Abstract
Jawed vertebrates (gnathostomes) have been the dominant lineage of deuterostomes for nearly three hundred fifty million years. Only a few lineages of jawless vertebrates remain in comparison. Composed of lampreys and hagfishes (cyclostomes), these jawless survivors are important systems for understanding the evolution of vertebrates. One focus of cyclostome research has been head skeleton development, as its evolution has been a driver of vertebrate morphological diversification. Recent work has identified hyaline-like cartilage in the oral cirri of the invertebrate chordate amphioxus, making cyclostomes critical for understanding the stepwise acquisition of vertebrate chondroid tissues. Our knowledge of cyclostome skeletogenesis, however, has lagged behind gnathostomes due to the difficulty of manipulating lamprey and hagfish embryos. In this review, we discuss and compare the regulation and histogenesis of cyclostome and gnathostome skeletal tissues. We also survey differences in skeletal morphology that we see amongst cyclostomes, as few elements can be confidently homologized between them. A recurring theme is the heterogeneity of skeletal morphology amongst living vertebrates, despite conserved genetic regulation. Based on these comparisons, we suggest a model through which these mesenchymal connective tissues acquired distinct histologies and that histological flexibility in cartilage existed in the last common ancestor of modern vertebrates.
1. Introduction
The evolution of vertebrates involved substantial elaboration of the ancestral chordate body plan. Many of these changes were made possible by the origin and deployment of neural crest cells (NCCs), which migrate throughout body and differentiate into a variety of tissues [1,2,3,4,5]. The major derivative of cranial NCCs is cartilage, the structural basis of the developing head skeleton. While it has been demonstrated that cartilage was likely present in the last common ancestor of chordates [6,7,8] and possibly metazoans [9], the vertebrate lineage has considerably expanded its deployment, providing the initial scaffolding for the head and limb skeletons. Even in bony vertebrates (Osteichthyes), cartilage persists in areas of the skeleton, particularly in most joints, where it is known as articular cartilage. On the molecular and histological levels, these cartilage cells (chondrocytes) contrast with those of their non-chordate counterparts, as the cells are more compact, produce a thicker extracellular matrix (ECM) of proteoglycans and collagens, and embed themselves into said ECM [10]. The origin of the vertebrate lineage also coincides with genome expansion, as they are thought to have collectively undergone at least one whole-genome duplication and likely segmental duplications as well [11,12,13,14,15]. This suggests that the novel composition of vertebrate cartilage and its deployment may have been partly driven by the evolution of new vertebrate genes [16]. Learning how the ancestral chondrocyte gene regulatory network (GRN) has been modified over evolutionary time is thus important for our understanding of cartilage development, deployment, and maintenance.
Unfortunately, the diversity of living vertebrates, which is dominated by gnathostomes, cannot provide us a complete explanation for the stepwise acquisition and modification of the cartilaginous skeleton. An important evolutionary event associated with the skeleton was the origin of the jaw, a modification of the first pharyngeal arch. Changes in the dorsoventral patterning of this arch is thought to have contributed to the formation of a medial joint which helped facilitate respiration as well as the capture of prey [17,18,19,20,21]. Additionally, it has been suggested that new skeletal cell types had evolved as well to support the joint, composed of articular cartilage surrounded by the synovium [20]. The evolution of the jaw thus provides one opportunity to understand the overall evolution of vertebrate chondrocytes, and related cell types. Unfortunately, the vast majority of extant vertebrates have jaws and therefore tell us little about this stepwise process. The few remaining jawless vertebrate taxa are known as cyclostomes, and they are an important lineage for understanding the evolution of vertebrate chondrogenesis. This superclass consists of lampreys and hagfishes of approximately 110 species, and great progress has been made in recent decades to understand their development. The evolutionary relationship between lampreys and hagfishes was contentious for decades, with classical morphological analyses placing hagfishes as a sister clade to lampreys and gnathostomes [22,23]. However, both molecular and morphological data in recent years confidently support lampreys and hagfishes as a monophyletic group. [24,25,26,27,28,29]. These species are very distantly related nonetheless, with modern estimates suggesting a divergence likely more than four hundred million years ago [30]. Fossil cyclostomes like Priscomyzon riniensis [31,32] and Myxinikela siroka [33,34] suggest that the adult morphology of lampreys and hagfishes has been largely conservative for at least three hundred million years and that many these differences in form may have arose not long after their divergence. In our understanding of the stepwise evolution of the vertebrate skeleton, we are therefore presented with two challenges: (1) identifying core conserved features of vertebrate skeletal tissue; (2) identifying features that are likely evolutionary novelties in both jawed and extant jawless vertebrates.
