Abstract
Fenugreek (Trigonella foenum-graecum L.) is one of the oldest cultivated plants grown for its leaves and seeds that are used for both culinary and medicinal purpose. This study aims to evaluate the effect of ethanol concentration (30, 50, 70 and 96% (v/v) of ethanol in water) as a solvent for the extraction of total phenolic content (TPC) and antioxidant properties (antiradical activity (ARA), transition metal reducing power (TMRP), iron chelating ability (ICA)) of seed extracts of spring variety Ovari 4 (FSV) and winter variety PSZ.G.SZ (FWV) fenugreek, and separate and identify the major phenolics of the extracts by HPLC-ESI-MS. The results indicated that 70% ethanol solution resulted in the maximum amount of TPC for both FSV and FWV seeds. The TPC decreased in the treatments in the following order: 70% ethanol > 96% ethanol > 50% ethanol > 30% ethanol, whereas extraction yield changed in a different manner: 30% ethanol > 50% ethanol > 70% ethanol > 96% ethanol. The extracts from seeds of both fenugreek varieties obtained with 70% and 96% ethanol showed equal high RSA while superior TMRP and ICA were observed in 70% ethanol extracts. The TMRP and ICA were strongly correlated with TPC for both varieties. The correlation between RSA and TPC was high, but not significant. Thus, the obtained data indicate the 70% ethanol solvent suitability for efficient extraction of phenolic compounds from seeds of the FWV and FSV. According to an HPLC-ESI-MS analysis, the polyphenolic profiles of fenugreek are presumably formed by flavone C-glycosides with apigenin or luteolin as aglycone linked with different glycones. High antioxidant activity of FWV seeds can be an adaptation to cold stress of the winter variety aimed at strengthening the antioxidant defense of the germinating seeds.
1. Introduction
Oxidative stress develops due to excess production of reactive oxygen species (ROS) relative to endogenous antioxidant defense [1]. Oxidative stress appears to be involved in non-communicable diseases, such as cancer, atherosclerosis, ischemia, hypertension, cardiomyopathy, cardiac hypertrophy, several neurological diseases including Parkinson’s and Alzheimer’s disease, asthma and chronic obstructive pulmonary disease, rheumatoid arthritis, glomerulo- and tubule-interstitial nephritis, and renal failure, all which are linked to oxidative stress [2,3,4,5]. ROS can negatively affect several cellular structures, such as membranes, lipids, proteins, lipoproteins, RNA and DNA [1,6]. The DNA base guanosine can be oxidized to 8-oxoguanosine which no longer base-pairs with cytosine but with adenosine. This can lead to mutations and, as a consequence, to genetic diseases such as cancer. In order to prevent such health conditions, food rich in natural oxidants is encouraged for a healthy nutrition [7].
Non-enzymatic natural antioxidants, primarily such as vitamins A, E, C, phenolics, carotenoids, allicin, isothiocyanates, chlorophyll, and glutathione contribute to oxidative stress prevention [8,9,10], reducing risk of numerous diseases [11,12]. This background stimulates increasing interest in finding new sources of antioxidants and investigation of antioxidant activity of plants used for food and medicinal purposes [12,13,14].
Fenugreek (Trigonella foenum-graecum L.) is an aromatic plant of the family Fabaceae cultivated for a long time in many countries of the world. Fenugreek seeds are used as an important spice in food preparations due to strong flavor and aroma in Mediterranean, Oriental and Asian countries. The leaves are used as a vegetable in India. Currently, fenugreek is extensively grown in southeastern and central Europe, as well as India, China, North Africa, and Western Canada. Fenugreek is also a known medicinal plant with a large number of medicinal properties and has a long history of medical uses in Ayurvedic and Chinese medicine. Fenugreek is rich in many valuable biologically active secondary metabolites such as phenolics, alkaloids, saponins, carbohydrates, vitamins, and volatile oils [15,16,17,18,19].
In recent decades, fenugreek has been extensively studied for its pharmacological properties and biological activities in various in vivo and in vitro models. According to the results obtained, fenugreek has been reported to exhibit antidiabetic, antitumor, anti-inflammatory, hypotensive, anti-atherosclerotic, antihyperglycemic, hypocholesterolaemic, antimicrobial, antioxidant, and hepatoprotective properties [15,16,17,18,19,20,21].
The pharmacological effects of any plant are due to presence of mixtures of secondary metabolites. Many of fenugreek pharmacological activities and its strong antioxidant properties mentioned above are associated with phenolic compounds [22,23,24,25,26,27,28,29,30,31].
Summarizing the results of several studies, the anti-inflammatory, analgesic, and antipyretic properties of fenugreek extracts are related not only to alkaloids and steroid glycosides, but also phenolics (flavonoids) [21,32,33]. Polyphenols are apparently responsible for the antidiabetic effect of fenugreek. In accordance with [34], polyphenols of fenugreek seeds can improve the sensitivity of the insulin signaling system thereby stimulating the cellular action of insulin in the animal-model of acquired insulin resistance.
Polyphenolic associated protective effects of fenugreek seed extracts have been reported against ethanol-induced toxicity in human Chang liver cells [27] and cypermethrin-induced damage to the liver and kidneys of rats [35]. Fenugreek seed extracts play a major role in liver cirrhosis of alcohol-damaged liver. The extracts prevent the formation of collagen crosslinking in the liver, which may be due to their effect on interleukin 6, tumor necrosis factor α and the enzymatic system responsible for the synthesis and degradation of collagen [26]. Tewari et al. (2020) assumed that fenugreek seeds as dietary supplements can positively influence the activities of the hepatic antioxidant defense enzymes in aging mice [36].
In addition, phenolics extracted from fenugreek seeds can prevent oxidative hemolysis and lipid peroxidation induced in human erythrocytes by H2O2 [28]. The extracts demonstrated anti-inflammatory activity against bleomycin-induced lung fibrosis model in rats [21] and the protective effects against selenite-induced cataract in rats [37] that were due to phenolics and also associated with their antioxidant properties. The ability of fenugreek seeds to strengthen the immune system is related to phenolics such as flavonoids [38]. Phenolic metabolites are also responsible for the manifestation of the antibacterial effect against Bacillus subtilis Escherichia coli, Helicobacter pylori, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhi and Staphylococcus aureus [39,40,41]. Luan et al. suggested that flavonoid glycosides of fenugreek seeds control glycolipid metabolism by improving mitochondrial function in 3T3-L1 adipocites [42]. In summary, all these properties are mainly associated with the antioxidant activity of fenugreek seeds.
It is obvious from these data that fenugreek seeds are a source of highly active phenolics that have great potential in prevention and therapy of various diseases related to oxidative stress.
Because new varieties of fenugreek are being developed, it is important to study the phenolic composition and antioxidant properties of these new varieties. In the present study we investigated seeds of two fenugreek varieties developed at The University of West Hungary: tax. conc. ”winter” cultivar (c. v. variety) PSZ.G.SZ (FWV) and tax. conc. ”spring-summer” cultivar (c. variety) Ovari 4 (FSV).
Although fenugreek seeds are characterized by a high content of phenolics [19,24], the efficiency of the extraction of these compounds from seeds depends to a large extent on the extraction conditions and mainly on the solvent [43].
Polar organic solvents, such as methanol, ethanol, ethyl acetate, acetone and their combinations with water are generally used for the extraction of phenolics. Among these solvents, ethanol has an important advantage. It is relatively safe for human consumption and consequently this solvent is widely used to extract phytochemicals and bioactive compounds from plant material. The mixtures of ethanol and water are more polar compared to absolute ethanol which may positively influence yields of free phenolics [20,44,45]. Usually, the optimal solvent concentrations are determined empirically. Therefore, the main objectives of this study were to estimate the influence of ethanol concentrations in solvent solutions on the yield of polyphenolics and their corresponding antioxidant activity. The extracts with highest total phenolics were selected for separation and identification of polyphenols in FWV and FSV by HPLC-ESI-MS.
