52 | 0.2 ± 0.0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinrichs, S.; Dölle, M.; Vor, T.; Balcar, P.; Schmidt, W. Understory Vegetation Dynamics in Non-Native Douglas Fir Forests after Management Abandonment—A Case Study in Two Strict Forest Reserves in Southwest Germany. Diversity 2022, 14, 795. https://doi.org/10.3390/d14100795
Heinrichs S, Dölle M, Vor T, Balcar P, Schmidt W. Understory Vegetation Dynamics in Non-Native Douglas Fir Forests after Management Abandonment—A Case Study in Two Strict Forest Reserves in Southwest Germany. Diversity. 2022; 14(10):795. https://doi.org/10.3390/d14100795
Chicago/Turabian StyleHeinrichs, Steffi, Michaela Dölle, Torsten Vor, Patricia Balcar, and Wolfgang Schmidt. 2022. "Understory Vegetation Dynamics in Non-Native Douglas Fir Forests after Management Abandonment—A Case Study in Two Strict Forest Reserves in Southwest Germany" Diversity 14, no. 10: 795. https://doi.org/10.3390/d14100795
APA StyleHeinrichs, S., Dölle, M., Vor, T., Balcar, P., & Schmidt, W. (2022). Understory Vegetation Dynamics in Non-Native Douglas Fir Forests after Management Abandonment—A Case Study in Two Strict Forest Reserves in Southwest Germany. Diversity, 14(10), 795. https://doi.org/10.3390/d14100795






