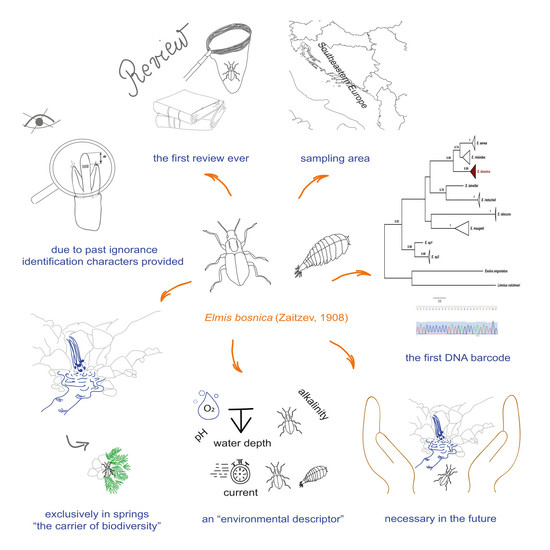
Ubiquitous but Ignored? A Case of Water Beetle in Southeastern Europe
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling and Data Collection
2.3. Data Analysis
2.4. DNA Extraction, PCR Amplification, Sequencing and Analyses
3. Results and Discussion
3.1. Identification and Distribution
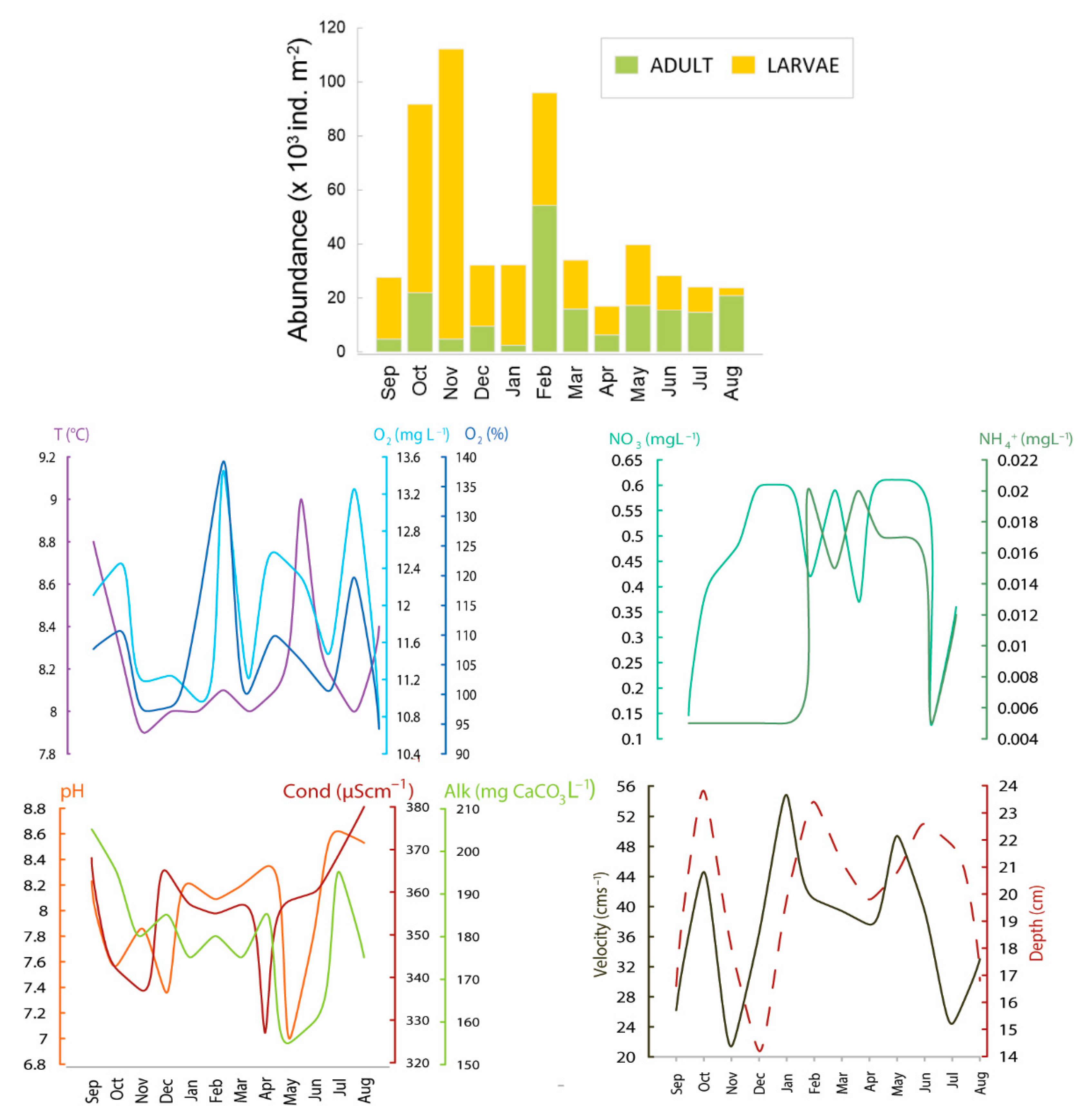
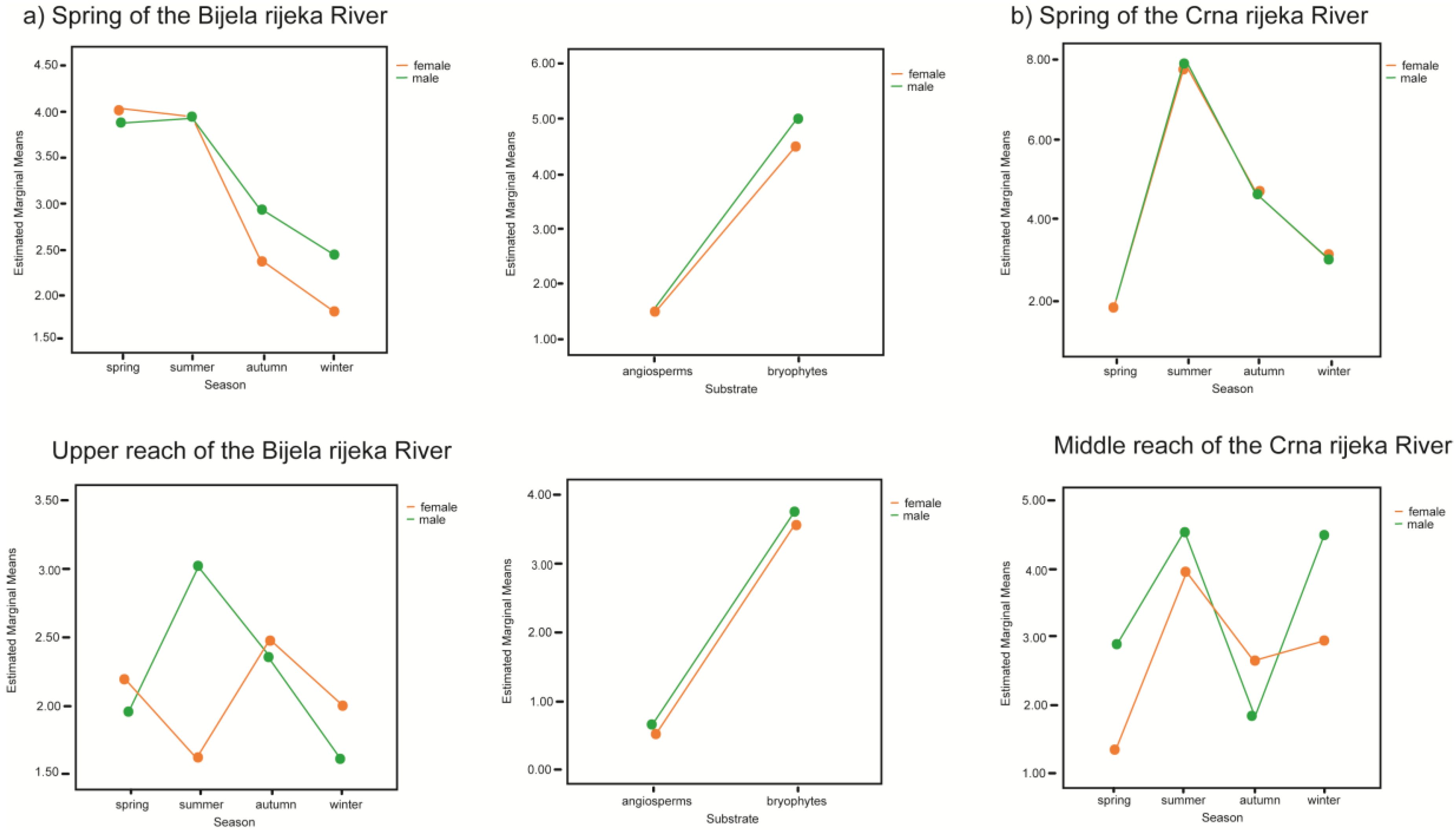
3.2. Population Traits and Seasonal Dynamics
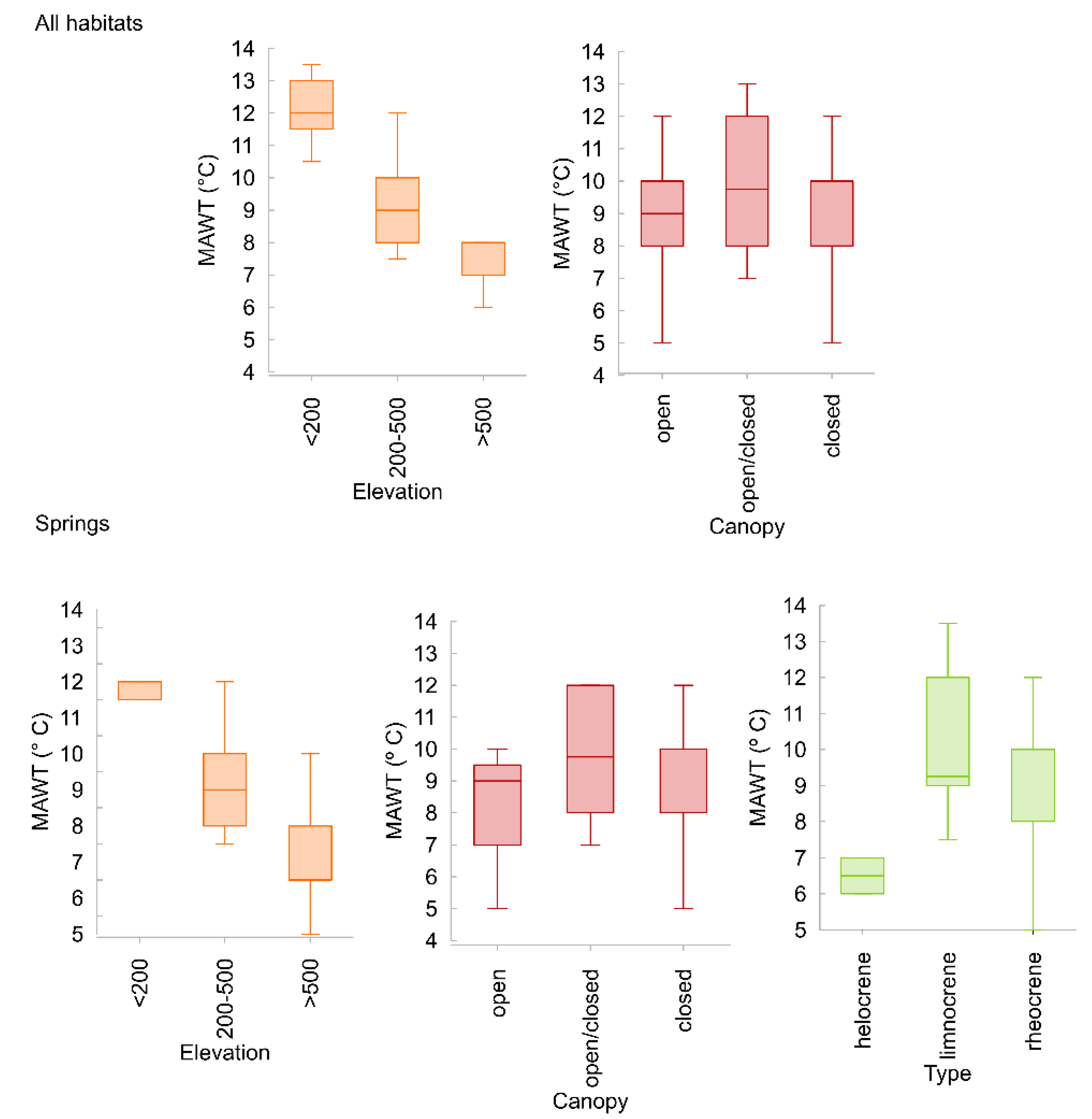
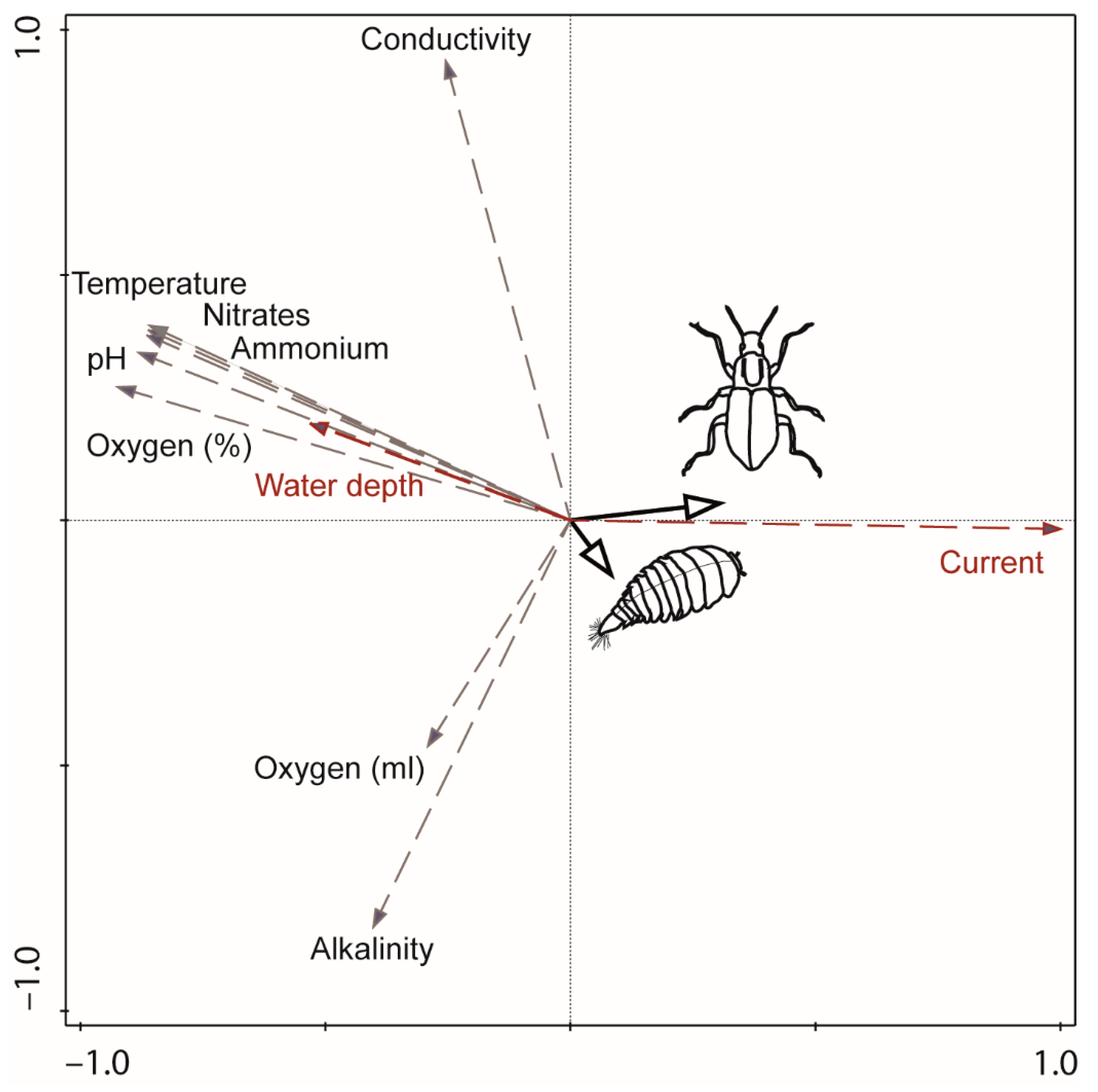
3.3. Ecological Traits
3.4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newbold, T.; Oppenheimer, P.; Etard, A.; Williams, J.J. Tropical and Mediterranean biodiversity is disproportionately sensitive to land-use and climate change. Nat. Ecol. Evol. 2020, 4, 1630–1638. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Corbera, J.; Domene, X.; Sayol, F.; Sabater, F.; Preece, C. Nitrate pollution reduces bryophyte diversity in Mediterranean springs. Sci. Total Environ. 2020, 705, 135823. [Google Scholar] [CrossRef]
