Bill Variation of Captive and Wild Chukar Partridge Populations: Shape or Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Molecular Sexing
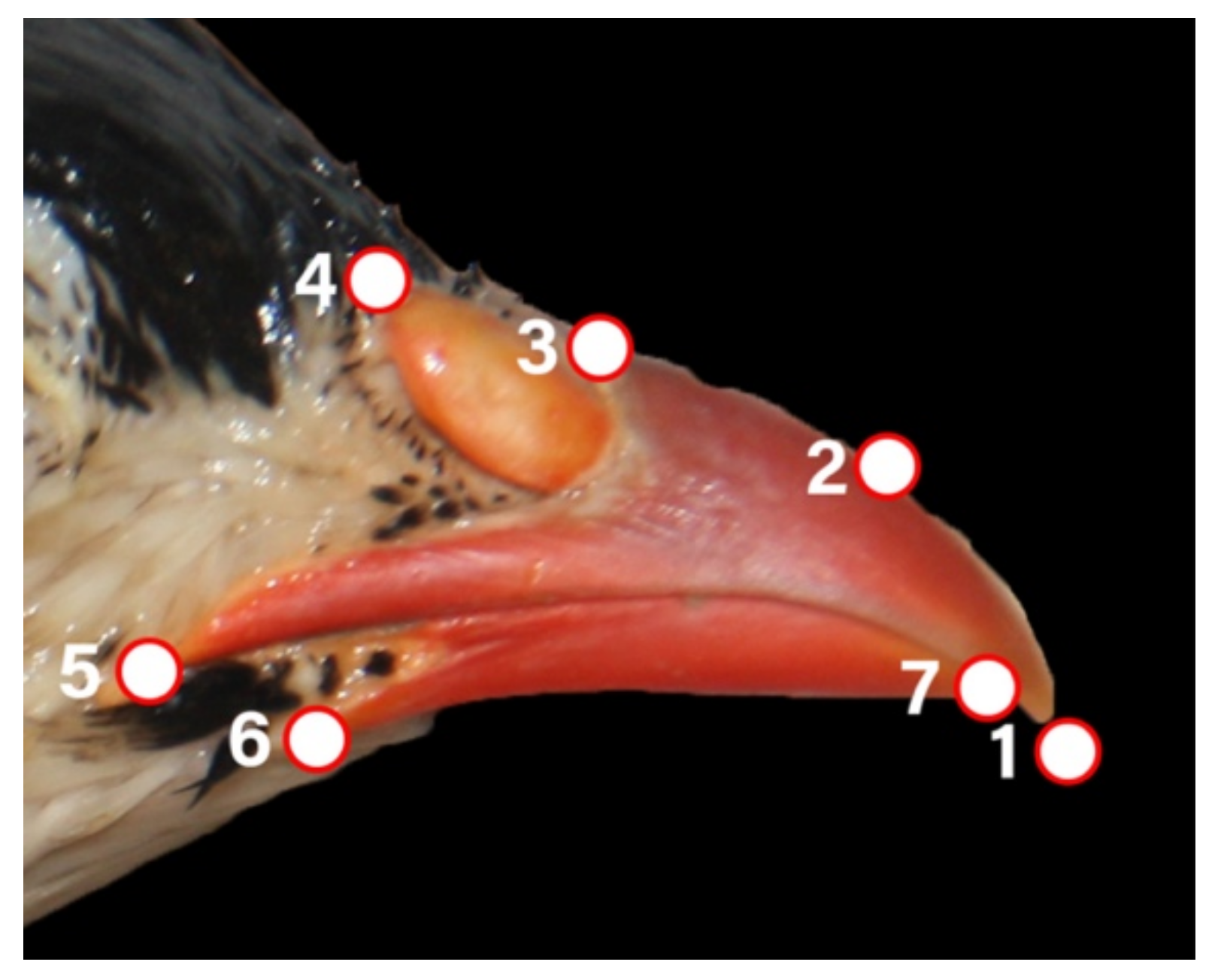
2.3. Morphometry
2.4. Geometric Morphometry
3. Results
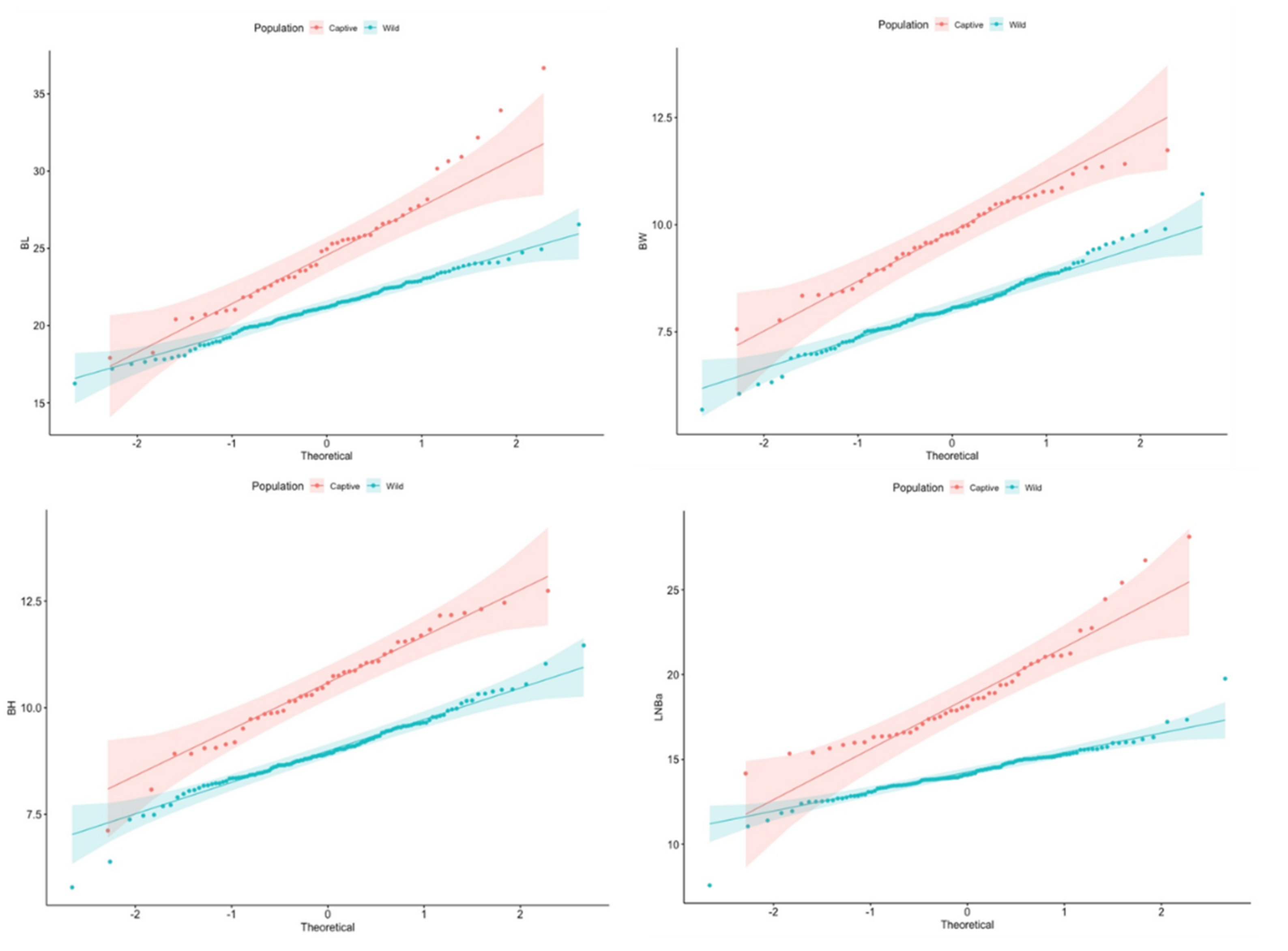
3.1. Population Differences
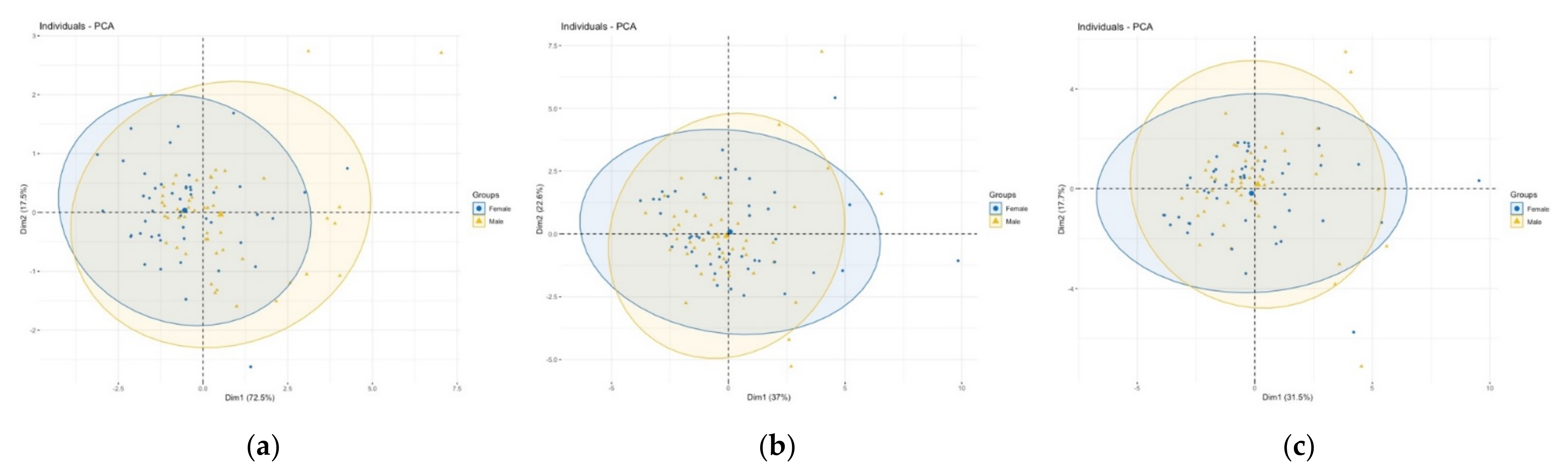
3.2. Comparison of Size and Shape
4. Discussion
4.1. Population Differences
4.2. Comparison of Size and Shape
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivas, N.; Sánchez Espíndola, M.E.; Camacho, A.D.; Moreno, E.R.; Rocha-Gómez, M.A.; Aguilar, R.A. Morphology and morphometry of the scutellum of six species in the genus Meccus (Hemiptera: Triatominae). J. Vector Ecol. 2014, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Papežík, P.; Kubala, M.; Jablonski, D.; Doležálková-Kaštánková, M.; Choleva, L.; Benovics, M.; Mikulíček, P. Morphological differentiation of endemic water frogs (Ranidae: Pelophylax) from the southwestern balkans. Salamandra 2021, 57, 105–123. [Google Scholar]
- Kadry, M.A.M.; Al-Qahtani, A.R.; Amer, S.A.M. Morphometric and molecular differentiation between Egyptian Stellagama stellio vulgaris and S. stellio salehi (Reptilia: Agamidae). Zool. Middle East 2020, 66, 295–301. [Google Scholar] [CrossRef]
- Islam, M.M.; Farag, E.; Mahmoudi, A.; Hassan, M.M.; Atta, M.; Mostafavi, E.; Alnager, I.A.; Farrag, H.A.; Eljack, G.E.A.; Bansal, D.; et al. Morphometric study of Mus musculus, Rattus norvegicus, and Rattus rattus in Qatar. Animals 2021, 11, 2162. [Google Scholar] [CrossRef]
