Butterfly Community Diversity in the Qinling Mountains
Abstract
1. Introduction
2. Materials and Methods
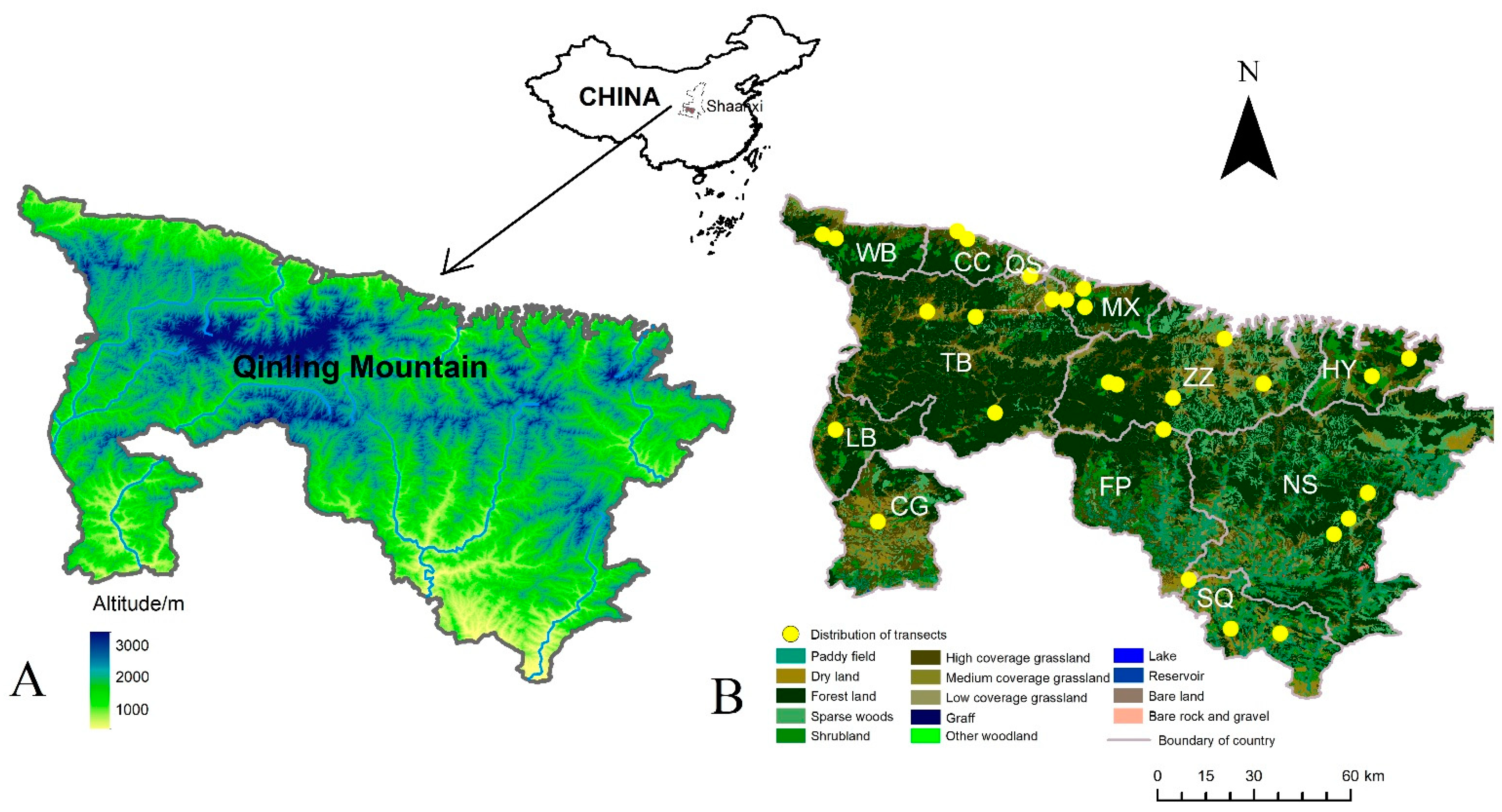
2.1. Study Region
2.2. Data Collection
2.3. Analysis Methods
3. Results
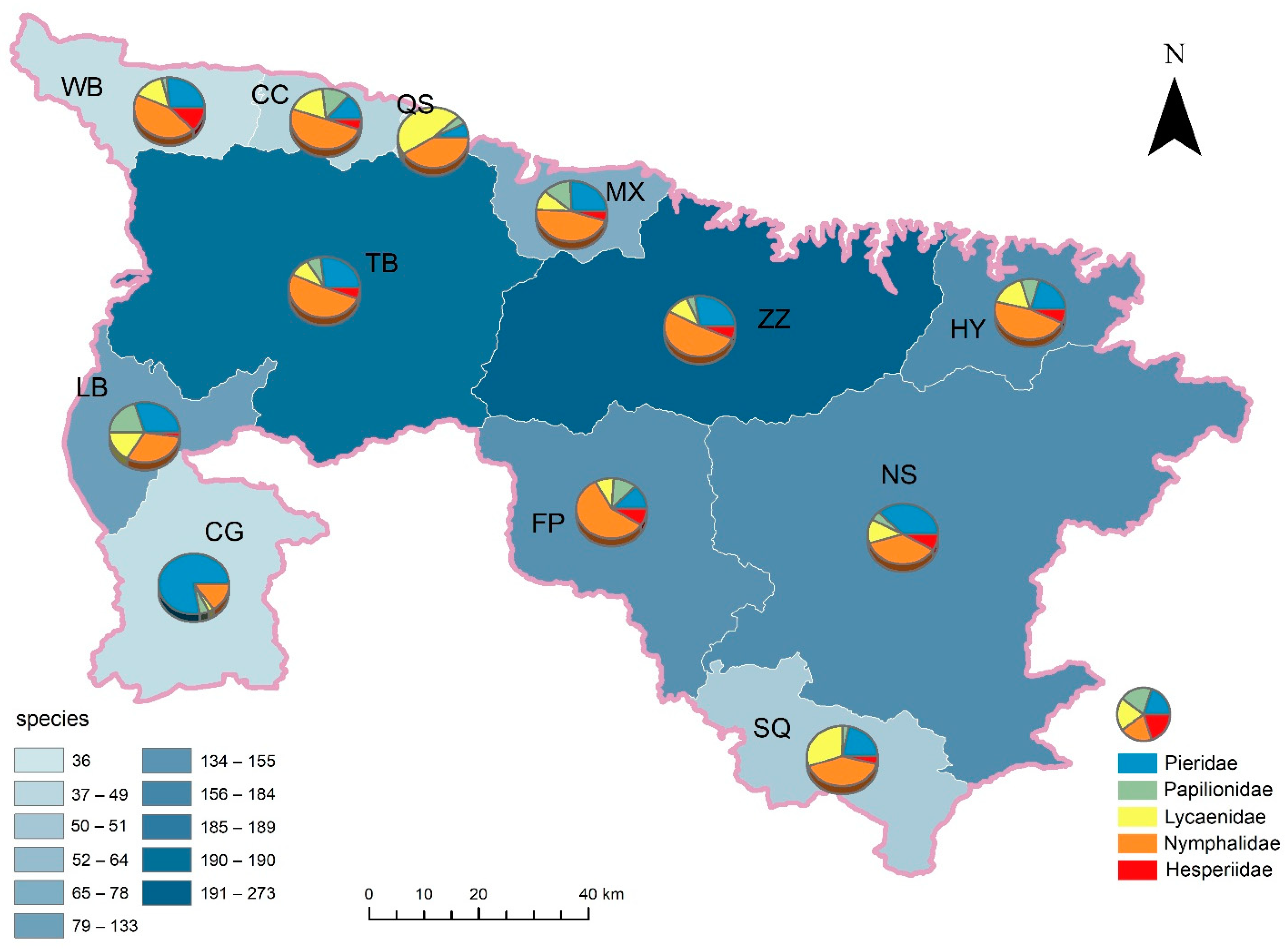
3.1. Species Richness and Its Spatial Pattern
3.2. Butterfly Alpha Diversity Patterns
3.3. Butterfly Beta Diversity Pattern
3.4. Evaluation of Endangered and Protected Butterflies in the Middle Qinling Mountains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, K. New opportunities for mainstreaming biodiversity conservation. Biodivers. Sci. 2015, 23, 557–558. [Google Scholar] [CrossRef][Green Version]
- Paul, V.M.; Ho, V.C. Farmers, biodiversity and plant protection: Developing a learning environment for sustainable tree cropping systems. Int. J. Agric. Sustain. 2004, 2, 67–76. [Google Scholar]
- Crist, E.; Mora, C.; Engelman, R. The interaction of human population, food production, and biodiversity protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- You, M.S. Conservation and utilization of the insect diversity in China. Chin. Biodivers. 1997, 5, 135–141. [Google Scholar]
- Sayer, C.A.; Carr, J.A.; Darwall, W.R.T. A critical sites network for freshwater biodiversity in the Lake Victoria Basin. Fish. Manag. Ecol. 2018, 26, 435–443. [Google Scholar] [CrossRef]
- Kaschner, K.; Tittensor, D.P.; Ready, J.; Gerrodette, T.; Worm, B. Current and Future Patterns of Global Marine Mammal Biodiversity. PLoS ONE 2011, 6, e19653. [Google Scholar] [CrossRef] [PubMed]
- Linquist, S.; Varner, G.; Newman, J. Precis of defending biodiversity. Biol. Philos. 2020, 35, 1–4. [Google Scholar] [CrossRef]
- Pilotto, F.; Kühn, I.; Adrian, R.; Alber, R.; Alignier, A.; Andrews, C.; Bäck, J.; Barbaro, L.; Beaumont, D.; Beenaerts, N.; et al. Meta-analysis of multidecadal biodiversity trends in Europe. Nat. Commun. 2020, 11, 3486. [Google Scholar] [CrossRef]
- Nowicki, P.; Settele, J.; Henry, P.-Y.; Woyciechowski, M. Butterfly Monitoring Methods: The ideal and the Real World. Isr. J. Ecol. Evol. 2008, 54, 69–88. [Google Scholar] [CrossRef]