2. Materials and Methods
2.1. Materials
Seeds of FSW and FWV were from our own collection (Professor Sándor Makai). Folin–Ciocalteu reagent (AppliChem GmbH, Darmstadt, Germany), 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich GmbH, Steinheim, Germany), ferrozin (ABCR GmbH, Karlsruhe, Germany), ascorbic acid (Sigma-Aldrich GmbH, Hamburg, Germany), ethylendiaminetetraacetic acid (AppliChem GmbH, Darmstadt, Germany), cinnamic acid (Sigma-Aldrich GmbH, Steinheim, Germany), methylcinnamic acid (Sigma-Aldrich GmbH, Steinheim, Germany), gallic acid (ABCR GmbH, Karlsruhe, Germany), ferulic acid (Sigma-Aldrich GmbH, Steinheim, Germany), caffeic acid (Sigma-Aldrich GmbH, Steinheim, Germany), chlorogenic acid (Sigma-Aldrich GmbH, Steinheim, Germany), quercetin (Sigma-Aldrich GmbH, Steinheim, Germany), rutin (Sigma-Aldrich GmbH, Steinheim, Germany), arbutin (Sigma-Aldrich GmbH, Steinheim, Germany). All other chemicals and solvents such as ethanol, methanol, formic acid, acetonitrile, petroleum ether, ethyl acetate, n-hexane, ferric chloride, sodium carbonate, sulfuric acid, monobasic potassium phosphate, ammonium molybdate were of the highest analytical grade and used as supplied.
2.2. Sample Preparation
Fenugreek of Ovari 4 and PSZ.G.SZ varieties was cultivated in Mosonmagyaróvár (Hungary). Collected mature seeds were cleaned and dried before storage in a dry, dark place. Within the next six months fenugreek seeds were ground into powder using a kitchen milling machine and passed through a 1 mm sieve. The powder (100 g) was defatted by n-hexane. The solid–liquid mixture was filtered with Whatman filter paper, and the residue was dried in a drying oven for 8 h at 50 °C. The dried defatted fenugreek seed material was stored at 4 °C within 2 weeks.
2.3. Extraction Procedure and Extraction Yield
Extraction consisted of cold and hot stages. In cold process, 10 g of dried defatted seed powder was mixed with 100 mL of the solvent in a 500 mL flask. Closed flask was stirred on a rotary shaker at 150 rpm at 25 °C for 24 h. The solvent used was ethanol with varying concentrations, namely 30%, 50%, 70% and 96% (v/v). In the hot process, the mixture was heated to 70 °C for 2 h in a water-bath and was then centrifuged (10,000× g, 25 °C) for 10 min. The supernatant obtained was filtered using Whatman filter paper. The filtrate from each solvent was evaporated in a rotary evaporator under reduced pressure at 50 °C until a thick extract was obtained. The thick extract was dried for 24 h at 50 °C in a water-bath in air conditions and was then weighed to determine extraction yield. The yields of the extracts were calculated as percent of initial dry weight of the defatted fenugreek seed material (dry basis, d.b.) and expressed as a percentage. Dried extracts were placed in Eppendorf tubes, and kept until further analysis at −12 °C.
2.4. Total Phenolic Content (TPC)
Total phenolics determination in each extract followed [46] with modifications using Folin–Ciocalteu method. Each dried extract (0.1 g) was dissolved in 10 mL of ethanol. Then, 0.2 mL of the solution was mixed with 1.6 mL of distilled water, 0.3 mL of 20% (w/w) Na2CO3 aqueous solution, and 0.1 mL of Folin–Ciocalteu reagent. The mixture was kept in the dark under ambient conditions for 1 h to complete the reaction and was then diluted to 4 mL using distilled water. The absorbance was measured at 750 nm using a UV-VIS spectrophotometer (Cary 50 Bio; Varian, Australia). Gallic acid was used as a standard. The calibration curve was established using gallic acid at concentrations of 0.01 to 0.1 mg/mL. The relationship between the concentration of gallic acid (x) and its absorbance (y) was plotted to produce a linear line equation (y = 3.7997 x; R2 = 0.9929). Results of total phenolic content determination were expressed as mg of gallic acid equivalents per gram of dry extract (mg GAE/g dry extract).
2.5. DPPH Radical Scavenging Activity
The radical scavenging activity of the extractions was determined with the DPPH method as described [47] with modifications. A solution of 0.3 mM DPPH was freshly prepared with 96% ethanol. The extracts (2.5 mL) with varying concentrations (0.05 to 0.25 mg dry residue of the extract/mL analysis solution) were mixed with 1.0 mL of DPPH solution in a test tube. The mixtures were left to stand for 30 min at room temperature under dark conditions. Absorption was measured at 520 nm using a UV-VIS spectrophotometer (Cary 50 Bio; Varian, Australia). Blank solution was 96% ethanol. DPPH solution (1.0 mL, 0.3 mM) mixed with 96% ethanol (2.5 mL) served as a negative control. Ascorbic acid was employed as a positive control. The half-maximal inhibitory concentration (IC50) was calculated as the amount of antioxidant required to decrease the initial DPPH concentration by 50%. The IC50 was obtained using linear regression analysis.
2.6. Transition Metal Reducing Power (TMRP)
The phosphomolybdenum method was used in investigation of the metal ion reducing capacity of the extracts. This parameter of the extracts was evaluated according to [48] with slight modifications. The dried extract was diluted with distilled water (0.05 to 0.25 mg dry residue of the extract/mL analysis solution). The diluted extract (0.3 mL) was combined with 3.0 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, 4 mM ammonium molybdate) in a capped plastic tube. Tubes containing the reaction solution were incubated in a water bath at 90 °C for 90 min, then cooled down to room temperature. The absorbance of this solution was measured at 695 nm using a UV-VIS spectrophotometer (Cary 50 Bio; Varian, Bungarra, Australia) against a blank. The blank solution contained 3.0 mL of reagent solution and the appropriate volume of the same solvent used for the sample and it was incubated under same conditions as the samples. Ascorbic acid (0.05 to 0.25 mg/mL) was used as the standard. TMRC was expressed as absorbance at 695 nm.
2.7. Iron Chelating Ability
Iron chelating ability of the extracts was determined according to [49] with minor modifications. One milliliter of each sample solution (0.05 to 0.25 mg dry residue of the extract/mL analysis solution) was mixed with 3.7 mL of 96% ethanol and 0.1 mL of 2.0 mM FeCl2, in a test tube. The reaction was initiated by the addition of 0.2 mL of 5.0 mM ferrozine. The solutions were well mixed and the mixtures left for 10 min at room temperature. The iron chelating ability (%) was obtained by measuring the absorbance of the Fe2+-ferrozine complex at 562 nm using a UV-VIS spectrophotometer (Cary 50 Bio; Varian, Australia). Instead of sample solution, 96% ethanol (1.0 mL) was used as a negative control. Ethylendiaminetetraacetic acid (EDTA) was used as the positive control. The half-maximal iron chelating ability (IC50) was calculated using linear regression analysis.
2.8. HPLC-ESI-MS Analysis
2.8.1. Sample Preparation
Plant extracts contain a mixture of substances other than phenolics. These compounds differ in the solubility dependent of the polarity of solvent [43]. Therefore, in order to separate polar components from non-polar, solvents with different polarities were used. Extracts of seeds were prepared according to the previously described method with the use of 70% ethanol solvent that gave highest yield of phenolics.
Ethanol was evaporated from extract solution by a rotary evaporator under reduced pressure at 50 °C until a thick extract was obtained. To get rid of non-polar substances, petroleum ether was added to the liquid water rest of extracts, and then removed from the mixture by heating at 50 °C in a rotary evaporator at 50 °C. This procedure was repeated 5 times. Further, water rest was treated 5 times with ethyl acetate to separate phenolic compounds. Ethyl acetate solutions were combined and dried. Dried samples were placed in Eppendorf tubes, and kept until further analysis at −12°C. During sample preparation dried ethyl acetate fractions were redissolved in 1 mL of methanol and centrifuged for 5 min at 10,000 rpm.
2.8.2. HPLC-ESI-MS Conditions
LC-ESI-MS analysis was performed using Thermo Finnigan (Thermo Electron Corporation, Waltham, MA, USA) coupled with an LCQ Duo ion trap mass spectrometer with an ESI source (ThermoQuest). A reversed-phase C18 column with particle size of 5 µm, 4.6 × 250 mm was used. The mobile phase composition was 0.5% (v/v) formic acid in water (solvent A) and acetonitrile (solvent B). The gradient of eluents was formed by mixing solvents A and B as follows: 0–10% B (v/v) from 0 to 10.0 min, 10% B (v/v) from 15.0–30.0 min, 10–100% B (v/v) from 30.0–50.0 min. The flow rate was 1 mL/min. Samples dissolved in methanol (10 mg/mL) were injected on the column using the HPLC pump. The mass spectrometry system was applied in the negative ionization mode.