- Kayvanfar, N.; Aliabadian, M.; Ghasempouri, S.M. Morphometric and morphological differentiation of the subspecies of Phasianus colchicus (Linnaeus, 1758) on the Iranian Plateau (Aves: Galliformes). Zool. Middle East 2015, 61, 9–17. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, J.; Gou, J.; Gao, X. Geographic variation in the skull morphometry of four populations of Batrachuperus karlschmidti (Urodela: Hynobiidae). Asian Herpetol. Res. 2020, 11, 194–204. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef]
- Myczko, Ł.; Mizerová, Z.; Kubicka, A.M.; Sparks, T.H.; Hromada, M. Bill morphology and biometrics of three sibling woodpecker species from sympatric populations. Bird Study 2020, 67, 8–18. [Google Scholar] [CrossRef]
- Angst, D.; Barnoud, J.; Cornette, R.; Chinsamy, A. Sex and Ontogenetic Variation in the Crest of Numida meleagris: Implications for Crested Vertebrates. Anat. Rec. 2020, 303, 1018–1034. [Google Scholar] [CrossRef]
- Dalton, H.A.; Wood, B.J.; Widowski, T.M.; Guerin, M.T.; Torrey, S. An analysis of beak shape variation in two ages of domestic turkeys (Meleagris gallopavo) using landmark-based geometric morphometrics. PLoS ONE 2017, 12, e0185159. [Google Scholar] [CrossRef]
- Foster, D.J.; Podos, J.; Hendry, A.P. A geometric morphometric appraisal of beak shape in Darwin’s finches. J. Evol. Biol. 2008, 21, 263–275. [Google Scholar] [CrossRef]
- Souza, A.T.; Soukalová, K.; Děd, V.; Šmejkal, M.; Blabolil, P.; Říha, M.; Jůza, T.; Vašek, M.; Čech, M.; Peterka, J.; et al. Ontogenetic and interpopulation differences in otolith shape of the European perch (Perca fluviatilis). Fish. Res. 2020, 230, 105673. [Google Scholar] [CrossRef]