To accomplish this, a comprehensive analysis of chondrogenesis in both cyclostomes and gnathostomes chondrogenesis is needed. While data from jawed vertebrates are abundant, we have considerably less information from lampreys and hagfishes. The objective of this review is to summarize and detail these findings, from the initial morphological and histological findings of the 19th century to more recent work with gene expression and other genetic and molecular methods. The wealth of gnathostome data can be a framework for comparing cartilage development in both lineages, providing insights not only into chondrogenesis in general but also the development of related connective tissues. Between the archetypal hyaline cartilage of gnathostomes as well as other cartilages and cartilage-like tissues, we see many shared characteristics with the cyclostome skeleton. Overall, we see an intrinsic malleability of the vertebrate chondrocyte GRN, with varying features of non-skeletogenic connective tissues. These comparisons indicate that skeletal heterogeneity was likely a feature in the last common ancestor of gnathostomes and cyclostomes, and these cell types have inherent similarities with tissues like the perichondrium and tendons.
2. Overview of the Cartilaginous Skeleton of Lampreys and Hagfishes
There are vast differences between the head skeleton of cyclostomes and gnathostomes, enough so that few elements of the differentiated head skeleton can be confidently homologized between the two groups [35,36]. Even within cyclostomes, skeletal anatomy differs considerably (Figure 1), but they are thought to be derived from similar developmental precursors [35,36]. It has been proposed that the cyclostome skeleton is divided into seven components of either neural crest or mesoderm origin: emanating from the neural crest would be the anterior nasal cartilages, post-hypophyseal cartilages, mandibular, velar, and branchial cartilages, while the mesoderm would give rise to the trabeculae and otic capsules (Figure 1) [2,3,4,35,36,37].
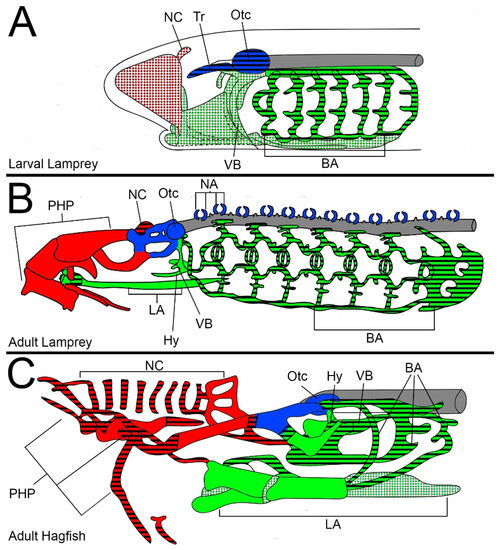
Figure 1.
Overview of cyclostome cartilaginous head skeletons. (A) Larval lamprey. (B) Adult lamprey. (C) Adult hagfish. Colors correspond to suggested tissue contributions. Red: Pre-mandibular neural crest. Green: Mandibular/Post-mandibular neural crest. Blue: Mesoderm. Textures correspond to distinctions in cartilage type. Solid color: hard cartilage. Color with black stripes: soft cartilage. White with colored spots: cartilage-like tissues. Cartilage distinctions for larval and adult lampreys are adapted from Parker (1888) and Johnel (1948) illustrations and descriptions. Cartilage distinctions for hagfishes are adapted from Cole (1905) illustrations and descriptions. Keywords: BA: branchial arches; Hy: hyoid; LA: lingual apparatus; NA: neural arches; NC: nasal cartilage(s); Otc: otic capsule; PHP: post-hypophyseal processes; Tr: trabecula; VB: velar bar.
The anterior nasal cartilages and post-hypophyseal cartilages are of great interest in the evolution of jaws, as they originate from the pre-mandibular neural crest, a lineage of NCCs which is thought to have sparsely contributed to the ancestral vertebrate head skeleton [37]. While the anterior nasal cartilages of lampreys comprise a single nasal capsule, they are part of a more elaborate nasal skeleton in hagfish of several ring cartilages surrounding a rostrally-projected nasal duct. The post-hypophyseal cartilages in both lineages, although similarly abundant, serve different functions. The post-hypophyseal skeleton of hagfishes largely supports the nasal duct and the sensory tentacles while the equivalent cartilages in lampreys support the feeding apparatus. Although the anterior nasal cartilages and post-hypophyseal cartilages are a potential synapomorphy of the cyclostome lineage or symplesiomorphic for all vertebrates, the differences between these tissues in lampreys and hagfishes leave us unable to determine the ancestral distribution for these cartilages.