- Bes, M.; Corbera, J.; Sayol, F.; Bagaria, G.; Jover, M.; Preece, C.; Viza, A.; Sabater, F.; Fernández-Martínez, M. On the influence of water conductivity, pH and climate on bryophyte assemblages in Catalan semi-natural springs. J. Bryol. 2018, 40, 149–158. [Google Scholar] [CrossRef]
- Cantonati, M.; Füreder, L.; Gerecke, R.; Jüttner, I.; Cox, E.J. Crenic habitats, hotspots for freshwater biodiversity conservation: Toward an understanding of their ecology. Freshw. Sci. 2012, 31, 463–480. [Google Scholar] [CrossRef]
- Pascual, R.; Nebra, A.; Gomà, J.; Pedrocchi, C.; Cadiach, O.; García, G.; Solé, J. First data on the biological richness of Mediterranean springs. Limnetica 2020, 39, 121–139. [Google Scholar] [CrossRef]
- Jäch, M.A.; Balke, M. Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia 2008, 595, 419–442. [Google Scholar] [CrossRef]
- Jäch, M.A.; Kodada, J.; Brojer, M.; Shepard, W.D.; Čiampor, F. Coleoptera: Elmidae and Protelmidae, World Catalogue of Insects; Brill: Leiden, The Netherlands, 2016; Volume 14, p. 318. [Google Scholar]
- Elliott, J.M. The ecology of riffle beetles (Coleoptera: Elmidae). Freshw. Rev. 2008, 1, 189–203. [Google Scholar] [CrossRef]
- Jäch, M.A. Annotated check list of aquatic and riparian/littoral beetle families of the world. In Water Beetles of China; Jäch, M.A., Ji, L., Eds.; Zoologisch-Botanische Gesellschaft in Österreich und Wiener Koleopterologen Verein: Wien, Austria, 1998; pp. 25–42. [Google Scholar]
- Moog, O.; Hartmann, A. Fauna Aquatica Austriaca, 3rd ed.; Federal Ministry of Agriculture, Forestry, Environment and Water Management: Wien, Austria, 2017; Available online: http://www.ecoprof.at/index.php/faunaaquaticaaustriaca.html (accessed on 12 May 2021).