- Wells, C.; Munn, A.; Woodworth, C. Geomorphic Morphometric Differences between Populations of Speyeria diana (Lepidoptera: Nymphalidae). Florida Entomol. 2018, 101, 195–202. [Google Scholar] [CrossRef]
- Slice, D.E. Geometric morphometrics. Annu. Rev. Anthropol. 2007, 36, 261–281. [Google Scholar] [CrossRef]
- Ariza-Marín, E.R.; De Luna, E. Linear and geometric morphometric analyses of variation of the plectrum in four species of bess beetles, tribe Proculini (Coleoptera: Passalidae). Arthropod Struct. Dev. 2020, 59, 100994. [Google Scholar] [CrossRef]
- Corruccini, R.S. Shape in morphometrics: Comparative analyses. Am. J. Phys. Anthropol. 1987, 73, 289–303. [Google Scholar] [CrossRef]
- Zelditch, M.; Swiderski, D.; Sheets, H.; Fink, W. Geometric Morphometrics for Biologists; Elsevier: New York, NY, USA, 2004; ISBN 9780127784601. [Google Scholar]
- Cardini, A. Modern morphometrics and the study of population differences: Good data behind clever analyses and cool pictures? Anat. Rec. 2020, 303, 2747–2765. [Google Scholar] [CrossRef]
- Cardini, A.; Elton, S.; Kovarovic, K.; Strand Viđarsdóttir, U.; Polly, P.D. On the Misidentification of Species: Sampling Error in Primates and Other Mammals Using Geometric Morphometrics in More than 4000 Individuals. Evol. Biol. 2021, 48, 190–220. [Google Scholar] [CrossRef]