2.8.3. Statistical Analysis
All experiments were performed in triplicate. Results were expressed as mean ± standard error. Differences in mean values were tested using a one-way Analysis of Variance (ANOVA) and differences were considered to be significant at p < 0.05. Correlation between TPC and the values of the antioxidant activity parameters was evaluated using Pearson’s correlation coefficient that was calculated with Microsoft Excel 2013.
3. Results and Discussion
3.1. Extraction Yield and Total Phenolic Content
The extraction of bioactive compounds from plant materials can be influenced by many factors such as the nature of the phytochemicals, composition of the sample, the extraction method used, chemical nature and polarity of solvents, sample particle size, solid–solvent ratio and physical extraction conditions. Under the same extraction time and temperature, the solvent is known as the most important parameter [50,51]. Therefore, in the current study ethanol at concentrations of 30, 50, 70 and 96% (v/v) was employed to determine the optimal extraction procedure.
Ethanol has the ability to extract phenolics and its glycosides [52] as well as non-phenolic substances such as terpenoids, alkaloids, polyacetylenes, and sterols [44,53]. Water in water–ethanol mixtures favors the extraction of carbohydrates, organic acids, and amino acids [44,52,53,54]. Consequently, total number of compounds extracted usually exceeds the amount of phenolic compounds extracted. Considering this fact, total extraction yields of the extracts of fenugreek seeds were determined together with total phenolics.
Figure 1 demonstrates that the extraction yield changed in a similar way for extracts of FWV and FSV seeds, depending on the ethanol concentration used: 30% ethanol > 50% ethanol > 70% ethanol > 96% ethanol. The highest extraction yields observed were 25.34 ± 2.28 and 20.25 ± 1.42% per dried defatted seed material of FSV and FWV, respectively (p < 0.05). No differences were observed for the parameters between the treatments of both fenugreek varieties when ethanol was employed at concentrations of 50%, 70%, and 96% while the yield at 30% ethanol solution was found to be higher in seeds of FSV compared to that of FWV (p < 0.05). Hikmavanty et al. (2021) reported similar results, suggesting that 50% ethanol extracted more compounds from the dry katuk leaves (37.77%) than 70% and 96% ethanol [45]. Kim and Chin (2017) investigated the extraction yield from tomato powder with the use of mixtures of ethanol and water in different concentrations (0%, 25%, 50%, 75%, and 100% ethanol). Solutions containing ethanol at concentrations of 0 to 75% gave similar total yields (from 3.14 to 3.87%). The 100% ethanol showed the lowest total yield (0.52%) [55]. Do et al. (2014) reported that the extraction yields of Limnophila aromatica by various solvents decreased in the order: 50% aqueous acetone > 50% aqueous ethanol > 75% aqueous methanol > 50% aqueous methanol > 75% aqueous acetone > 75% aqueous ethanol > 100% methanol > 100% ethanol > 100% acetone. Extraction yield ranged from 12.33% for acetone extract to 33.67% for 75% aqueous acetone extract. According to these results the authors concluded that the extraction yield increases with increasing polarity of the extract solvent used [56].
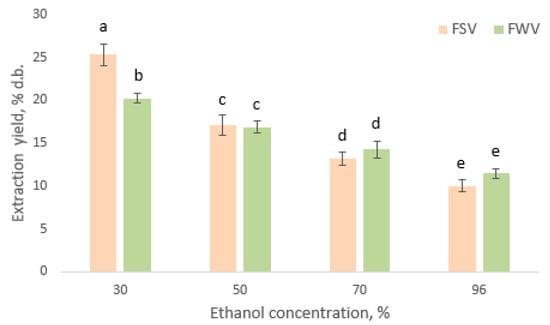
Figure 1.
Extraction yield (% dry basis) of seed extracts of FSV and FWV at different ethanol concentrations in the extract solutions. Means not bearing a same letter are significantly different (p < 0.05).
Based on the results in Figure 2, TPC in the extracts of both fenugreek varieties decreased in a similar order: 70% ethanol > 96% ethanol > 50% ethanol > 30% ethanol. The values of total phenolics reaching 120.3 ± 6.1 mg GAE/g dry extract for FSV and 102.6 ± 5.1 mg GAE/g dry extract for FWV when 70% ethanol were used for extraction (p < 0.05). TPC in 50–96% ethanol extracts of seeds of FSV was higher than that of the extract of FWV seeds (p < 0.05), whereas extracts of seeds of FSV and FWV prepared with 30% ethanol showed nearly equal amounts of total phenolics which were lowest compared to other treatments.
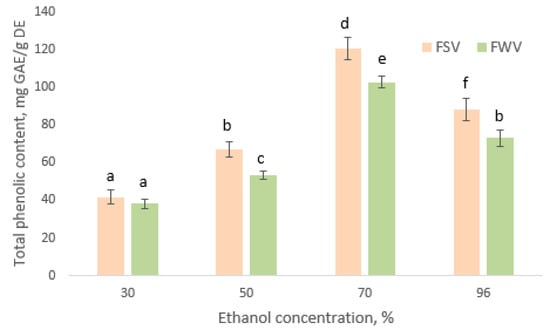
Figure 2.
Total phenolic content (mg GAE/g dry extract) in seed extracts of FSV and FWV at different ethanol concentrations. Means not bearing a same letter are significantly different (p < 0.05).
In general, the results of similar studies vary greatly, depending on plant material tested as well as the design of each study. Thus, Mani and Thomas (2014) showed that aqueous extracts of Pittosporum dasycaulon Miq. stem bark resulted in higher TPC (182.2 mg of GAE/g of extract) than methanol [57]. In tomato powder, with ethanol at all concentrations tested, total phenolics ranged from 1.57 to 2.02 g/100 g DW with the lowest value seen in 100% ethanol [55]. The TPC of Limnophila aromatica extracts ranged from 6.25 mg GAE/g DF for water extract to 40.5 mg GAE/g DFLA for 100% ethanol extract [56]. According with Rababah et al. (2004), TPC in 60% ethanol extracts from black tea, green tea, grape seed, ginger, rosemary, gotu cola, and ginkgo ranged from 24.8 to 92.5 mg ChAE/g DW [58].
Several previous studies estimated total phenolics in fenugreek seeds with differing extraction solvents [21,25,28,30,31,41,54,58]. Table 1 summarizes these data.

Table 1.
TPC in fenugreek seeds that reported in the previous studies.
According to our findings, the best extraction solvent for total phenolics was 70% ethanol, and this parameter of the extracts decreased with increasing water content in the aqueous solvent. However, the total yield of extraction was highest in the 30% ethanol solution. As opposed to our results, Bukhary et al. (2008) reported that in the extracts obtained by the Soxhlet method both highest extraction yield and TPC were observed in ethanol and methanol extracts of fenugreek while extracts with ethyl acetate, hexane, acetone, and dichloro methane as a solvent showed lower yields and total phenolics [31].
There is one other study that should be noted. Norziah et al. (2015) also investigated influence of different solvents (ethanol, methanol, water) on extraction yield of non-sorted fenugreek seeds and total phenolics. Similar to our results, they found that the highest yield was in water solution (about 38%), while in ethanol extracts the total yield was lowest (16.54%), but at the same time the TPC was highest among the treatments (45.0 mg GAE/g DW). The authors explained the results by the fact that increase in the water ratio contributed to the extraction of more galactomannan that resulted in an increase of extraction yield [41]. Galactomannan is a water-soluble heteropolysaccharide. According to Garti et al. (1997), fenugreek seeds possess the unique galactomannan having a mannose backbone grafted with galactose units at an average ratio of one [59]. Jiang et al. (2007) reported that fenugreek gum (seed endosperm) contains 73.6% galactomannans [60]. Solvents with high polarity such as water and water–ethanol solutions with predominant water content have the ability to also extract a class of compounds with a wider polarity (peptides, amino acids, carbohydrates) besides galactomannans [56]. Thus, compounds other than phenolics may have been extracted in high amount by 30 and 50% ethanol that contributed to higher total yield, while ethanol of 70 and 96% extracts more phenolics with lower recovery of water-soluble compounds such as, for instance, galactomannans.
3.2. Antioxidant Activity
The beneficial health effects of phenolics are mainly attributed to their antioxidant abilities [61].