- Schlosser, J.K. Fauna Kornjašah Trojedne Kraljevine; Yugoslav Academy of Sciences and Arts: Zagreb, Croatia, 1877; pp. 88–118. (In Croatian) [Google Scholar]
- Apfelbeck, A. Fauna Insectorum Balcanica. Beiträge zur Kenntnis der Balkanfauna; Wissenschaftliche Mitteilungen aus Bosnien und der Hercegowina: Sarajevo, Bosnia and Herzegovina, 1894; Volume 2, pp. 24–32. [Google Scholar]
- Mičetić Stanković, V.; Jäch, M.A.; Kučinić, M. Annotated checklist of Croatian riffle beetles (Coleoptera: Elmidae). Nat. Croat. Period. Musei Hist. Nat. Croat. 2015, 24, 93–109. [Google Scholar] [CrossRef]
- Mičetić Stanković, V.; Jäch, M.A.; Ivković, M.; Stanković, I.; Kružić, P.; Kučinić, M. Spatio-temporal distribution and species traits of water beetles along an oligotrophic hydrosystem: A case study. Ann. Limnol. 2019, 55, 16. [Google Scholar] [CrossRef]
- Mičetić Stanković, V.; Bruvo Mađarić, B.; Jäch, M.A.; Kučinić, M. Elmis rietscheli Steffan, 1958 (Insecta: Coleoptera: Elmidae) in Croatia: First record and DNA barcoding. Nat. Croat. Period. Musei Hist. Nat. Croat. 2018, 27, 185–194. [Google Scholar] [CrossRef]
- Mičetić Stanković, V.; Jäch, M.A.; Vučković, I.; Popijač, A.; Kerovec, M.; Kučinić, M. Ecological traits of water beetles in a karstic river from the Eastern Mediterranean region. Limnologica 2018, 71, 75–88. [Google Scholar] [CrossRef]
- Novaković, B.B.; Mesaroš, G. First recent record of Macronychus quadrituberculatus Müller, 1806 (Elmidae: Coleoptera) in Serbia. Water Resour. Manag. 2014, 4, 35–37. [Google Scholar]
- Novaković, B.B.; Teofilova, T.M.; Pandakov, P.G.; Živić, I. New distributional records of rare riffle beetles (Coleoptera: Elmidae) from the Balkan Peninsula. Arch. Biol. Sci. 2020, 72, 129–135. [Google Scholar] [CrossRef]
- Novaković, B.B.; Marković, V.; Mesaroš, G.; Živić, I. The riffle beetle Macronychus quadrituberculatus Müller, 1806 (Coleoptera: Elmidae): Recent findings in Serbia with ecological notes. Biologia 2020, 75, 1891–1897. [Google Scholar] [CrossRef]
- Mičetić Stanković, V.; Stanković, I.; Vilenica, M.; Delić, A.; Kučinić, M. Water Beetles (Insecta: Coleoptera) in Spring of the Dinaric Karst: Ecology and Biology; Croatian Natural History Museum: Zagreb, Croatia, 2022. [Google Scholar]
- Matoničkin, I.; Pavletić, Z.; Tavčar, V.; Krkač, N. Limnološka istraživanja reikotopa i fenomena protočne travertinizacije u Plitvičkim jezerima. Prirodosl. Istraž. Acta Biol. 1971, 7, 1–88. (In Croatian) [Google Scholar]
- Habdija, I.; Primc-Habdija, B.; Belinić, I. Functional community organization of macroinvertebrates in lotic habitats of the Plitvice Lakes. Acta Hydrochim. Hydrobiol. 1994, 22, 85–92. [Google Scholar] [CrossRef]
- Zaitzev, P.A. Catalogue des Coléoptères Aquatiques des Familles des Dryopidae, Georyssidae, Cyathoceridae, Heteroceridae et Hydrophilidae, Horae Societatis Entomologicae Rossicae, Variis Sermonibus in Rossia Usitatis Editae; Besobrasovi & Comp.: Saint Petersburg, Russia, 1908; Volume 38, pp. 283–420. [Google Scholar]
- Chen, Z.; Auler, A.S.; Bakalowicz, M.; Drew, D.; Griger, F.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Richts, A.; Stevanović, Z.; et al. The world karst aquifer mapping project: Concept, mapping procedure and map of Europe. Hydrogeol. J. 2017, 25, 771–785. [Google Scholar] [CrossRef]
- Bonacci, O. Karst hydrology and water resources—Past, present and future. In Water for the Future: Hydrology in Perspective, Proceedings of the Rome Symposium, Rome, Italy, 20–22 October 1987; Rodda, J.C., Matalas, N.C., Eds.; International Association of Hydrological Sciences: Rome, Italy, 1987; pp. 205–213. [Google Scholar]
- Reed, J.M.; Kryštufek, B.; Eastwood, W.J. The physical geography of the Balkans and nomenclature of place names. In Balkan Biodiversity; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 9–22. [Google Scholar] [CrossRef]
- Bonacci, O.; Pipan, T.; Culver, D.C. A framework for karst ecohydrology. Environ. Geol. 2009, 56, 891–900. [Google Scholar] [CrossRef]
- Sánchez-Fernández, D.; Abellán, P.; Velasco, J.; Millán, A. Selecting areas to protect the biodiversity of aquatic ecosystems in a semiarid Mediterranean region using water beetles. Aquat. Conserv. 2004, 14, 465–479. [Google Scholar] [CrossRef]
- Bonacci, O.; Andrić, I. Karst spring catchment: An example from Dinaric karst. Environ. Earth Sci. 2015, 74, 6211–6223. [Google Scholar] [CrossRef]
- Earman, S.; Dettinger, M. Potential impacts of climate change on groundwater resources—A global review. J. Water Clim. Chang. 2011, 2, 213–229. [Google Scholar] [CrossRef]
- Nerantzaki, S.D.; Nikolaidis, N.P. The response of three Mediterranean karst springs to drought and the impact of climate change. J. Hydrol. 2020, 591, 125296. [Google Scholar] [CrossRef]
- Gaston, K.J.; David, R. Hotspots across Europe. Biodivers. Lett. 1994, 2, 108–116. [Google Scholar] [CrossRef]
- Kryštufek, B.; Buzan, E.V.; Hutchinson, W.F.; Hanfling, B. Phylogeography of the rare Balkan endemic Martino’s vole, Dinaromys bogdanovi, reveals strong differentiation within the western Balkan Peninsula. Mol. Ecol. 2007, 16, 1221–1232. [Google Scholar] [CrossRef]
- Previšić, A.; Walton, C.; Kučinić, M.; Mitrikeski, P.T.; Kerovec, M. Pleistocene divergence of Dinaric Drusus endemics (Trichoptera, Limnephilidae) in multiple microrefugia within the Balkan Peninsula. Mol. Ecol. 2009, 18, 634–647. [Google Scholar] [CrossRef]
- Palandačić, A.; Bonacci, O.; Snoj, A. Molecular data as a possible tool for tracing groundwater flow in karst environment: Example of Delminichthys adspersus in Dinaric karst system. Ecohydrology 2011, 5, 791–797. [Google Scholar] [CrossRef]
- Ivković, M.; Plant, A. Aquatic insects in the Dinarides: Identifying hotspots of endemism and species richness shaped by geological and hydrological history using Empididae (Diptera). Insect Conserv. Divers. 2015, 8, 302–312. [Google Scholar] [CrossRef]
- Bregović, P.; Fišer, C.; Zagmajster, M. Contribution of rare and common species to subterranean species richness patterns. Ecol. Evol. 2019, 9, 11606–11618. [Google Scholar] [CrossRef]
- Manenti, R.; Piazza, B. Between darkness and light: Spring habitats provide new perspectives for modern researchers on groundwater biology. PeerJ 2021, 9, e11711. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.G.; Padedda, B.M.; Ector, L.; Wetzel, C.E.; Lugliè, A.; Cantonati, M. Mediterranean karst springs: Diatom biodiversity hotspots under the pressure of hydrological fluctuations and nutrient enrichment. Plant Biosyst. 2019, 154, 673–684. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, L.; Morinière, J.; Haszprunar, G.; Hebert, P.D.N.; Hausmann, A.; Köhler, F.; Balke, M. A comprehensive DNA barcode database for Central European beetles with a focus on Germany: Adding more than 3500 identified species to BOLD. Mol. Ecol. Resour. 2015, 15, 795–818. [Google Scholar] [CrossRef] [PubMed]
- Jovović, L.; Bruvo Mađarić, B.; Mičetić Stanković, V.; Jäch, M.A.; Kučinić, M. Phylogeny and phylogeography of genus Elmis (Coleoptera, Elmidae) in karstic running waters in southeastern Europe. In Proceedings of the 12th Croatian Biological Congress with International Participation, Sveti Martin na Muri, Croatia, 18–23 September 2015; pp. 173–174. [Google Scholar]
- Múrria, C.; Bonada, N.; Vellend, M.; Zamora-Muñoz, C.; Alba-Tercedor, J.; Elisa Sainz-Cantero, C.; Garrido, J.; Acosta, R.; El Alami, M.; Barquín, J.; et al. Local environment rather than past climate determines community composition of mountain stream macroinvertebrates across Europe. Mol. Ecol. 2017, 26, 6085–6099. [Google Scholar] [CrossRef]
- German Barcode of Life. 2021. Available online: https://bolgermany.de/home/en/german-barcode-of-life-2 (accessed on 23 February 2021).