- Gray, J.A.; McDowell, M.C.; Hutchinson, M.N.; Jones, M.E.H. Geometric morphometrics provides an alternative approach for interpreting the affinity of fossil lizard jaws. J. Herpetol. 2017, 51, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Kaliontzopoulou, A. Geometric morphometrics in herpetology: Modern tools for enhancing the study of morphological variation in amphibians and reptiles. Basic Appl. Herpetol. 2011, 25, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Tatsuta, H.; Takahashi, K.H.; Sakamaki, Y. Geometric morphometrics in entomology: Basics and applications. Entomol. Sci. 2018, 21, 164–184. [Google Scholar] [CrossRef] [Green Version]
- Sumruayphol, S.; Chaiphongpachara, T. Geometric morphometrics as a tool for three species identification of the firefly (Coleoptera: Lampyridae) in Thailand. Biodiversitas 2019, 20, 2388–2395. [Google Scholar] [CrossRef]
- Bright, J.A.; Marugán-Lobón, J.; Cobb, S.N.; Rayfield, E.J. The shapes of bird beaks are highly controlled by nondietary factors. Proc. Natl. Acad. Sci. USA 2016, 113, 5352–5357. [Google Scholar] [CrossRef] [Green Version]
- Carvalho Provinciato, I.C.; Araújo, M.S.; Jahn, A.E. Drivers of wing shape in a widespread Neotropical bird: A dual role of sex-specific and migration-related functions. Evol. Ecol. 2018, 32, 379–393. [Google Scholar] [CrossRef] [Green Version]
- Gündemir, O.; Özkan, E.; Dayan, M.O.; Aydoğdu, S. Sexual analysis in Turkey (Meleagris gallopavo) neurocranium using geometric morphometric methods. Turkish J. Vet. Anim. Sci. 2020, 44, 681–687. [Google Scholar] [CrossRef]
- Tokita, M.; Yano, W.; James, H.F.; Abzhanov, A. Cranial shape evolution in adaptive radiations of birds: Comparative morphometrics of Darwin’s finches and Hawaiian honeycreepers. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20150481. [Google Scholar] [CrossRef] [Green Version]