- Forister, M.L.; McCall, A.C.; Sanders, N.J.; Fordyce, J.A.; Thorne, J.H.; O’Brien, J.; Waetjen, D.P.; Shapiro, A.M. Com-pounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- González-Megías, A.; Menéndez, R.; Roy, D.; Brereton, T.O.M.; Thomas, C.D. Changes in the composition of British butterfly assemblages over two decades. Glob. Change Biol. 2008, 14, 1464–1474. [Google Scholar] [CrossRef]
- Breed, G.A.; Stichter, S.; Crone, E.E. Climate-driven changes in northeastern U.S. butterfly communities. Nat. Clim. Change 2012, 3, 142–145. [Google Scholar] [CrossRef]
- Checa, M.F.; Rodríguez, J.; Willmott, K.R.; Liger, B. Microclimate variability significantly affects the composition, abundance and phenology of butterfly communities in a highly threatened neotropical dry forest. Fla. Entomol. 2014, 97, 1–13. [Google Scholar] [CrossRef]
- Munyuli, M.B.T. Odore Butterfly Diversity from Farmlands of Central Uganda. Psyche A J. Èntomol. 2012, 2012, 1–23. [Google Scholar] [CrossRef][Green Version]
- Xie, Z.; Wang, Y.; Cao, J.; Wang, J.; An, J. Ecological resilience of pollination in the face of pollinator decline: Content, mechanism and perspective. Biodivers. Sci. 2021, 29, 980–994. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, X.; Zhou, J.; Huang, C.; Deng, J.; Tang, X.; Luo, S. Pollination of the Orchid Habenaria rhodocheila by the Swallowtail Butterfly Papilio helenus in Subtropical Evergreen Broad-Leaved Forests in Southern China. Flora-Morphol. Distrib. Funct. Ecol. Plants 2021, 274, 151736. [Google Scholar] [CrossRef]
- Tzortzakaki, O.; Kati, V.; Panitsa, M.; Tzanatos, E.; Giokas, S. Butterfly diversity along the urbanization gradient in a densely-built Mediterranean city: Land cover is more decisive than resources in structuring communities. Landsc. Urban Plan. 2019, 183, 79–87. [Google Scholar] [CrossRef]
- Ramirez, N. Pollination specialization and time of pollination on a tropical Venezuelan plain: Variations in time and space. Bot. J. Linn. Soc. 2004, 145, 1–16. [Google Scholar] [CrossRef]
- Vodă, R.; Dapporto, L.; Dinca, V.E.; Vila, R. Cryptic matters: Overlooked species generate most butterfly beta-diversity. Ecography 2014, 38, 405–409. [Google Scholar] [CrossRef]
- Yue, M. The plant altitudinal spectrum of Qinling Mountains is complete and complex. For. Hum. 2015, 2, 76–81. [Google Scholar]