- Austrian Barcode of Life. 2021. Available online: https://www.abol.ac.at/ (accessed on 23 February 2021).
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Fossen, E.I.; Ekrem, T.; Nilsson, A.N.; Bergsten, J. Species delimitation in northern European water scavenger beetles of the genus Hydrobius (Coleoptera, Hydrophilidae). ZooKeys 2016, 564, 71–120. [Google Scholar] [CrossRef]
- Bilton, D.T.; Turner, L.; Foster, G.N. Frequent discordance between morphology and mitochondrial DNA in a species group of European water beetles (Coleoptera: Dytiscidae). PeerJ 2017, 5, e3076. [Google Scholar] [CrossRef][Green Version]
- Bilton, D.T.; Ribera, I. A revision of Meladema diving beetles (Coleoptera, Dytiscidae), with the description of a new species from the central Mediterranean based on molecules and morphology. ZooKeys 2017, 702, 45–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joffre, S.; Stanners, D. Europe: The continent. In Europe’s Environment: The Dobřĭš Assessement; Stanners, D., Bourdeau, P., Eds.; European Environment Agency: Copenhagen, Denmark, 1995; pp. 18–24. [Google Scholar]
- European Environment Agency 2021. Biogeographical Regions in Europe. Available online: https://www.eea.europa.eu/data-and-maps/figures/biogeographical-regions-in-europe-2 (accessed on 26 February 2021).
- Bilandžija, H.; Morton, B.; Podnar, M.; Ćetković, H. Evolutionary history of relict Congeria (Bivalvia: Dreissenidae): Unearthing the subterranean biodiversity of the Dinaric Karst. Front. Zool. 2013, 10, 5. [Google Scholar] [CrossRef]
- Gottstein, S.; Žganec, K.; Kerovec, M.; Ternjej, I.; Lajtner, J.; Mihaljević, Z.; Popijač, A.; Previšić, A.; Ivković, M.; Mičetić, V. ThePotential Effect of the Hydroelectric Power Plant Lešće on Spring Macroinvertebrate Assemblages of the Upper Course of the Gojačka Dobra River; University of Zagreb, Faculty of Science: Zagreb, Croatia, 2009. (In Croatian) [Google Scholar]
- Kerovec, M.; Kučinić, M. Bioindikatorska i Ekološka Obilježja te Rasprostranjenost i Gustoća Populacija Faune Tulara (Trichoptera, Insecta) duž Toka Rijeke Cetine; University of Zagreb, Faculty of Science: Zagreb, Croatia, 2007; 158p. (In Croatian) [Google Scholar]
- Šerić Jelaska, L.; Temunović, M.; Mičetić Stanković, V.; Hlavati, D. Ground beetles, diving beetles, whirligig beetles, water scavengers, riffle beetles, scarab beetles, dung beetles, hister beetles, carrion beetles—The beetles (Insecta, Coleoptera) of the travertine waterfalls, Mediterranean ponds and grasslands of Krka National Park. In Abstract Book: Vision and Challenges of Managing Protected Natural Areas in the Republic of Croatia: Active Protection and Sustainable Management in Krka National Park, Šibenik, Croatia; Marguš, D., Ed.; The Public Institution of Krka National Park: Šibenik, Croatia, 2017; pp. 208–247. [Google Scholar]
- Mičetić Stanković, V. Water Beetles (Insecta: Coleoptera) in Microhabitats of Karstic Springs and Rivers. Ph.D. Thesis, Faculty of Science, University of Zagreb, Zagreb, Croatia, 2012. [Google Scholar]
- Hynes, H.B.N. The Ecology of Running Waters; University of Toronto Press: Toronto, ON, Canada, 1970; p. 555. [Google Scholar] [CrossRef]
- Berthélemy, C. Elmidae de la région paléarctique occidentale: Systématique et répartition (Coleoptera Dryopoidea). Ann. Limnol. 1979, 15, 1–102. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Ordination. In Data Analysis in Community and Landscape Ecology; Jongman, R.H.G., ter Braak, C.J.F., van Tongeren, O.F.R., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 91–173. [Google Scholar]
- SPSS Inc. SPSS Statistics for Windows, Version 17.0; Released 2008; SPSS Inc.: Chicago, IL, USA, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- GrapherTM; Golden Software, LLC.: Golden, CO, USA, 2021. Available online: www.goldensoftware.com (accessed on 24 September 2021).
- Folmer, O.; Black, M.; Wr, H.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial Cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Geneious 8.1.4. Available online: https://www.geneious.com (accessed on 15 March 2021).
- BOLD Identification Tool. Available online: https://www.boldsystems.org/index.php/IDs (accessed on 20 May 2021).
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Multiple Alignment Program for Amino Acid or Nucleotide Sequences. Available online: https://mafft.cbrc.jp/alignment/server/index.html (accessed on 29 May 2021).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- PhyML 3.0: New Algorithms, Methods and Utilities. Available online: http://www.atgc-montpellier.fr/phyml (accessed on 10 June 2021).
- Lefort, V.; Longueville, J.-E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef]
- FigTree v.1.4.3. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 June 2021).
- Olmi, M. Fauna d’Italia. Coleoptera: Dryopidae, Elminthidae; Edizioni Calderini: Bologna, Italy, 1976; Volume XII, p. 272. [Google Scholar]
- Pentinsaari, M.; Hebert, P.D.N.; Mutanen, M. Barcoding beetles: A regional survey of 1872 species reveals high identification success and unusually deep interspecific divergences. PLoS ONE 2014, 9, e108651. [Google Scholar] [CrossRef]
- Oláh, J.; Andersen, T.; Chvojka, P.; Coppa, G.; Graf, W.; Ibrahimi, H.; Previšić, A.; Valle, M. The Potamophylax nigricornis group (Trichoptera, Limnephilidae): Resolution of phylogenetic species by fine structure analysis. Opusc. Zool. Bp. 2013, 44, 167–200. [Google Scholar]
- Hebauer, F. Entwurf einer Entomosoziologie aquatischer Coleoptera in Mitteleuropa (Insecta, Coleoptera, Hydradephaga, Hydrophiloidea, Dryopoidea). Lauterbornia 1994, 19, 43–76. [Google Scholar]
- Hebauer, F. Katalog der bayerischen Wasserkäfer, ihrer Ökologie, Verbreitung, Gefährdung. Ber. Akad. Nat. Schutz Landsch. Pfl. 1994, 18, 47–59. [Google Scholar]
- Brojer, M.; Jäch, M.A.; Kodada, J.; Moog, O. Coleoptera: Water Beetles s.l. In Fauna Aquatica Austriaca, 3rd ed.; Moog, O., Hartmann, A., Eds.; Federal Ministry of Agriculture, Forestry, Environment and Water Management: Wien, Austria, 2017; p. 49. [Google Scholar]
- Zollhöfer, J.M. Spring Biotopes in Northern Switzerland: Habitat Heterogeneity, Zoobenthic Communities and Colonization Dynamics. Ph.D. Thesis, Swiss Federal Institute of Science and Technology, Zürich, Switzerland, 1999. [Google Scholar]
- Smith, H.; Wood, P.J. Flow permanence and macroinvertebrate community variability in limestone spring systems. Hydrobiologia 2002, 487, 45–58. [Google Scholar] [CrossRef]
- Mičetić Stanković, V. Croatian Natural History Museum, Zagreb, Croatia. Personal observation.