- Haidr, N.S.; Acosta Hospitaleche, C. Fossil penguin beaks from the Eocene of Antarctica: New materials from La Meseta Formation. Contrib. MACN 2017, 1, 57–68. [Google Scholar]
- Kass, N.; Montalti, D.; Acosta Hospitaleche, C. Comparison of the skull of Brown Skua (Catharacta antarctica lonnbergi) and South Polar Skua (Catharacta maccormicki): Differentiation source identification and discriminant analysis. Polar Biol. 2018, 41, 1049–1053. [Google Scholar] [CrossRef]
- Ottvall, R.; Gunnarsson, G. Morphological and molecular sex identification of Redshanks Tringa totanus. Bird Study 2007, 54, 127–129. [Google Scholar] [CrossRef]
- Albayrak, T.; Besnard, A.; Erdogan, A. Morphometric Variation and Population Relationships of Kruper’s Nuthatch (Sitta krueperi) in Turkey. Wilson J. Ornithol. 2011, 123, 734–740. [Google Scholar] [CrossRef]
- Madge, S.; McGowan, P. Pheasants, Partridges, and Grouse: A Guide to the Pheasants, Partridges, Quails, Grouse, Guineafowl, Buttonquails, and Sandgrouse of the World; Christopher Helm, A & C Black: London, UK, 2002. [Google Scholar]
- Svensson, L. Identification Guide to European Passerines; British Trust for Ornithology: Norfolk, UK, 1992. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org (accessed on 3 March 2021).
- Rohlf, F.J. TpsUtil, Version 1.60; Department of Ecology and Evolution, State University of New York: Stone Brook, NY, USA, 2013.
- Rohlf, F.J. TpsDig2, Version 2.18; Department of Ecology and Evolution, State University of New York: Stone Brook, NY, USA, 2015.