- Liu, X.; Zhang, Y.; Zhao, X.; He, X.; Cai, Q.; Zhu, Y.; He, B.; Jiu, Q. Introduction to the wildlife camera-trapping database of the middle Qinling Mountains. Biodivers. Sci. 2020, 28, 1075–1080. [Google Scholar] [CrossRef]
- Zhang, R.Z. Relict distribution of land vertebrates and Quaternary glaciation in China. Acta Zool. Sin. 2004, 50, 841–851. [Google Scholar]
- Kang, M.Y.; Zhu, Y. Discussion and analysis on the geo-ecological boundary in Qinling Range. Acta Ecol. Sin. 2007, 7, 118–128. [Google Scholar]
- Cao, F.; Wang, H.; Xia, G. Leafhopper (Hemiptera, Cicadellidae) community composition and diversity in different forest zones of Taibai Mountains. J. Northwest For. Univ. 2016, 6, 197–203. [Google Scholar]
- He, H.; Wei, C.; Liu, Y. The species diversity of ants in different habitats in Mt. Taibai. J. Northwest A F Univ. 2003, 31, 141–144. [Google Scholar]
- Yan, W.; Ji, S.; Shuai, L.; Zhao, L.; Zhu, D.; Zeng, Z.; Reserve, F.N.N. The spatial distribution patterns of mammal species diversity in Yangxian county on the southern slope of the Qinling Mountains. Biodivers. Sci. 2019, 27, 177–185. [Google Scholar] [CrossRef]
- Zhao, T.; Bai, H.; Deng, C.; Meng, Q.; Guo, S.; Qi, G. Topographic differentiation effect on vegetation cover in the Qinling Mountains from 2000 to 2016. Acta Ecol. Sin. 2019, 12, 4499–4509. [Google Scholar]
- Wu, H.; Xiao, N.N.; Lin, T.T. Relationships between functional diversity and species diversity of pine-oak mixed forest in Qinling Mountains and their environmental explanations. Ecol. Environ. Sci. 2020, 6, 1090–1100. [Google Scholar]
- Zhang, M.X.; Wang, D.X.; Peng, S.L.; Huang, Y.K.; Zhang, G.G. Community stability analysis for the oak-pine mixed forest in Qinling Mountains. Acta Ecol. Sin. 2015, 35, 2564–2573. [Google Scholar]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef]
- Chazot, N.; Willmott, K.R.; Endara, P.G.S.; Toporov, A.; Hill, R.I.; Jiggins, C.D.; Elias, M. Mutualistic mimicry and filtering by altitude shape the structure of Andean butterfly communities. Am. Nat. 2014, 183, 26–39. [Google Scholar] [CrossRef]
- Gallou, A.; Baillet, Y.; Ficetola, G.F.; Després, L. Elevational gradient and human effects on butterfly species richness in the French Alps. Ecol. Evol. 2017, 7, 3672–3681. [Google Scholar] [CrossRef] [PubMed]