- Bournaud, M.; Richoux, P.; Usseglio-Polatera, P. An approach to the synthesis of qualitative ecological information from aquatic coleoptera communities. Regul. Rivers Res. Manag. 1992, 7, 165–180. [Google Scholar] [CrossRef]
- Eyre, M.D.; Pilkington, J.G.; Carr, R.; McBlane, R.P.; Rushton, S.P.; Foster, G.N. The running-water beetles (Coleoptera) of a river catchment in northern England. Hydrobiologia 1993, 264, 33–45. [Google Scholar] [CrossRef]
- Berthélemy, C.; de Riols, J. Les larves ď Elmis du groupe ď E. maugetii (Coléoptères Drypoidea). Ann. Limnol. 1965, 1, 21–38. [Google Scholar] [CrossRef][Green Version]
- Elliott, J.M. Critical periods in the life cycle and the effects of a severe spate vary markedly between four species of elmid beetles in a small stream. Freshw. Biol. 2006, 51, 1527–1542. [Google Scholar] [CrossRef]
- Giller, P.S.; Malmqvist, B. The Biology of Streams and Rivers, 1st ed.; Oxford University Press: Oxford, UK, 1998; p. 304. [Google Scholar]
- Jäch, M.A. Daytime swarming of rheophilic water beetles in Austria (Coleoptera: Elmidae, Hydraenidae, Haliplidae). Latissimus 1997, 9, 10–11. [Google Scholar]
- Jäch, M.A. Observations on rheophilic water beetles swarming in lower Austria in 2003 (Hydraenidae, Elmidae). Latissimus 2003, 1, 20–22. [Google Scholar]
- Seagle, H.H. Flight periodicity and emergence patterns in the Elmidae (Coleoptera: Dyropoidea). Ann. Entomol. Soc. Am. 1980, 73, 300–306. [Google Scholar] [CrossRef]
- Girondot, M.; Pieau, C. Effects of sexual differences of age at maturity and survival on population sex ratio. Evol. Ecol. 1993, 7, 645–650. [Google Scholar] [CrossRef]
- Papach, A.; Gonthier, J.; Williams, G.R.; Neumann, P. Sex ratio of small hive beetles: The role of pupation and adult longevity. Insects 2019, 10, 133. [Google Scholar] [CrossRef]
- Miserendino, M.L.; Archangelsky, M. Aquatic coleoptera distribution and environmental relationships in a large Patagonian River. Int. Rev. Hydrobiol. 2006, 91, 423–437. [Google Scholar] [CrossRef]
- Matoničkin, I.; Pavletić, Z. Hidrologija protočnog sistema Plitvičkih jezera i njegove ekološko-biocenološke značajke. Carsus Iugosl. 1967, 5, 83–126. (In Croatian) [Google Scholar]
- Habdija, I.; Primc-Habdija, B.; Matoničkin, R.; Kučinić, M.; Radanović, I.; Miliša, M.; Mihaljević, Z. Current velocity and food supply as factors affecting the composition of macroinvertebrates in bryophyte habitats in karst running water. Biologia 2004, 59, 577–593. [Google Scholar]
- Brown, H.P. Biology of riffle beetles. Annu. Rev. Entomol. 1987, 32, 253–273. [Google Scholar] [CrossRef]
- Bonada, N.; Rieradevall, M.; Prat, N. Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiologia 2007, 589, 91–106. [Google Scholar] [CrossRef]
- Datry, T.; Larned, S.T.; Tockner, K. Intermittent rivers: A challenge for freshwater ecology. Bioscience 2014, 64, 229–235. [Google Scholar] [CrossRef]
- Vilenica, M.; Mičetić Stanković, V.; Sartori, M.; Kučinić, M.; Mihaljević, Z. Environmental factors affecting mayfly assemblages in tufa-depositing habitats of the Dinaric Karst. Knowl. Manag. Aquat. Ec. 2017, 418, 14. [Google Scholar] [CrossRef][Green Version]
- Vilenica, M.; Ivković, M.; Sartori, M.; Mihaljević, Z. Mayfly emergence along an oligotrophic Dinaric karst hydrosystem: Spatial and temporal patterns, and species–environment relationship. Aquat. Ecol. 2017, 51, 417–433. [Google Scholar] [CrossRef]
- Ortega, J.C.G.; Geijer, J.; Bergsten, J.; Heino, J.; Herrmann, J.; Johansson, F.; Bini, L.M. Spatio-temporal variation in water beetle assemblages across temperate freshwater ecosystems. Sci. Total Environ. 2021, 792, 148071. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L.; Flores, F.; Moneo, M. Challenges to manage the risk of water scarcity and climate change in the Mediterranean. Water Resour. Manag. 2007, 21, 775–788. [Google Scholar] [CrossRef]

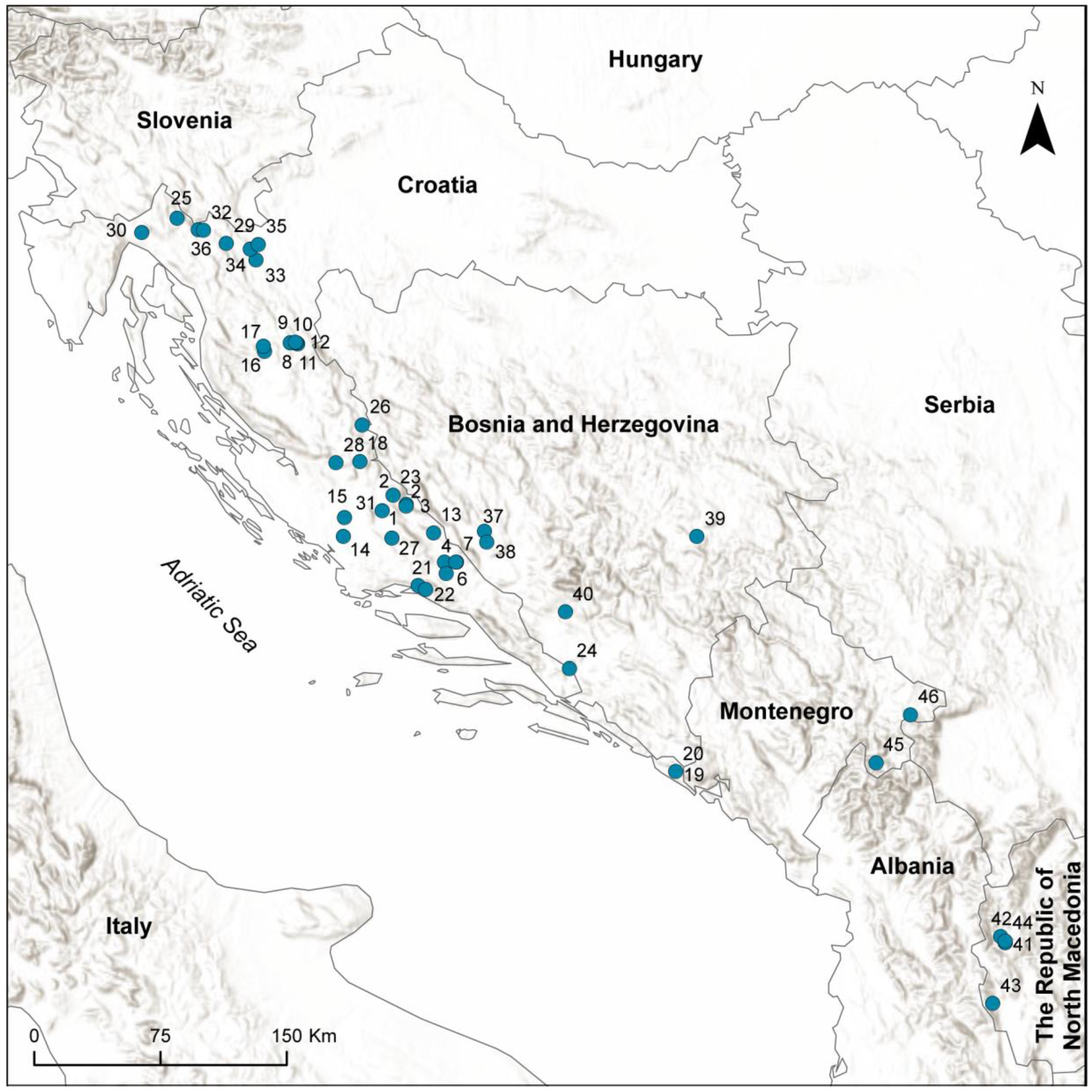


| C | Sampling Site | Latitude | Longitude | Altitude (m) | Habitat Type | Water Constancy | App. MWT (°C) |
|---|---|---|---|---|---|---|---|
| CROATIA | |||||||
| 1 | Cetina River, Glavaš spring | N43°58′36.00″ | E16°25′48.00″ | 385 | limnocrene spring | permanent | 9.00 |