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Christensen, G.C. Chukar (Alectoris chukar), version 1.0. In Birds of the World; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, Greece, 2020. [Google Scholar]
- Ewen, J.G.; Clarke, R.H.; Moysey, E.; Boulton, R.L.; Crozier, R.H.; Clarke, M.F. Primary sex ratio bias in an endangered cooperatively breeding bird, the black-eared miner, and its implications for conservation. Biol. Conserv. 2001, 101, 137–145. [Google Scholar] [CrossRef]
- Robertson, B.C.; Elliott, G.P.; Eason, D.K.; Clout, M.N.; Gemmell, N.J. Sex allocation theory aids species conservation. Biol. Lett. 2006, 2, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forcina, G.; Guerrini, M.; Khaliq, I.; Khan, A.A.; Barbanera, F. Human-modified biogeographic patterns and conservation in game birds: The dilemma of the black francolin (Francolinus francolinus, Phasianidae) in Pakistan. PLoS ONE 2018, 13, e0205059. [Google Scholar] [CrossRef] [Green Version]
- Aradis, A.; Landucci, G.; Tagliavia, M.; Bultrini, M. Sex determination of Eurasian Woodcock Scolopax rusticola: A molecular and morphological approach. Avocetta 2015, 39, 83–89. [Google Scholar]
- Kabasakal, B.; Albayrak, T. Offspring sex ratios and breeding success of a population of the Great Tit, Parus major (Aves: Passeriformes). Zool. Middle East 2012, 57, 27–34. [Google Scholar] [CrossRef]
- Kark, S.; Mukerji, T.; Safriel, U.N.; Noy-Meir, I.; Nissani, R.; Darvasi, A. Peak morphological diversity in an ecotone unveiled in the chukar partridge by a novel Estimator in a Dependent Sample (EDS). J. Anim. Ecol. 2002, 71, 1015–1029. [Google Scholar] [CrossRef]
- Albayrak, T.; Pekgöz, A.K. Heavy metal effects on bird morphometry: A case study on the house sparrow Passer domesticus. Chemosphere 2021, 276, 130056. [Google Scholar] [CrossRef]
- Asthon, K.G. Patterns of Within-Species Body Size Variation of Birds: Strong Evidence for Bergmann’s. Glob. Ecol. Biogeogr. 2002, 11, 505–523. [Google Scholar]
- Barbosa, A.; Moreno, E. Evolution of foraging strategies in shorebirds: An ecomorphological approach. Auk 1999, 116, 712–725. [Google Scholar]
- Lachlan, R.F.; Verhagen, L.; Peters, S.; Ten Cate, C. Are There Species-Universal Categories in Bird Song Phonology and Syntax? A Comparative Study of Chaffinches (Fringilla coelebs), Zebra Finches (Taenopygia guttata), and Swamp Sparrows (Melospiza georgiana). J. Comp. Psychol. 2010, 124, 92–108. [Google Scholar] [CrossRef]




| Captive | Wild | Overall | |||||
|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | p | |
| (n = 12) | (n = 10) | (n = 36) | (n = 40) | (n = 48) | (n = 50) | ||
| BL | ** | ||||||
| Mean (SD) | 23.8 (3.18) | 26.9 (4.48) | 20.3 (1.85) | 21.8 (1.42) | 21.2 (2.71) | 22.9 (3.07) | |
| Median [Min, Max] | 23.5 [17.9, 30.6] | 26.1 [20.8, 36.7] | 20.1 [16.3, 23.4] | 21.6 [18.9, 24.9] | 21.1 [16.3, 30.6] | 21.9 [18.9, 36.7] | |