- Homeier, J.; Breckle, S.-W.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich Ecuadorian montane rain forest. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2004, 8, 224–239. [Google Scholar] [CrossRef]
- Molina-Martínez, A.; León-Cortés, J.L.; Regan, H.M. Climatic and geometric constraints as driving factors of butterfly species richness along a Neotropical elevational gradient. J. Insect Conserv. 2013, 17, 1169–1180. [Google Scholar] [CrossRef]
- Pyrcz, T.W.; Janusz, W.; Raf, A.G. Diversity and distribution patterns of Pronophilina butterflies (Lepidoptera: Nymphalidae: Satyrinae) along an altitudinal transect in north-western Ecuador. Neotrop. Entomol. 2009, 38, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, G.M.; Luna, P.; Guevara, R.; Dáttilo, W.; Freitas, A.V.L.; Ribeiro, S.P. Temporal shifts in butterfly diversity: Responses to natural and anthropic forest transitions. J. Insect Conserv. 2020, 24, 353–363. [Google Scholar] [CrossRef]
- Zhong, Y.Z.; Li, K.H. A primary study on the climatic boundary effect of the join zone between Qinling Mountain and Huanghuai Plain. Geogr. Res. 1996, 4, 66–73. [Google Scholar]
- Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Morris, M.G.; Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation. J. Appl. Ecol. 1995, 32, 673. [Google Scholar] [CrossRef]
- Gall, L.F. Measuring the size of Lepidopteran populations. J. Res. Lepid 1985, 24, 97–116. [Google Scholar]
- Basset, Y.; Eastwood, R.O.D.; Sam, L.; Lohman, D.J.; Novotny, V.; Treuer, T.I.M.; Miller, S.E.; Weiblen, G.D.; Pierce, N.E.; Bunyavejchewin, S.; et al. Cross-continental comparisons of butterfly assemblages in tropical rainforests: Implications for biological monitoring. Insect Conserv. Divers. 2013, 6, 223–233. [Google Scholar] [CrossRef]
- Ma, F.Z.; Xu, H.G.; Chen, M.M.; Tong, W.J.; Wang, C.B.; Cai, L. Progress in construction of China Butterfly Diversity Observation Network (China BON-Butterflies). J. Ecol. Rural. Environ. 2018, 34, 27–36. [Google Scholar]
- Chou, I. Monographia Rhopalocerorum Sinensium; Henan Science and Technology Publishing House: Zhengzhou, China, 1994; pp. 12–453. [Google Scholar]