| 2 | Cetina River, Crveni Most | N43°58′05.00″ | E16°25′53.00″ | 365 | upper reach | permanent | 9.00 |
| 3 | Cetina River, Obrovac Sinjski | N43°40′01.00″ | E16°41′04.00″ | 300 | middle reach | permanent | 10.00 |
| 4 | Cetina River, Preočki Most | N43°57′59.00″ | E16°25′53.00″ | 370 | upper reach | permanent | 9.00 |
| 5 | Cetina River, Trilj | N43°36′21.00″ | E16°43′46.00″ | 295 | middle reach | permanent | 10.00 |
| 6 | Ruda River, spring | N43°40′07.00″ | E16°47′56.00″ | 320 | limnocrene spring | permanent | 12.00 |
| 7 | Ruda River, 500 m downstream of spring | N43°40′07.50″ | E16°47′49.20″ | 330 | upper reach | permanent | 12.00 |
| 8 | Plitvice Lakes NP, Bijela rijeka River spring | N44°50′05.00″ | E15°33′43.00″ | 720 | helocrene spring | permanent | 7.00 |
| 9 | Plitvice Lakes NP, Bijela rijeka River Upper Reach | N44°50′04.00″ | E15°33′33.00″ | 715 | upper reach | permanent | 7.50 |
| 10 | Plitvice Lakes NP, Crna rijeka River spring | N44°49′44.46″ | E15°36′50.05″ | 680 | rheocrene spring | permanent | 8.00 |
| 11 | Plitvice Lakes NP, Crna rijeka River Middle Reach | N44°50′10.00″ | E15°36′30.00″ | 670 | middle reach | permanent | 8.00 |
| 12 | Plitvice Lakes NP, Crna rijeka River Lower Reach | N44°50′22.00″ | E15°35′59.00″ | 665 | lower reach | permanent | 8.00 |
| 13 | Vojskova River | N43°49′25.60″ | E16°37′54.60″ | 360 | upper reach | permanent | 10.00 |
| 14 | Krka River NP, Skradinski Buk waterfall | N43°48′11.26″ | E15°57′56.29″ | 41 | lower reach | permanent | 12.00 |
| 15 | Krka River NP, Roški Slap waterfall | N43°54′20.00″ | E15°58′22.00″ | 55 | lower reach | permanent | 13.00 |
| 16 | Gacka River, Tonkovića spring | N44°47′17.35″ | E15°22′01.66″ | 465 | limnocrene spring | permanent | 9.50 |
| 17 | Gacka River, Majer spring | N44°48′51.52″ | E15°21′31.46″ | 459 | limnocrene spring | permanent | 9.00 |
| 18 | Zrmanja River, spring | N44°12′18.13″ | E16°05′05.21″ | 280 | rheocrene spring | permanent | 9.00 |
| 19 | Ljuta River, Konaovski dvori | N42°32′16.41″ | E18°22′43.59″ | 80 | upper reach | permanent | 8.5 |
| 20 | Ljuta River, spring | N42°32′11.00″ | E18°22′41.00″ | 70 | rheocrene spring | permanent | 11.50 |
| 21 | Jadro River, spring | N43°32′30.30″ | E16°31′11.80″ | 35 | limnocrene spring | permanent | 12.00 |
| 22 | Žrnovnica River, spring | N43°31′22.13″ | E16°34′25.95″ | 70 | rheocrene spring | permanent | 12.00 |
| 23 | Krčić River, spring | N44°01′31.45″ | E16°19′52.71″ | 370 | rheocrene spring | temporary | 9.00 |
| 24 | Norin River, Prud spring | N43°05′41.44″ | E17°37′12.24″ | 3 | limnocrene spring | permanent | 13.50 |
| 25 | Kupa River, spring brook | N45°29′26.75″ | E14°41′26.47″ | 385 | limnocrene spring | permanent | 7.50 |
| 26 | Una River, spring | N44°23′58.01″ | E16°06′13.93″ | 396 | limnocrene spring | permanent | 10.00 |
| 27 | Čabranka River, spring | N43°47′50.69″ | E16°19′26.43″ | 570 | rheocrene spring | permanent | 8.00 |
| 28 | Krupa River, spring | N44°11′48.38″ | E15°54′32.45″ | 1058 | rheocrene spring | permanent | 10.00 |
| 29 | Kamačnik River, spring | N45°21′38.45″ | E15°03′59.77″ | 483 | limnocrene spring | permanent | 8.00 |
| 30 | Rječina River, spring | N45°24′31.30″ | E14°25′30.01″ | 370 | rheocrene spring | permanent | 8.00 |
| 31 | Kosovčica River, spring | N43°56′28.00″ | E16°15′10.00″ | 260 | limnocrene spring | permanent | 9.00 |
| 32 | Curak Stream, after HP Munjara | N45°25′38.12″ | E14°53′34.45″ | 339 | lower reach | permanent | 8.00 |
| 33 | Bistrica Spring, Dobra River | N45°16′27.80″ | E15°17′28.10″ | 230 | rheocrene spring | permanent | 10.50 |
| 34 | Ribnjak Spring, Dobra River | N45°20′05.00″ | E15°15′07.00″ | 260 | rheocrene spring | permanent | 10.00 |
| 35 | Podumol Spring, Dobra River | N45°21′27.3″ | E15°18′31.20″ | 184 | limnocrene spring | permanent | 10.50 |
| 36 | Kupica River, spring | N45°25′47.34″ | E14°51′11.96″ | 310 | limnocrene spring | permanent | 7.50 |
| BOSNIA AND HERZEGOVINA | |||||||
| 37 | Bistrica River, spring | N43°49′55.76″ | E17°00′31.21″ | 777 | rheocrene spring | permanent | 8.00 |
| 38 | Sturba River, spring | N43°46′26.18″ | E17°01′24.43″ | 753 | rheocrene spring | permanent | 8.00 |