| BW | ns | ||||||
| Mean (SD) | 9.50 (0.907) | 10.3 (0.861) | 7.81 (0.814) | 8.25 (0.766) | 8.23 (1.11) | 8.66 (1.14) | |
| Median [Min, Max] | 9.56 [7.77, 11.2] | 10.6 [8.34, 11.4] | 7.89 [5.68, 9.85] | 8.09 [6.05, 9.90] | 8.02 [5.68, 11.2] | 8.21 [6.05, 11.4] | |
| BH | |||||||
| Mean (SD) | 9.96 (1.01) | 11.3 (1.04) | 8.52 (0.704) | 9.39 (0.715) | 8.88 (1.00) | 9.77 (1.09) | ** |
| Median [Min, Max] | 10.0 [8.08, 11.3] | 11.6 [9.19, 12.5] | 8.67 [5.79, 9.93] | 9.44 [7.69, 11.5] | 8.76 [5.79, 11.3] | 9.57 [7.69, 12.5] | |
| LNBa | * | ||||||
| Mean (SD) | 17.6 (1.60) | 20.8 (2.99) | 14.0 (1.07) | 14.7 (1.02) | 14.9 (2.01) | 15.9 (2.92) | |
| Median [Min, Max] | 16.9 [16.0, 21.0] | 20.0 [17.5, 28.1] | 13.9 [11.4, 16.0] | 14.6 [12.7, 17.3] | 14.4 [11.4, 21.0] | 15.1 [12.7, 28.1] | |
| Bill | |||||
|---|---|---|---|---|---|
| Location | n | BL | BH | BW | LNBa |
| Western | |||||
| BUR | 13 | 21.8 (1.6) | 9.2 (0.7) | 8.5 (0.8) | 14.4 (0.8) |
| CAN | 9 | 20.4 (1.9) | 8.8 (1.4) | 7.5 (0.8) | 13.9 (1.6) |
| ESK | 21 | 20.4 (1.6) | 8.6 (0.8) | 7.9 (0.4) | 13.6 (0.9) |
| MUG | 8 | 20.3 (2.6) | 9.3 (0.6) | 8.9 (0.7) | 13.7 (2.9) |
| Centre | |||||
| CNK | 14 | 21.1 (1.3) | 8.7 (0.6) | 7.7 (0.9) | 14.6 (1.1) |
| NIG | 3 | 20.5 (0.7) | 8.7 (0.4) | 8.2 (0.9) | 13.8 (1.2) |
| SIV | 11 | 21.2 (1.6) | 9.5 (0.9) | 8.4 (0.7) | 14.2 (0.9) |
| Eastern | |||||
| BIT | 12 | 21.8 (1.7) | 8.9 (0.5) | 7.9 (0.5) | 14.7 (0.9) |
| ERZ | 12 | 20.9 (1.6) | 8.8 (0.8) | 7.9 (0.6) | 14.1 (1.1) |
| VAN | 11 | 22.8 (1.5) | 9.3 (0.6) | 7.9 (0.9) | 14.9 (0.9) |
| Unknown | 14 | 22.1 (3.1) | 9.2 (0.8) | 8.4 (1.0) | 14.7 (2.9) |
| Captive | |||||
| BSA | 6 | 25.4 (3.5) | 9.0 (1.0) | 8.2 (0.5) | 19.0 (2.9) |
| BSK | 21 | 26.4 (4.1) | 11.0 (1.1) | 10.1 (0.8) | 20.2 (3.4) |
| BSM | 17 | 22.8 (2.6) | 10.4 (0.9) | 10.0 (0.9) | 17.0 (1.4) |
| Total | |||||
| Wild | 128 | 21.3 (1.9) | 8.9 (0.8) | 8.1 (0.8) | 14.3 (1.5) |
| Captive | 44 | 24.9 (3.8) | 10.5 (1.2) | 9.8 (1.0) | 18.8 (3.1) |
| Total | 172 | 22.2 (3.0) | 9.4 (1.1) | 8.5 (1.1) | 15.4 (2.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albayrak, T.; Aytek, A.İ. Bill Variation of Captive and Wild Chukar Partridge Populations: Shape or Size. Diversity 2022, 14, 48. https://doi.org/10.3390/d14010048
Albayrak T, Aytek Aİ. Bill Variation of Captive and Wild Chukar Partridge Populations: Shape or Size. Diversity. 2022; 14(1):48. https://doi.org/10.3390/d14010048
Chicago/Turabian StyleAlbayrak, Tamer, and Ahmet İhsan Aytek. 2022. "Bill Variation of Captive and Wild Chukar Partridge Populations: Shape or Size" Diversity 14, no. 1: 48. https://doi.org/10.3390/d14010048
APA StyleAlbayrak, T., & Aytek, A. İ. (2022). Bill Variation of Captive and Wild Chukar Partridge Populations: Shape or Size. Diversity, 14(1), 48. https://doi.org/10.3390/d14010048