The number and position of hydroxyl groups in a molecule of phenolic compound affects its antioxidant properties. These compounds are capable of donating hydrogen atoms or electrons from their hydroxyl groups and thereby inhibit the free radical process [62,63]. The ion chelating activity of plant extracts may be also attributed to the presence of phenolic compounds [64,65]. Phenolic acids possess a metal binding site—the 3′,4′-dihydroxy group on the B-ring [55]. Flavonoids with 3′,4′-catechol, 4-oxo, and 5-OH arrangements may inhibit Fenton-induced oxidation. Chelating complexes with divalent cations may form between the 5-OH and 4-oxo group, or between the 3′- and 4′-OH [64,66,67]. All of these functional groups have iron-chelating ability due to their electron-donating abilities [68].
In this study three common assays were used to evaluate antioxidant properties of the seed extracts of FSV and FWV.
3.2.1. Radical Scavenging Activity
Testing was carried out using DPPH radicals. The changes in the free radical scavenging ability in a concentration-dependent way of the ethanol extracts of fenugreek seeds are presented in Figure 3. The extracts prepared using 70 and 96% ethanol yielded the highest DPPH radical scavenging activity at concentrations 0.15 to 0.25 mg/mL and 0.20 to 0.25 mg/mL for extracts from seeds of FWV and FSV, respectively (p < 0.05). However, for both fenugreek varieties no significant difference was observed in the radical scavenging ability of 70 and 96% ethanol extractions although total phenolics in extracts with 70% ethanol was found to be higher than that of the extracts with 96% ethanol. It is evident from the Figure 3 that among all the treatments the extract with 30% ethanol had the lowest free radical scavenging capacity for both fenugreek varieties (p < 0.05). As for percentage of DPPH scavenging activity of fenugreek seeds with 70% acetone extract, Yacoubi et al. (2011) showed that to be 74.71%, corresponding to 73.5 mM TrE/g DW [21].
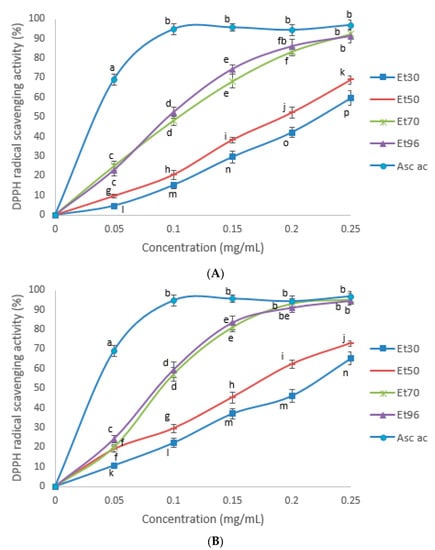
Figure 3.
DPPH radical scavenging activity (%) of the ascorbic acid and seed extracts of FSV (A) and FWV (B) at different ethanol concentrations in the extract solutions. Means not bearing a same letter are significantly different (p < 0.05). Asc ac = Ascorbic acid. Treatments: Et30 = extract with 30% ethanol; Et50 = extract with 50% ethanol; Et70 = extract with 70% ethanol; Et96 = extract with 96% ethanol.
The IC50 values in DPPH radical scavenging activity were also determined. The higher the IC50 value the weaker the antioxidant activity of a compound. The antiradical activity of the 30% extracts from FSV and FWV seeds were significantly weaker (p < 0.05) than that of all other extracts tested (Table 2). The 70 and 96% extracts of both varieties had the most potent antiradical activity with the lowest IC50 values (p < 0.05) (0.093 ± 0.005 mg and 0.088 ± 0.006 mg for 70 and 96% extracts of FWV, respectively; 0.108 ± 0.04 mg and 0.100 ± 0.06 mg for 70 and 96% extracts of FSV, respectively). The IC50 value of the ascorbic acid standard was 0.036 ± 0.003, which is more potent compared to all extracts tested (p < 0.05).

Table 2.
IC50 values evaluated by the DPPH assay (mg/mL) of the ascorbic acid (standard) and the extracts of FSV and FWV seeds at different ethanol concentrations in the extract solutions.
According to Chatterjee et al. (2011), IC50 for free radical scavenging activity measured by DPPH assay was found to be 366.52 ± 5.99 μg/mL for 80% methanol extract from fenugreek seeds [50]. In accordance with the results of Kaviarasan et al. (2007), the IC50 value for 80% aqueous methanol extract of fenugreek seed was 350 μg/mL [25].
3.2.2. Transition Metal Reducing Power
It is evident from the results presented in Figure 4 that the TMRP of all treatments increased with an increase in the amount of the extracts, which indicates their antioxidant potential (p < 0.05). The TMRP of seed extracts of both fenugreek varieties decreased, depending on ethanol content in solutions in the order: 70% ethanol > 96% ethanol > 50% ethanol > 30% ethanol. TMRP as absorbance at 695 nm of 0.25 mg/mL extracts (maximum concentration tested) is presented in Table 3. As opposed to results of DPPH assay, TMRP of the 70 and 96% extracts was significantly higher (p < 0.05) than that of ascorbic acid standard, indicating that these extracts possessed strong reducing capacity.
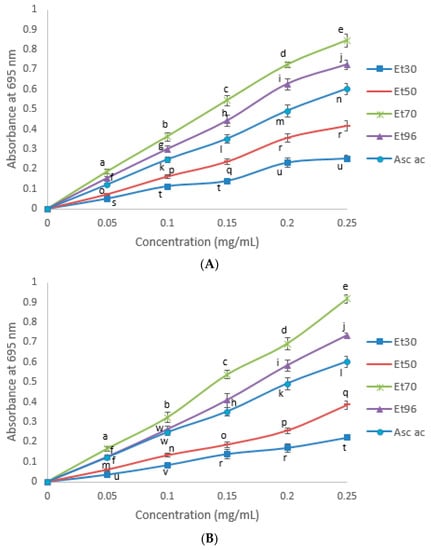
Figure 4.
Transition metal reducing power (abs at 695 nm) of the ascorbic acid and the seed extracts of FSV (A) and FWV (B) at different ethanol concentrations in the extract solutions. Means not bearing a same letter are significantly different (p < 0.05). Asc ac = Ascorbic acid. Treatments: Et30 = extract with 30% ethanol; Et50 = extract with 50% ethanol; Et70 = extract with 70% ethanol; Et96 = extract with 96% ethanol.

Table 3.
Transition metal reducing power (abs at 695 nm) at the highest concentration tested (0.25 mg/mL) of ascorbic acid (standard) and the extracts of FSV and FWV seeds at different ethanol concentrations in the extract solutions.
Subhashini et al. (2011) reported that TMRP (phosphomolybdenum assay) of fenugreek seeds was 6 µg TE/mg [30]. The TMRP of the various solvent extracts of Tagetes erecta leaves decreased in this order: petroleum ether > chloroform > methanol > water [69]. Methanolic extracts of both Cleome speciosa and Pittosporum dasycaulon Miq. had the highest reducing power compared to other solvents used for extraction [57,70].
3.2.3. Iron Chelating Ability
Figure 5 provides results for iron chelating ability. The percent of inhibition of Fe2 + -ferrozine formation by all samples increased with increasing of concentrations of extracts up to 0.25 mg/mL (p < 0.05). The difference in iron chelating activity was observed among the extract of the various combinations of water and ethanol. The results of chelating activity were in agreement with TPC and TMRP when the highest values of these parameters were found in the 70% ethanol extracts while lowest values were in 30% solutions (p < 0.05). Iron chelating activity of the extracts of FWV and FSV prepared with the same solvent was broadly comparable. The IC50 of chelating activity was determined for all treatments and standard (Table 4). The 70% extracts of both varieties had the most potent iron chelating activity in accordance with the IC50 values (p < 0.05). The metal chelating effect of extracts was lower than the EDTA standard used (0.054 ± 0.003 mg).
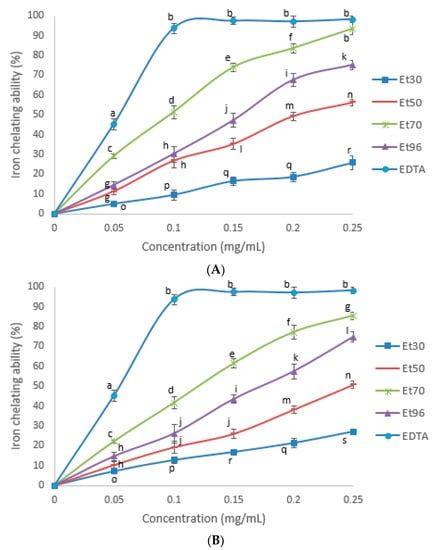
Figure 5.