| 39 | Miljacka River, spring | N43°47′07.00″ | E18°34′25.00″ | 1026 | rheocrene spring | permanent | 6.50 |
| 40 | Lištica River, spring | N43°23′46.87″ | E17°35′50.23″ | 322 | rheocrene spring | permanent | 8.00 |
| REPUBLIC OF NORTH MACEDONIA | |||||||
| 41 | Lazaropole, Hygropetric beside the road | N41°33′38.10″ | E20°41′39.00″ | 916 | rheocrene spring | permanent | 8.00 |
| 42 | Galička River, spring | N41°35′36.40″ | E20°39′52.30″ | 1407 | rheocrene spring | permanent | 5.00 |
| 43 | Vevčani Springs | N41°14′22.50″ | E20°35′03.70″ | 951 | rheocrene spring | permanent | 8.00 |
| 44 | Rosočka River, Rosoki spring | N41°34′10.00″ | E20°41′35.90″ | 1024 | rheocrene spring | permanent | 7.00 |
| MONTENEGRO | |||||||
| 45 | Alipaša Springs, Gusinje | N42°33′00.50″ | E19°49′33.10″ | 932 | helocrene spring | permanent | 6.00 |
| 46 | Ibra River, spring | N42°47′51.00″ | E20°05′25.30″ | 1256 | rheocrene spring | permanent | 5.00 |
| Sample | Sampling Site | BOLD-ID | GenBank Accession No. | Voucher |
|---|---|---|---|---|
| VMS-B14 | Plitvice Lakes NP (CRO) | CROBF009-19 | OL874461 | CROBB9 |
| VMS-B61 | Žrnovnica River, spring (CRO) | CROBF010-19 | OL874463 | CROBB10 |
| VMS-B30 | Žrnovnica River, spring (CRO) | CROBF006-19 | OL874460 | CROBB6 |
| VMS-B102 | Kupica River, spring (CRO) | CROBF008-19 | OL874462 | CROBB8 |
| EB144-MK | Galička River, spring (RNM) | CROEL024-21 | OL874459 | CROBB879 |
| Microhabitat/Month | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plitvice Lakes NP, CRO | February | March | April | May | June | July | August | September | October | November | December | January |
| Spring of the Crna rijeka River, bryophytes | only ♂ | 0 | 0 | 0.67:1 | 0.93:1 | 0.87:1 | 1.46:1 | 0.8:1 | 1.07:1 | 0 | 1.11:1 | 0 |
| Middle reach of the Crna rijeka River, bryophytes | 0.5:1 | 0 | only ♂ | 1 | 3:1 | 2:1 | 1 | only ♀ | 5:1 | 0 | only ♂ | 1.5:1 |
| Spring of the Bijela rijeka River, angiosperms | 0 | 0.33:1 | 0.5:1 | only ♂ | 0 | only ♀ | 1 | 0.5:1 | 0 | only ♂ | 0 | 0 |
| Spring of the Bijela rijeka River, bryophytes | 1.42:1 | 1 | 0.42:1 | 0.33:1 | 2.08:1 | 0.9:1 | 9:1 | only ♂ | 1.67:1 | only ♀ | 0.17:1 | only ♂ |
| Upper reach of the Bijela rijeka River, angiosperms | 0 | only ♀ | 0 | 0 | 0 | only ♂ | only ♂ | only ♂ | 0 | only ♀ | only ♂ | only ♀ |
| Upper reach of the Bijela rijeka River, bryophytes | 5:1 | 2.67:1 | 0.33:1 | 2.17:1 | only ♂ | 1 | 2.17:1 | 1.75:1 | 0.67:1 | 0.4:1 | 2.5:1 | only ♀ |
| Spring of the Cetina River, CRO | ||||||||||||
| Angiosperms | 0 | 0 | 0 | 0 | 0 | 0 | 1 | only ♂ | 0 | 0 | 0 | 0 |
| Pebbles | 0 | only ♀ | 0 | 0 | 0 | only ♀ | 0 | 0 | 0 | 0 | 0 | 0 |
| Spring of the Bistrica River, B&H | ||||||||||||
| Bryophytes main course | 1.3:1 | 0.74:1 | 0.78:1 | 0.99:1 | 0.93:1 | 1.84:1 | 1.02:1 | 1.24:1 | 0.62:1 | 1.47:1 | 1.76:1 | 0 |
| Bryophytes side channel | 0.59:1 | 0.53:1 | 0.23:1 | 0.77:1 | 0.61:1 | 0.17:1 | 0.91:1 | 1.77:1 | 1.2:1 | 2 | only ♀ | 1.07:1 |
| Bryophytes side course | 1.45:1 | 0.95:1 | 0.85:1 | 1.45:1 | 1.68:1 | 1.46:1 | 1.02:1 | 1.48:1 | 1.24:1 | 2 | 1.09:1 | 0 |
| Pebbles main course | 1.16:1 | 1.28:1 | 1 | 0.5:1 | 3.5:1 | 0.83:1 | 0 | 0.31:1 | 0 | 1.44:1 | 1 | 1.5:1 |
| Sand and gravel main course | 0.82:1 | 0.87:1 | 0.86:1 | 1.33:1 | 0.65:1 | 0.69:1 | 1.04:1 | 1.67:1 | 1.13:1 | 0.44:1 | 1 | 1.37:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mičetić Stanković, V.; Bruvo Mađarić, B.; Kučinić, M. Ubiquitous but Ignored? A Case of Water Beetle in Southeastern Europe. Diversity 2022, 14, 26. https://doi.org/10.3390/d14010026
Mičetić Stanković V, Bruvo Mađarić B, Kučinić M. Ubiquitous but Ignored? A Case of Water Beetle in Southeastern Europe. Diversity. 2022; 14(1):26. https://doi.org/10.3390/d14010026
Chicago/Turabian StyleMičetić Stanković, Vlatka, Branka Bruvo Mađarić, and Mladen Kučinić. 2022. "Ubiquitous but Ignored? A Case of Water Beetle in Southeastern Europe" Diversity 14, no. 1: 26. https://doi.org/10.3390/d14010026
APA StyleMičetić Stanković, V., Bruvo Mađarić, B., & Kučinić, M. (2022). Ubiquitous but Ignored? A Case of Water Beetle in Southeastern Europe. Diversity, 14(1), 26. https://doi.org/10.3390/d14010026