Another intriguing feature that is shared in cyclostomes is the lingual apparatus, a set of cartilages which use a pulley-like motion for feeding. A paired set of keratinous teeth rests on top of the anterior end of the lingual apparatus. Lampreys have additional medial teeth which are dorsal to those. Although hagfish developmental studies support that the lingual apparatus is derived from the mandibular arch [35,36], it remains unclear from which exact pre-metamorphic ammocoete structures the adult lamprey lingual cartilages are derived. The presence of a tooth-covered “tongue” skeleton for feeding in both lineages, regardless, strongly supports that it existed in the last common ancestor of cyclostomes and was an independent innovation since their split from jawed vertebrates [28].
The presence or absence of vertebral elements in lampreys and hagfishes is another important reminder that both lineages have independently deviated from gnathostomes and the ancestral vertebrate body plan. Dorsal vertebrae are well documented in adult lamprey, but the development of these tissues remains largely unknown. Conversely, rudiments of ventral vertebral elements in the post-anal tail have been identified in the inshore hagfish E. burgeri but not in the Atlantic hagfish M. glutinosa [38,39,40], supporting variation not only between lampreys and hagfishes but also within these lineages. In contrast to gnathostomes, the vertebra rudiments in both lampreys and hagfishes do not envelop the notochord, a trait present in the vertebral column of all jawed vertebrates. Taken together, these data support both dorsal and ventral elements in the vertebral skeleton in the last common ancestor of cyclostomes and gnathostomes, with each lineage losing part of this vertebral skeleton [38,39].
There are considerable differences in the morphology of lampreys and hagfishes, and their life histories contrast as well. Whereas hagfish have direct development, modern lampreys have a larval stage lasting several years during which their morphology is noticeably different from their adult form. During this larval (ammocoete) period, lampreys are burrowing filter feeders that use their velar skeleton to pump and siphon detritus and algae [41,42,43]. Much of the pre-mandibular skeleton at this point still supports the feeding apparatus, but the branchial basket is more rudimentary. During metamorphosis, the head skeleton de-differentiates and is remodeled [44]. Whether an extended filter feeding larval stage is a vertebrate symplesiomorphy or a lamprey-specific innovation remains controversial, as ammocoetes have not been found for stem lampreys [32]. Regardless, the early development of lampreys remains an important area of research for vertebrate skeletal evolution, as their embryos are more accessible than those of hagfishes, and comparisons can be made between this larval form and the more direct-developing hagfishes despite these differences in life history.
The paucity of extant jawless vertebrates means that we must take a more nuanced approach in understanding the cyclostome skeleton, one that considers both their shared and divergent characteristics between each other as well as with gnathostomes. Lampreys and hagfishes have considerable differences in their ecology, and their morphologies reflect this. Despite these differences, a molecular and cellular approach can help reconcile what anatomy cannot. We will review the ways in which past and present work has elucidated the evolutionary significance of the cyclostome skeleton, from broader histological and histochemical observations to more precise gene expression and functional studies.
2.1. History of Cyclostome Cartilage Research
Research on the general morphology and composition of cyclostome skeletons began in the early 19th century, Johannes Müller making perhaps the first comprehensive analysis of both lamprey and hagfish skeletons [45]. Once evolutionary theory was mainstream among biologists, morphologists tried to homologize parts of the cyclostome skeleton with their gnathostome counterparts. Though many of these homology arguments were controversial at the time, and most have since been largely rejected, early morphologists arrived at similar conclusions regarding the histology of the cyclostome skeleton, dividing its contents into “hard” and “soft” cartilages with different features [45,46,47,48] [Figure 1]. These cartilage types in a particular cyclostome were contrasted with gnathostome cartilage. This contrast between skeletal types, however, often lacked thorough criteria, meaning that certain parts of the lamprey and hagfish skeletons were debated as to whether they were “hard” or “soft” cartilages, even across different species of the same group [48]. Our understanding of the cyclostome skeleton was further elaborated by the identification of soft and hard “pseudo-cartilages” throughout the hagfish head, whose characteristics were thought to resemble less a bona fide cartilage but rather sesamoids, bones that develop within tendons or muscles [48,49]. Cartilage-like tissues were also identified throughout the anterior portion of the larval lamprey skeleton, a cell type which would become known as “mucocartilage” [50,51]. For these early histologists, the cartilage-like tissues of cyclostomes lacked any single unifying feature, but rather had various combinations of features which they shared with cartilage-like tissues. The nature of these tissues will be further discussed here, but for now, it is important to understand that, even before the advent of modern genetic and molecular methods, 19th- and early 20th-century morphologists recognized skeletal heterogeneity in cyclostomes and were interested in its evolutionary significance.