Iron chelating ability (%) of EDTA and the seed extracts of FSV (A) and FWV (B) at different ethanol concentrations in the extract solutions. Means not bearing a same letter are significantly different (p < 0.05). EDTA = Ethylendiaminetetraacetic acid. Treatments: Et30 = extract with 30% ethanol; Et50 = extract with 50% ethanol; Et70 = extract with 70% ethanol; Et96 = extract with 96% ethanol.

Table 4.
IC50 in iron chelating ability (mg/mL) of EDTA (standard) and the extracts of FSV and FWV seeds at different ethanol concentrations in the extract solutions.
Kim et al. reported the increasing of iron chelating activity of tomato extracts with increasing concentration of ethanol in extract solvent up to 75%. The 50 and 75% ethanol extracts were found to show higher chelating ability than the other concentrations [55]. According to Subhashini et al. (2011) iron chelating ability of 70% ethanol extract of fenugreek seeds was 0.105 ± 0.007 mg/mL [30].
3.3. Correlation Analysis
In general, there is a positive correlation between TPC and TMRP, because TMRP tends to go up in response to increasing of TPC, whereas there is a negative correlation between TPC and IC50 in antiradical activity and between TPC and IC50 in iron chelating ability, because IC50 for these parameters goes down when TPC goes up. An excellent correlation (R ≥ 0.90 or R ≤ −0.90) was found between TPC and the IC50 in the iron chelating ability (R = −0988 for FSV, R = −0904 for FWV, p < 0.05) as well as the values of TMRP at maximal extract concentration tested (R = 0971 for FSV, R = 0977 for FWV, p < 0.05) for all variants of extract solutions used. The high correlations indicate a major role of phenolics as iron chelators and reducing agents contributing to the antioxidant activity of fenugreek seeds.
At the same time the correlation analysis did not show such a significant correlation between TPC and the IC50 in DPPH radical scavenging activity (R = −0881 for FSV, R = −0882 for FWV, p < 0.05) that is related to the almost equal antiradical activity of the samples extracted with 70 and 96% ethanol although the TPC was found to be higher in the extract of 70% ethanol for both fenugreek varieties.
A possible explanation of high antiradical properties of 96% ethanol extracts is ethanol in that concentration extracted a higher amount of more active phenolic radical scavengers compared to 70% ethanol. In addition to that, DPPH radicals may be also neutralized by non-phenolic antioxidants [71] which might be better extracted from seeds by 96% solvent than 70% ethanol.
The other studies that were also dedicated to finding of best solvent for extraction of phenolics showed positive correlation between total phenolics in the extracts of all variants of ethanol concentration and their antiradical properties. Thus, the 100% ethanol extract of Limnophila aromatica demonstrated the strongest DPPH radical activity (IC50 = 0.07 mg/mL) that was corresponding to its highest total phenolics among all extraction solvents tested. This extract also showed the highest TAA [56].
The 50% ethanol extract of katuk leaves had the strongest antioxidant activity as well as the highest phenolic content compared to ethanol extracts of 70 and 96% ethanol, while the strongest antiradical and iron chelating activities were observed in tomato powders extracted with 50 and 75% ethanol compared to other solvents used (0, 25, 100% of ethanol). DPPH scavenging activity and iron chelating ability were highly correlated with total phenolics content [45,55].
Opposite results were also reported. Aparadh et al. (2012) investigated some Cleome species for TPC and antioxidant activity by several assays, including DPPH, TMRP, and metal chelating assay. It was found that although the methanolic extract of Cleome speciosa had the lowest TPC among Cleome species tested, it showed the highest TMRP [70]. Mani and Thomas (2014) found that the TMRP of the methanol extract of Pittosporum dasycaulon Miq. was higher than that of the aqueous extract. However, TPC was much lower in the methanol extract than in the aqueous extract. Therefore, the authors concluded that the TMRP of the methanol extract was associated with the presence of not only phenolics, but also of other metabolites, such as alkaloids and saponins [57].
As for non-sorted fenugreek, the previous studies also showed that antioxidant activity of extracts depended on total phenolics. For instance, Kenny et al. (2013) investigated the extracts of fenugreek seeds prepared using non-polar and polar solvents. They found that ethyl acetate crude extract had the highest values of both DPPH scavenging activity and TPC [72]. According to [20], among ethanol, methanol, hexane, chloroform, and aqueous extracts of fenugreek leaves which were tested, ethanol extract showed better DPPH radical scavenging activity (59.7 ± 0.46%). There was a linear correlation between the antioxidant activity and total phenol content of fenugreek leaves. Subhashini et al. (2011) reported results of antioxidant activity of 70% ethanol extract of fenugreek with in vitro assays which included DPPH, phosphomolybdenum method, and metal chelating. In all the methods the extract showed strong antioxidant activity in a concentration-dependent manner. The values of antioxidant activities correlated with the TPC [30]. It was also reported that there was a positive correlation between the total phenolics in fenugreek seeds extract of 80% methanol and its ability to scavenge DPPH radical [54].
The results indicate that antioxidant activity of the seed extracts of both fenugreek varieties increased with increasing of ethanol concentration up to 70% and generally depended on total phenolic content. There was no significant difference between highest values of DPPH radical scavenging activity, total antioxidant capacity and iron chelating ability found for the seed extracts of FSV and FWV.
3.4. HPLC-ESI-MS Analysis
The extracts of 70% ethanol were found to show the highest TPC. Therefore, the 70% ethanol extracts were subjected to HPLC-ESI-MS analysis.
Figure 6 shows LC-MS profiles of purified 70% ethanol extracts of seeds of fenugreek varieties, while retention time (RT) and [M-H] values of separated constituents as well as nature of the compounds tentatively identified are presented in Table 5.
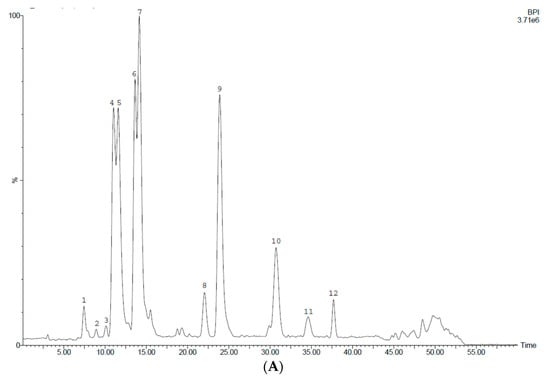
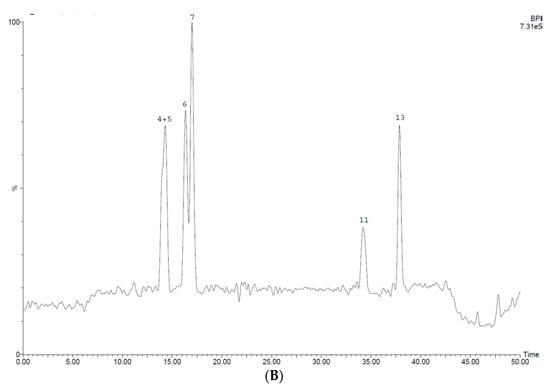
Figure 6.
HPLC chromatograms of the extract samples obtained after sequential processing of the 70% ethanol extracts of fenugreek seeds with chloroform and ethyl acetate. (A) Seeds of FWV; (B) seeds of FSV.

Table 5.
Phenolic compounds analyzed by HPLC-ESI-MS in purified ethanol extracts of FWV and FSV seeds.
It is evident from the profiles there was a difference in phenolic composition of purified seed extracts of FSV and FWV. Twelve phenolic compounds (peaks 1–12) were separated for FWV seeds, whereas, six compounds (peaks 4–7, 11, 13) were only found in the purified samples of FSV seeds. Compounds 4–7 and 11 were common phenolics for purified extracts of seeds of both fenugreek varieties.
Profile A exhibits five dominant peaks corresponding to compounds 4, 5, 6, 7, and 9 with a slight predominance of compound 7, while Profile B demonstrates one major peak that corresponds to compound 7.
Thus, there are significant differences in the composition and number of components of the extract samples from the seeds of the fenugreek varieties studied. According to Boubakri et al. (2017), significant quantitative and qualitative differences in the phenolic fraction of four barley varieties were shown [73].