- Chou, I. Classification and Identification of Chinese Butterflies; Henan Science and Technology Publishing House: Zhengzhou, China, 1998; pp. 27–487. [Google Scholar]
- Wu, C.S.; Xu, Y.F. Butterflies of China; The Straits Publishing House: Fuzhou, China, 2017; pp. 5–2036. [Google Scholar]
- National Animal Collection Resource Center. Available online: http://museum.ioz.ac.cn/ (accessed on 9 June 2021).
- China Teaching Specimen Standardized Integration and Resource Sharing Platform. Available online: http://mnh.scu.edu.cn/ (accessed on 15 July 2021).
- Roberts, J.J.; Best, B.D.; Dunn, D.C.; Treml, E.A.; Halpin, P.N. Marine Geospatial Ecology Tools: An integrated framework for ecological geoprocessing with ArcGIS, Python, R, MATLAB, and C++. Environ. Model. Softw. 2010, 25, 1197–1207. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecol. 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A.; McInerny, G. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.; Li, C.; Kusumoto, B.; Yasuhara, M.; Thorn, S.; Wei, C.; Costello, M.J.; et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- Chai, Z.; Wang, D. A comparison of species composition and community assemblage of secondary forests between the birch and pine-oak belts in the mid-altitude zone of the Qinling Mountains, China. PeerJ 2016, 4, e1900. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R. Vegan: Community Ecology Package, R Package Version 2.5–7; 2019. Available online: http://vegan.r-forge.r-project.org/ (accessed on 5 May 2021).
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.Q.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; De-Santis, T.; Warrington, J.; et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef]
- Prentice, I.C.; van der Maarel, E. Theory and Models in Vegetation Science; Springer: Heidelberg, Germany, 1987; Volume 69, pp. 57–68. [Google Scholar]
- Minchin, P.R. An evaluation of the relative robustness of techniques for ecological ordination. Plant Ecol. 1987, 69, 89–107. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, S.T.; Wang, Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2019-3. 2019. Available online: http://www.iucnredlist.org/ (accessed on 9 October 2019).