As molecular and histological methods improved, so did our understanding of hagfish and lamprey anatomy. Alongside ribosomal RNA evidence which supported cyclostome monophyly [24,26,27], more recent work identified sclerotome-derived rudiments of skeleton in the post-anal tail of the inshore hagfish E. burgeri [38,39,40], and fossil evidence further supports cyclostome monophyly and modern hagfish morphology as degenerate rather than primitive for vertebrates [29]. Together, these highlight the importance of genetics, development, and fossil evidence to corroborate morphological data, providing a more robust framework for exploring the evolution of the cyclostome skeleton.
2.2. The “Hard” and “Soft” Cartilages of Cyclostomes
Despite the variety of cartilage and cartilage-like tissues found in both lampreys and hagfishes, the consensus for early authors was that these categories of “soft” and “hard” cartilages were homologous between cyclostomes and that the “hard” category of tissue in both groups was likely homologous to the hyaline cartilage found in gnathostomes [46,48,50,51] [Figure 2C–F]. The main defining criteria for this classification were based on a combination of ground substance and particular staining affinities [48,50]. While there were some descriptions of individual cell morphology for hard and soft cartilages [48,51], these have been met with skepticism by modern researchers due to the prevalence of fixation and other artefacts in classical studies [52]. These classical studies lacked the cellular and molecular resolution of modern analyses, but more recent histology allows us to compare the features and distribution of these skeletal subtypes in cyclostomes, nonetheless.
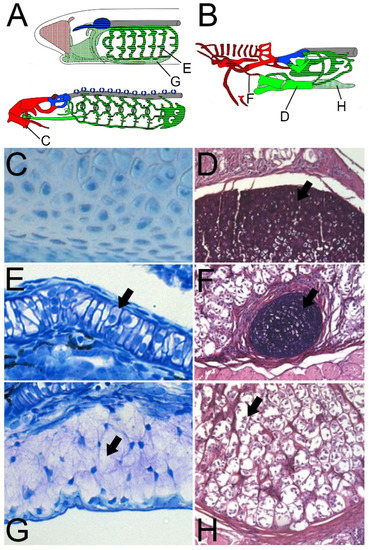
Figure 2.
Variants of cartilage in cyclostomes. (A) Lamprey ammocoete (top) and adult lamprey (bottom). (B) Adult hagfish. Letters correspond with their respective panels. (C) Hard cartilage from adult lamprey annular cartilage. (D) Hard cartilage from adult hagfish medial basal plate. Both hard cartilages are surrounded by abundant ECM. (E) Soft cartilage from lamprey ammocoete branchial arch. (F) Soft cartilage from adult hagfish lateral tentacular cartilages. Both soft cartilages are compact and have sparse ECM. (G) Mucocartilage from lamprey ammocoete ventral pharynx. (H) Pseudo-cartilage from adult hagfish posterior basal plate. Both cartilage-like tissues have fibroblast-like morphology with cartilage features. Panels D, F, and H were reproduced via Miyashita (2012) with permission from Tetsuto Miyashita.
“Hard” cartilages in cyclostomes can be distinguished by scattered chondrocytes with abundant ECM (Figure 2C,D), whereas “soft” cartilages comprise dense cells with sparse ECM (Figure 2E,F). The most common pattern for “hard” and “soft” distributions is along the antero-posterior axis of lampreys and hagfishes. In both lineages, the branchial skeleton is composed of “soft” cartilages whereas the anterior pre-mandibular skeleton comprises “hard” cartilage [46,48,50,51]. There are some exceptions to this pattern, as the nasal skeleton of both lineages is primarily made of “soft” cartilage, although hagfishes contain some of both types [46,48,50,51]. An interesting skeletal topology can be observed in the lingual apparatus in both groups wherein the primary lingual elements are “hard” but are linked by “soft” cartilages or cartilage-like tissues [46,48,50,51]. There can also exist heterogeneity within a single skeletal element, as the lamprey hyoid bar and trabecular processes contain “hard” cartilage in its rostral end and “soft” cartilage along the caudal portion [46].