The compounds in the samples analyzed were identified on the basis of their MS spectra and retention time data, comparison with available standard compounds, and literature data. Phenolics such as cinnamic acid, methylcynamic acid, gallic acid, ferulic acid, coumaric acid, caffeic acid, chlorogenic acid, quercetin, rutin, and arbutin were used as standards. They were not identified in the purified extracts according HPLC-ESI-MS data.
From literature data, the peak No. 1 corresponding to RT 7.5 and [M-H]− 153 was tentatively identified as gentisic acid. Peaks No. 2 (RT 9.0, [M-H]− 593), No. 3 (RT 9.0, [M-H]− 593), No 4. (RT 11.0, [M-H]− 447), No. 5 (RT 11.5, [M-H]− 447), No. 6 (RT 13.6, [M-H]− 431), No. 7 (RT 14.2, [M-H]− 431), and No. 9 (RT 23.8, [M-H]− 593) were tentatively identified as apigenin 6-C-β-xylopyranosyl-8-C-β-galactopyranoside, apigenin 6-C-β-arabinopyranosyl-8-C-β-galactopyranoside, luteolin 8-C-β-glucopyranoside, luteolin 6-C-β-glucopyranoside, apigenin 8-C-β-glucopyranoside, apigenin 6-C-β-glucopyranoside, luteolin 8-C-(2′′-O-(E)-p-coumaroyl-β-glucopyranoside), respectively, on the basis of data of previous reports [74]. Compounds No. 8 (RT 22.0, [M-H]− 593), No. 10 (RT 30.7, [M-H]− 557), No. 11 (RT 34.7, [M-H]− 593), No. 12 (RT 37.7, [M-H]− 449), and No. 13 (RT 37.8, [M-H]− 577) could not be tentatively identified. Based on the literature data [54,72,74,75], unidentified substances are probably flavone C-glycosides with apigenin or luteolin as aglycon.
The data obtained in this study are not sufficient for accurate identification of compounds. Therefore, further studies of the qualitative composition of phenolic compounds of FWV and FSV seeds are required. At the same time, the analysis made it possible to identify likely predominant phenolic compounds that will be the basis for future studies of the qualitative composition of phenolics of fenugreek seeds.
A series of experiments were designed 50 years ago to determine phenolic composition in unsorted fenugreek seeds. Thus, Seshadri et al. (1973) reported apigenin-6-C-glucoside and apigenin-8-C-glucoside in seeds of fenugreek [76]. Wagner et al. (1973) found apigenin-6-C-xyloside-8-C-glucoside (viccnin I) and apigenin-6.8-di-C-glucoside (viccnin 2) in addition to apigenin-8-C-glucoside in fenugreek seeds. Later, vitexin-2′-C-xoumarate was isolated from fenugreek [77]. Shang et al. (1998) identified five different flavonoids, namely vitexin, tricin, naringenin, quercetin, and tricin-7-O-β-d-glucopyranoside [78].
Rayyan et al. (2010) reported the flavone C-glycosides apigenin 6-C-beta-chinovopyranosyl-8-C-β-galactopyranoside and apigenin 6-C-β-xylopyranosyl-8-C-(6′′′-O-(3-hydroxy-3-methylglutaroyl)-β-glucopyranoside), in addition to the known flavone C-glycosides, apigenin 6-C-β-xylopyranosyl-8-C-β-galactopyranoside, apigenin 6-C-beta-arabinopyranosyl-8-C-β-galactopyranoside, luteolin 8-C-β-glucopyranoside, apigenin 8-C-β-glucopyranoside, apigenin 6-C-β-glucopyranoside, and apigenin 8-C-(2′′-O-(E)-p-coumaroyl-β-glucopyranoside). Then, apigenin 6,8-C-di-β-galactopyranoside, luteolin 6-C-β-glucopyranoside, and luteolin 8-C-(2′′-O-(E)-p-coumaroyl-β-glucopyranoside) were found in fenugreek seeds for the first time [74].
Phenolics such as quercetin 3-O-β-d-glucosyl (1→2)-β-d-galactoside 7-O-β-d-glucoside, vitexin, kaempferol 3-O-β-d-glucosyl (1→2)-β-d-galactoside 7-O-β-d-glucoside, kaempferol 3-O-glucoside-7-O-rhamnoside, isovitexin, quercetin 3,7-O-R-l-dirhamnoside, 3-O-R-l-rhamnosyl quercetin, kaempferol 3,7-O-R-l-dirhamnoside, kaempferol-3-O-R-l-rhamnoside, quercetin, luteolin, kaempferol, and apigenin were identified in fenugreek seeds with HPLC by [54].
Benayad et al. (2014) found 32 phenolic compounds in crude seeds of fenugreek by HPLC-DAD-ESI/MS that were flavonoid glycosides (apigenin, luteolin and kaempferol as aglycons) and phenolic acids (hydroxycinnamic and caffeic acid) [75].
Ethyl acetate crude extracts of fenugreek seeds were investigated for phenolic compounds using HPLC-MS [72]. As a result, 18 phenolic compounds were found in fenugreek seeds. Among them, the flavonoids apigenin-7-O-glycoside and luteolin-7-O-glycoside were predominant components.
Apparently, our results are in agreement with the results of other similar studies of fenugreek seeds by HPLC-MS that indicated flavones as predominant phenolic compounds in extract samples.
On the other hand, according to the above-mentioned reports the seed samples of non-sorted fenugreek contained more phenolic components.
The difference in phenolics between seed samples of non-sorted fenugreek and its varieties may be attributed to variation in biochemical parameters of varietal and non-sorted plants as well as growing region [79]. It also may depend on features of extraction and extract purification procedure.
Our findings indicate that phenolic systems of 70% ethanol extracts of FWV and FSW seeds presumably consist of flavonoid glycosides. Several studies have reported that fenugreek seeds are rich in flavonoids (more than 100 mg/100 g) [78,80]. Kaviarasan et al. (2004) suggested that flavonoids were the possible candidates that may explain the high antioxidant activity of the extracts from fenugreek seeds [28].
Based on the data obtained by HPLC-ESI-MS we can assume that high antioxidant activity is associated with the polyphenol composition in the extracts. It is known that antioxidant properties of flavonoids vary greatly, depending on the arrangement of functional groups regarding the nuclear structure and glycosylation [64,81]. According to Hirano et al. (2001), the flavone luteolin was found to be a very weak scavenger of DPPH free radicals compared to the flavonols quercetin, myricetin, and kaempferol [64,82]. Aglycones of flavonoids show higher antioxidant activity than their corresponding glycosides. For instance, the luteolin aglycone significantly exceeded its 3-,4′- and 7-O-glucosides in retarding the accumulation of hydroperoxides in membrane bilayers [64,67].
Comparing fenugreek varieties, differences between FSV and FWV were found for some parameters. In particular, extraction yield of FWV seeds with 30% ethanol was lower than that of FSV seeds. There was a difference in the total phenolic content and composition of phenolic systems of purified 70% ethanol extracts of fenugreek varieties. Varietal differences in TPC were established for onion [83], potato [84], and lantana [85].
However, despite the fact that the phenolic content is significantly higher in the 70% extract of the FSV compared to the FWV, there were no differences in the iron chelating ability, antiradical activity, and transition metal reducing power. Increased antioxidant activity of the phenolic system of FWV seeds may be one of the genetically determined mechanisms for enhancing its tolerance to cold stress. According to Mirmiran et al. (2018), in the investigation of the cold tolerance of numbers of Iranian fenugreek ecotypes, it was found that most of the studied fenugreek ecotypes showed high sensitivity to freezing. Therefore, winter varieties are created for fenugreek growing in the regions with cold winter [86]. Winter varieties have a number of adaptation responses which allow handling cold stress at the molecular, cellular, physiological, and biochemical levels [87]. At the biochemical level, secondary metabolites play a role in plant tolerance against environmental stressors [88]. Phenolics are generally recognized as compounds which are involved in stress protection in plants [89].
Meena et al. (2017) indicated that phenolic compounds protect tomato (Solanum lycopersicum L.) against low temperature stress [90]. Rivero et al. (2001) reported that growing of tomato and watermelon plants at 15 °C caused the accumulation of soluble phenolics [91]. Olenichenko et al. (2006) found that low temperature induced the accumulation of phenolic compounds in winter wheat leaves. Glycosides of flavones such as luteolin and apigenin were predominant phenolics in the leaves [92]. That is in accordance with Samec et al. (2020), who reported that flavones were involved in abiotic stress response including cold temperature stress [89].