- Wang, S.; Xie, Y. China Species Red List. Vol. III, Invertebrates; Higher Education Press: Beijing, China, 2005; pp. 51–850. [Google Scholar]
- Deng, C.H.; Bai, H.Y.; Ma, X.Y.; Zhao, T. Variation characteristics and its north-south differences of the vegetation phenology by remote sensing monitoring in the Qinling Mountains during 2000–2017. Acta Ecol. 2021, 41, 1068–1080. [Google Scholar]
- Yan, J.P. Comparison of Environmental Response between North and South of Qinling Mountains; Chinese Science Press: Beijing, China, 2006; p. 112. [Google Scholar]
- Hamer, K.C.; Hill, J.K.; Benedick, S.; Mustaffa, N.; Chey, V.K.; Maryati, M. Diversity and ecology of carrion- and fruit-feeding butterflies in Bornean rain forest. J. Trop. Ecol. 2006, 22, 25–33. [Google Scholar] [CrossRef]
- Murakami, M.; Ichie, T.; Hirao, T. Beta-diversity of lepidopteran larval communities in a Japanese temperate forest: Effects of phenology and tree species. Ecol. Res. 2008, 23, 179–187. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, Y.; Xing, X. Butterfly community structure and diversity in Qinling National Botanical Garden, China. Biodivers. Sci. 2020, 28, 965–972. [Google Scholar] [CrossRef]
- Yi, L.; Dong, Y.; Miao, B.; Peng, Y. Diversity of butterfly communities in Gaoligong region of Yunnan. Biodivers. Sci. 2021, 29, 950–959. [Google Scholar] [CrossRef]

| Family | No. of Genera | No. of Individuals | Species Richness | Shannon Diversity | Simpson Diversity |
|---|---|---|---|---|---|
| Pieridae | 10 | 2660 | 58 ± 13.81 | 10.80 ± 0.30 | 5.71 ± 0.16 |
| Papilionidae | 11 | 833 | 36 ± 3.65 | 10.90 ± 0.43 | 7.49 ± 0.27 |
| Lycaenidae | 49 | 1412 | 126 ± 18.22 | 20.90 ± 0.89 | 9.93 ± 0.41 |
| Nymphalidae | 74 | 4263 | 238 ± 21.51 | 79.44 ± 1.70 | 38.09 ± 1.31 |
| Hesperiidae | 31 | 458 | 95 ± 20.95 | 39.54 ± 2.38 | 27.43 ± 1.61 |
| Factors | No. of Individuals | Species Richness | Shannon Diversity | Simpson Diversity | |
|---|---|---|---|---|---|
| Altitude | <1000 m | 2565 | 261 ± 19.36 | 45.27 ±1.50 | 14.39 ± 0.71 |
| 1000–2000 m | 5516 | 424 ± 25.57 | 110.50 ± 2.36 | 50.53 ± 1.29 | |
| >2000 m | 74 | 101 ± 34.51 | 66.98 ± 11.36 | 44.27 ± 8.34 | |
| Season | Summer | 6591 | 466 ± 28.43 | 133.18 ± 2.36 | 63.43 ± 1.52 |
| Spring | 2087 | 251 ± 17.14 | 37.98 ± 1.64 | 11.04 ± 0.52 | |
| Autumn | 928 | 140 ± 21.69 | 38.71 ± 1.75 | 22.36 ± 1.03 | |
| Habitat | Reserve | 4151 | 475 ± 33.74 | 144.45 ± 3.50 | 58.06 ± 2.06 |
| Ecotone | 2634 | 324 ± 19.57 | 85.92 ± 2.54 | 36.14 ± 1.60 | |
| Farm | 2841 | 280 ± 20.30 | 62.70 ± 1.98 | 25.62 ± 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Li, S.; He, M.; Zhang, Y. Butterfly Community Diversity in the Qinling Mountains. Diversity 2022, 14, 27. https://doi.org/10.3390/d14010027
Ren J, Li S, He M, Zhang Y. Butterfly Community Diversity in the Qinling Mountains. Diversity. 2022; 14(1):27. https://doi.org/10.3390/d14010027
Chicago/Turabian StyleRen, Jinze, Shuying Li, Mengdi He, and Yalin Zhang. 2022. "Butterfly Community Diversity in the Qinling Mountains" Diversity 14, no. 1: 27. https://doi.org/10.3390/d14010027
APA StyleRen, J., Li, S., He, M., & Zhang, Y. (2022). Butterfly Community Diversity in the Qinling Mountains. Diversity, 14(1), 27. https://doi.org/10.3390/d14010027