The differences in lamprey and hagfish skeletal morphology can be somewhat reconciled by these observations of “hard” and “soft” cartilages. It is likely that the last common ancestor of cyclostomes possessed a branchial skeleton which was made of “soft” cartilage and a pre-mandibular and lingual skeleton made mostly of “hard” cartilages. It is interesting to note that most of the cyclostome pre-mandibular skeleton is “hard” cartilage likely homologous to gnathostome hyaline cartilage yet is derived from pre-mandibular NCCs [35,36]. The modified nasal skeleton of hagfishes complicates our classification of these cartilages, as the derived tube-like cartilaginous rings are “soft” like the lamprey nasal capsule, but the hagfish nasal capsule itself is “hard”, although there are more modern histological analyses which suggest that it may in fact be “soft”. We therefore posit that the last common ancestor of cyclostomes had a nasal skeleton composed mostly of “soft” cartilage, but we cannot be certain whether “hard” elements were also present. The heterogeneity seen in the lamprey trabecular processes and hyoid bar are another conflicting finding, and we cannot ascertain whether this sort of multitype cartilaginous structure was present in the ancestor of lampreys and hagfishes. Nonetheless, these classic histological studies provide a basis for our understanding of skeletal heterogeneity in cyclostomes by dividing the skeleton into smaller components of shared traits which can be analyzed piecemeal with more robust modern methods.
2.3. Cyclostome Cartilage-Like Tissues: Hagfish Pseudo-Cartilage and Lamprey Mucocartilage
Early morphologists recognized that several types of cyclostome connective tissues had characteristics which did not correspond perfectly to one gnathostome cell type or another, and they were understood to have some relevance for the evolution of the vertebrate skeleton. Many of these findings are inadequate from a modern developmental perspective, as they often try to tie these tissues into ideas of directional evolution and are cited as “transitional tissues” [48,49]. The original word coined for these vertebrate cartilage-like tissues, “vorknorpel” (German: pre-cartilage) is thus also used to refer to connective tissues in invertebrate mollusks and vertebrate structures like the sesamoid nodule in the Achilles tendon [49,50]. Even still, these early findings regarding “vorknorpel” in cyclostomes may still be of relevance to us, as we can apply modern understandings of development to their descriptions. These cartilage-like tissues have a cell morphology similar to that of fibroblasts (Figure 2G,H) yet stain in similar ways to more traditional vertebrate cartilages. Like the “hard” and “soft” cartilages, we can use these early descriptions to group cell types based on histology, using future work to test these relationships.
The “vorknorpel” of hagfishes, later coined “pseudo-cartilages”, comprises two distinct types of tissues in the head skeleton (Figure 2H). Together, they are a minority of the skeletal tissue, located mostly in the lingual apparatus as well as parts of the branchial skeleton. Like the more traditional cartilages, these pseudo-cartilages have a “hard” and “soft” subtype, and both are considered structurally more similar to the “soft” cartilages in the branchial and fin skeletons [48]. Both pseudo-cartilages are enclosed in a thick perichondrium, contain branching fibers throughout, and are often multinucleated with granular nuclei. The main histological difference between these pseudo-cartilages is that the “hard” subtype is denser, and its fibers are more regularly arranged and ordered than its “soft” counterpart. The connection between the pseudo-cartilages and sesamoids is interesting, as one of the main locations for “soft” pseudo-cartilage in the hagfish skeleton is embedded in the protractor and retractor tendons of the lingual apparatus.
The “vorknorpel” of lampreys is also known as “mucocartilage”, as the intercellular substance of these cells is rich with basophilic mucous-like substance (Figure 2G) [50,51,53]. Unlike hagfishes, this cartilage-like tissue is only present in the larval ammocoete life stage of lampreys. During metamorphosis, the mucocartilage is thought to dedifferentiate into a loose mesenchyme which then re-differentiates into an adult hyaline cartilage type [44]. The mucocartilage has a mesenchymal morphology, is surrounded by a thick perichondrium, and contains multiple types of irregular fibers interspersed across the cell [51]. There are some exceptions to this, as the mucocartilage of the ventrolateral skeleton contains regular parallel fibers. The development of the mucocartilage cell and its surrounding fibrous network appear to occur in distinct phases. These fibers appear largely unique to lamprey, as some of them have elastin-like properties but are not considered true elastin [54].