4. Conclusions
The ethanol concentration in the extraction solvent affected extraction yield and recovery of phenolic compounds from seeds of the fenugreek varieties studied. With increasing water content in the solvent, the overall yield increased whereas TPC and antioxidant activity (antiradical activity, iron chelating ability, and TMRP) decreased. The 70% ethanol was the most suitable solvent providing maximal extraction of phenolics. Total phenolic content as well as the composition of the phenolic system of 70% ethanol extracts differed in seeds of FWV and FSV, while extracts of FWV and FSV seeds showed similar values for DPPH radical scavenging activity, iron chelating ability, and TMRP. The iron chelating ability and TMRP significantly correlated with total phenolics for all treatments. Summarizing the results of all in vitro tests used, 70% ethanol extracts had the highest antioxidant activity. Higher antioxidant activity of phenolic system of FWV may be an adaptation to cold stress. Based on HPLC-ESI-MS data, the phenolic profile of the purified 70% ethanol extracts of seeds of both fenugreek varieties predominantly contained the compounds of [M-H]− 431 to 593 tentatively identified as glycosides of the flavones apigenin and luteolin. High antioxidant activity allows the seeds of Ovari 4 and PSZ.G.SZ fenugreek varieties to be considered as sources of phenolic antioxidants along with non-sorted fenugreek.
Author Contributions
Conceptualization, H.L., M.S. and M.W.; methodology, H.L., M.S. and M.W.; software, H.L. and M.W.; validation, H.L., M.S. and M.W.; formal analysis, H.L. and M.S.; investigation, H.L.; resources, M.S. and M.W.; data curation, H.L.; writing—original draft preparation, H.L.; writing—review and editing, M.W.; visualization, H.L.; supervision, M.S. and M.W.; project administration, M.S. and M.W.; funding acquisition, M.S. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Belarusian Republican Foundation for Fundamental Research grant number 20131168 and by the German Academic Exchange Service (a research grant).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Ahmad Tahrani (IPMB, Heidelberg University) for the HPLC-ESI-MS procedure designing and the help with obtained data processing.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Shankar, K.; Mehendale, H.M. Oxidative stress. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Bethesda, MD, USA, 2014; pp. 735–737. [Google Scholar]
- Halliwell, B.; Cross, C.E. Oxygen-derived species: Their relation to human disease and environmental stress. Environ. Health Perspect. 1994, 102, 5–12. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Li, Z.; Wu, J.; Daleo, C.J. RNA damage and surveillance under oxidative stress. IUBMB Life 2006, 58, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Current understanding of modes of action of multicom multicomponent bioactive phytochemicals: Potential for nutraceuticals and antimicrobials. Annu. Rev. Food Sci. Technol. 2022, 13, 1–23. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Schröder, P.; Biesalski, H.K. Low molecular weight antioxidants. Handb. Environ. Chem. 2004, 20, 77–90. [Google Scholar]
- Horman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 652. [Google Scholar]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beveragesand spices: Antioxidant activity and health effects. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Board, N. Hand Book on Spices; Asia Pacific Business Press Inc.: Delhi, India, 2010; pp. 137–138. [Google Scholar]
- Basch, F.I.; Ulbricht, C.; Kuo, G.; Szapary, P.; Smith, M. Therapeutic applications of fenugreek. Altern. Med. Rev. 2003, 8, 20–27. [Google Scholar]
- Khorshidian, N.; Yousefi, M.; Arab, M. Fenugreek: Potential applications as a functional food and nutraceutical. Nutr. Food Sci. Res. 2016, 3, 5–16. [Google Scholar] [CrossRef]
- Yadav, U.S.C.; Baquer, N.Z. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef]
- Syed, Q.A.; Rashid, Z.; Ahmad, M.H.; Shukat, R.; Ishaq, A.; Muhammad, N.; Rahman, H.U.U. Nutritional and therapeutic properties of fenugreek (Trigonella foenum-graecum): A review. Int. J. Food Prop. 2020, 23, 1777–1791. [Google Scholar] [CrossRef]
- Premanath, R.; Sudisha, N.; Devi, N.L.; Aradhya, S.M. Antibacterial and anti-oxidant activities of fenugreek (Trigonella foenum graecum L.) leaves. Res. J. Med. Plant 2011, 5, 695–705. [Google Scholar] [CrossRef][Green Version]
- Yacoubi, L.; Rabaoui, L.; Hamdaoui, M.H.; Fattouch, S.; Serairi, R.; Kourda, N.; Khamsa, S.B. Anti-oxidative and anti-inflammatory effects of Trigonella foenum-graecum Linnaeus, (Fenugreek) seed extract in experimental pulmonary fibrosis. J. Med. Plants Res. 2011, 5, 4315–4325. [Google Scholar]
- Anuradha, C.V.; Ravikumar, P. Antilipid peroxidative activity of seeds of fenugreek (Trigonella foenum-graecum). Med. Sci. Res. 1998, 26, 317–321. [Google Scholar]
- Anuradha, C.V.; Ravikumar, P. Restoration of tissue anti-oxidants by fenugreek (Trigonella foenum-graecum) seeds in alloxan-diabetic. Indian J. Physiol. Pharmacol. 2001, 45, 408–420. [Google Scholar] [PubMed]
- El-Malky, W.A.; Gouda, H.A. Effect of green leaves and germination and boiling treatments of fenugreek and lupin seeds on chemical composition, serum glucose, lipid profile and hepatic enzymes of rats. Egypt. J. Biomed. Sci. 2007, 23, 39–59. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chemistry 2007, 103, 31–37. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Viswanathan, P.; Viswanathan, C.V. Fenugreek seed (Trigonella foenum-graecum) polyphenols inhibit ethanol-induced collagen and lipid accumulation in rat liver. Cell. Biol. Toxicol. 2007, 23, 373–380. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Ramamurty, N.; Gunasekaran, P.; Varalakshmi, E.; Anuradha, C.V. Fenugreek (Trigonella foenum-graecum) Seed Extract Prevents Ethanol-Induced Toxicity And Apoptosis In Chang Liver Cells. Alcohol Alcohol. 2006, 41, 267–273. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Vijayalakshmi, K.; Anuradha, C.V. Polyphenol-rich extract of fenugreek seeds protect erythrocytes from oxidative damage. Plant Foods Hum. Nutr. 2004, 59, 143–147. [Google Scholar] [CrossRef]
- Thirunavukkarasu, V.; Anuradha, C.V. Gastroprotective effect of fenugreek seeds (Trigonella foenum-graecum) on experimental gastric ulcer in rats. J. Herbs Spices Med. Plants 2006, 12, 13–25. [Google Scholar] [CrossRef]
- Subhashini, N.; Thangathirupathi, A.; Lavanya, N. Antioxidant activity of Trigonella foenum graecum using various in vitro and ex vivo models. Int. J. Pharm. Pharm. Sci. 2011, 3, 96–102. [Google Scholar]
- Bukhari, S.B.; Bhanger, M.I.; Memon, S. 83 Antioxidative activity of extracts from fenugreek seeds (Trigonella foenum-graecum). Pak. J. Anal. Environ. Chem. 2008, 9, 78–83. [Google Scholar]
- Parvizpur, A.; Ahmadiani, A.; Kamalinejad, M. Probable role of spinal purinoceptors in the analgesic effect of Trigonella foenum-graecum (TFG) leaves extract. J. Ethnopharmacol. 2006, 104, 108–112. [Google Scholar] [CrossRef]
- Parvizpur, A.; Ahmadiani, A.; Kamalinejad, M. Spinal serotonergic system is partially involved in antinociception induced by Trigonella foenum-graecum (TFG) leaf extract. J. Ethnopharmacol. 2004, 95, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, S.; Anuradha, C.V. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin and metformin in a rat model. Indian. J. Med. Res. 2009, 129, 401–408. [Google Scholar] [PubMed]
- Sushma, N.; Devasena, T. Aqueous extract of Trigonella foenum-graecum (fenugreek) prevents cypermethrin-induced hepatotoxicity and nephrotoxicity. Hum. Exp. Toxicol. 2010, 29, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Jóźwik, A.; Łysek-Gładysińska, M.; Grzybek, W.; Adamus-Białek, W.; Bicki, J.; Strzałkowska, N.; Kamińska, A.; Horbańczuk, O.K.; Atanasov, A.G. Fenugreek (Trigonella foenum-graecum L.) Seeds Dietary Supplementation Regulates Liver Antioxidant Defense Systems in Aging Mice. Nutrients 2020, 12, 2552. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kalaiselvan, V.; Srivastava, S.; Saxena, R.; Agrawal, S.S. Trigonella foenum-graecum (fenugreek) protects against selenite-induced oxidative stress in experimental cataractogenesis. Biol. Trace Elem. Res. 2010, 136, 258–268. [Google Scholar] [CrossRef]
- Sauvare, Y.; Pett, P.; Baissao, Y.; Ribes, G. Chemistry and pharmacology of fenugreek. In Herbs, Botanicals and Teas; Mazza, G., Oomah, B.D., Eds.; Technomic Publishing Company Inc.: Lancaster, PA, USA, 2000; pp. 107–129. [Google Scholar]
- Alwahebi, M.; Soliman, D. Evaluating the antibacterial activity of fenugreek (Trigonella foenum-graecum) seed extract against a selection of different pathogenic bacteria. J. Pure Appl. Microbiol. 2014, 8, 817–821. [Google Scholar]
- Randhir, R.; Lin, Y.T.; Shetty, K. Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac. J. Clin. Nutr. 2004, 13, 295–307. [Google Scholar]
- Norziah, M.H.; Fezea, F.A.; Bhat, F.A.; Bhat, R.; Ahmad, M. Effect of extraction solvents on antioxidant and antimicrobial properties of fenugreek seeds (Trigonella foenum-graecum L.). Int. Food Res. J. 2015, 22, 1261–1271. [Google Scholar]
- Luan, G.; Wang, Y.; Wang, Z.; Zhou, W.; Hu, N.; Li, G.; Wang, H. Flavonoid glycosides from fenugreek seeds regulate glycolipid metabolism by improving mitochondrial function in 3t3-l1 adipocytes in vitro. J. Agric. Food Chem. 2018, 66, 3169–3178. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Hikmawanti, N.P.E.; Fatmawati, S.; Asri, A.W. The Effect of Ethanol Concentrations as The Extraction Solvent on Antioxidant Activity of Katuk (Sauropus androgynus (L.) Merr.) Leaves Extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012060. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Wijekoon, M.M.J.O.; Bhat, R.; Karim, A.A. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack) inflorescence. J. Food Compos. Anal. 2011, 24, 615–619. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Houghton, P.J.; Raman, A. Laboratory Handbook for the Fractionation of Natural Extracts. In Laboratory Handbook for the Fractionation of Natural Extracts; Springer Science Business Media: London, UK, 1998. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Chatterjee, S.; Variyar, P.S.; Sharma, A. Effect of γ-irradiation on the antioxidant activity of fenugreek (Trigonella Foenum-graecum) seed extract. J. Environ. Agric. Food Chem. 2011, 10, 2798–2805. [Google Scholar]
- Kim, H.S.; Chin, K.B. Food Science of Animal Resources Evaluation of Antioxidative Activity of Various Levels of Ethanol Extracted Tomato Powder and Application to Pork Patties. Korean J. Food Sci. Anim. Resour. 2017, 37, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadj, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.; Thuruthiyil, D.T. Evaluation of the antioxidant potential of pittosporum dasycaulon miq. stem bark. Food Sci. Biotechnol. 2014, 23, 2045–2052. [Google Scholar] [CrossRef]
- Rababah, T.M.; Hettiarachchy, N.S.; Horax, R. Total Phenolics and Antioxidant Activities of Fenugreek, Green Tea, Black Tea, Grape Seed, Ginger, Rosemary, Gotu Kola, and Ginkgo Extracts, Vitamin E, and tert-Butylhydroquinone. Am. Chem. Soc. 2004, 52, 5183–5186. [Google Scholar] [CrossRef]
- Garti, N.; Madar, Z.; Aserin, A.; Sternheim, B. Fenugreek galactomannans as food emulsifiers. Food Sci. Technol. 1997, 30, 305–311. [Google Scholar] [CrossRef]
- Jiang, J.X.; Zhu, L.W.; Zhang, W.M.; Sun, R.C. Characterization of Galactomannan Gum from Fenugreek (Trigonella foenum-graecum) Seeds and Its Rheological Properties. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 1145–1154. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, M.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From In Vitro Results to In Vivo Evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Cheng, I.F.; Breen, K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. Biometals 2000, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ratty, A.K.; Das, N.P. Effects of flavonoids on nonenzymatic lipid peroxidation: Structure-activity relationship. Biochem Med Metab Biol 1988, 39, 69–79. [Google Scholar] [CrossRef]
- Mora, A.; Paya, M.; Rios, J.L.; Alcaraz, M.J. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem. Pharmacol. 1990, 40, 793–797. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Ironchelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Arefin, S.; Islam, T.; Hossain, T. Proximate analysis, phytochemical screening and antioxidant activity of Tagetes erecta flower growing in coastal area of Bangladesh. J. Glob. Biosci. 2015, 4, 2060–2066. [Google Scholar]
- Aparadh, V.T.; Naik, V.V.; Karadge, B.A. Antioxidative properties (TPC, DPPH, FRAP, metal chelating ability, reducing power and TAC) within some Cleome species. Ann. Bot. 2012, 2, 49–56. [Google Scholar]
- Foti, M.C.; Amorati, R. Non-phenolic radical-trapping antioxidants. J. Pharm. Pharmacol. 2009, 61, 1435–1448. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Hawage, C.M.; Brunton, N.P. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef]
- Boubakri, H.; Jdey, A.; Taamalli, A.; Taamalli, W.; Jebara, M.; Brini, F.; Riciputi, Y.; Pasini, F.; Cristian, M.; Verardo, V. Phenolic composition as measured by liquid chromatography/mass spectrometry and biological properties of Tunisian barley. Int. J. Food Prop. 2017, 20, 1783–1797. [Google Scholar] [CrossRef]
- Rayyan, S.; Fossen, T.; Andersen, Ø.M. Flavone C-Glycosides from Seeds of Fenugreek, Trigonella foenum-graecum L. J. Agric. Food Chem 2010, 58, 7211–7217. [Google Scholar] [CrossRef] [PubMed]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, T.R.; Varshney, I.P.; Sood, A.R. Glycosides from Trigonella corniculata and Trigonella foenum-graecum Linn, seeds. Curr. Sei. 1973, 42, 412–414. [Google Scholar]
- Wagner, H.; Iyengar, M.A.; Horhammer, L. Vicenin-1 and -2 in the seeds of Trigonella foenumgraecum. Phytochemistry 1973, 12, 2548. [Google Scholar] [CrossRef]
- Shang, M.; Cais, H.J.; Li, J.; Zhao, Y.; Zheng, J.; Namba, T.; Kadota, S.; Tezuka, Y.; Fan, W. Studies on flavonoids from fenugreek (Trigonella foenum graecum L). Zhongguo Zhong Yao Za Zhi 1998, 23, 614–639. [Google Scholar]
- Ram, S.G.; Thiruvengadam, V.; Vinod, K.K. Genetic diversity among cultivars, landraces and wild relatives of rice as revealed by microsatellite markers. J. Appl. Genet. 2007, 48, 337–345. [Google Scholar] [CrossRef]
- Gupta, P.C.; Nair, A.K. Antioxidant flavonoids in common Indian diet. South Asian J. Prev. Cardiol. 1999, 3, 83–94. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Meyers, K.J.; Heide, J.V.D.; Liu, R.H. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Al-Saikhan, M.S.; Howard, L.R.; Miller, J.C. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L.). J. Food Sci. 1995, 60, 341–343. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 22, 560. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, S.M.; Nezami, A.; Kafi, M. Evaluation of freezing tolerance in fenugreek (Trigonella foenum-graceum L.). IJFCS Res. 2018, 16, 299–315. [Google Scholar]
- Huber, A.E.; Bauerle, T.L. Long-distance plant signaling pathways in response multiple stressors: The gap in knowledge. J. Exp. Bot. 2016, 67, 2063–2079. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Samec, D.; Karalija, E.; Sola, I.; Bok, V.V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Meena, Y.K.; Khurana, D.S.; Kaur, N.; Singh, K. Phenolic compounds enhanced low temperature stress tolerance in tomato (Solanum lycopersicum L.). Br. J. Appl. Sci. Technol. 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Garcia, P.C.; Lopez-Lefebre, L.R.; Sanchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Olenichenko, N.A.; Ossipov, V.I.; Zagoskina, N. Effect of cold hardening on the phenolic complex of winter wheat leaves. Russ. J. Plant Physiol. 2006, 53, 495–500. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).