While the descriptions of lamprey mucocartilage and hagfish pseudo-cartilage appear similar, these descriptions could also be used to describe most fibroblasts. This overlap of characteristics with non-cartilaginous tissues is a theme which will be persistent throughout this review. Although pseudo-cartilage and mucocartilage may not be chondrocytes sensu stricto, they possess multiple features common to one connective tissue or another. As we will see with gnathostome cartilage, these cell types are not monolithic in properties either, and some gnathostome cartilages will share features with these cyclostome “cartilage-like” tissues. The presence of fibril networks throughout mucocartilage and pseudo-cartilage is reminiscent of fibrocartilage which can be found in the intervertebral discs and tendon insertion sites of gnathostomes [55]. While it seems too speculative to assume the homology between these connective tissues based on histology alone, it shows us the extent to which we can assess vertebrate cartilages.
2.4. Looking Forward: A Synthesis of Classic Cyclostome Histology
From the “hard” cartilage of lampreys and hagfishes to pseudo-cartilage and mucocartilage, we can see a spectrum on which the various cyclostome chondrogenic tissues have different levels of similarity to archetypal gnathostome cartilage. While classic morphologists were often keen on directional evolution and deducing “steps” along the course of vertebrate evolution, we can still use their basic histological framework to understand the evolution of the vertebrate skeleton. Hagfishes and lampreys are a sister group to gnathostomes and are a valuable reference point for understanding the stepwise acquisition of the cartilaginous skeleton, but we must be careful if the traits we observe are homologous to something in gnathostomes, symplesiomorphic for all vertebrates, or are a derived state unique to cyclostomes. A similar conundrum in vertebrates regards the evolution of hard tissues like bone, enamel, and dentine, where early vertebrates may have evolved several different types of these tissues before canalizing a select few later on [56]. While evolutionary origins of cartilage diversity are still unclear, these classic histological studies provide us a basis for comparing features between gnathostome and cyclostome cartilages to determine which, or if any, of these cell types are homologous.
3. Overview of Gnathostome Cartilage Development
A proper comparison of vertebrate cartilages requires both loose and strict parameters for understanding chondrocytes and their histogenesis, as we need to define a more universal chondrocyte GRN which all vertebrate cartilages utilize. Although there have been several genomic and morphological changes in both jawed and jawless lineages, comparative gene expression and function studies can partially reconcile these differences. Gnathostome chondrogenesis is well-studied and it provides a starting reference for understanding the more intricate parts of vertebrate cartilage development. It is from these studies in humans, zebrafish, chickens, and other gnathostomes that we can determine which chondrogenic genes should have priority in future cyclostome studies.
Most recent work on vertebrate skeletal evolution has focused on understanding the evolution of skeletal element morphology. While this is certainly important, it does not directly address the evolution of chondrogenesis and chondroid cell types. We focus here primarily on the genetic basis of mesenchymal condensation, chondrogenesis, cartilage proliferation, as well as chondrocyte maturation.
3.1. All Gnathostome Cartilages Develop Using a Conserved Gene Regulatory Network
The core components of the gnathostome cartilage GRN are thought to be the transcription factor sox9 and the extracellular matrix genes col2a1 and acan. Multiple studies have found putative sox9 target sites in the cis-regulatory regions of col2a1, col9a1, acan, and the transcription factors sox5 and sox6 [57,58,59,60,61]. Some of these enhancers are even thought to be chondrocyte specific as well [60,61]. Before sox9 activity, initial cartilage development is largely dependent on TGFβ signaling like bmp2, bmp4, and gdf5, as they regulate the initial mesenchyme condensation and also the pace and location of chondrocyte maturation (Figure 3) [62,63,64,65,66,67,68,69]. The receptors involved in the expansive TGFβ pathway, including TGFβRs and BMPs, overlap and compete during chondrogenesis. TGFβ signaling and BMP signaling collaborate to prevent differentiation. Simultaneously, TGFβ signaling also antagonizes BMP signaling to prevent the cartilage from entering hypertrophy [62,66]. FGF signaling occurs in conjunction with hedgehog signaling to maintain chondrocytes between proliferation and differentiation, and the downstream targets trps1, gli1, gli2, and gli3 all have variable roles in regulating sox9 expression [70,71,72,73]. Conversely, Wnt signaling is thought to negatively regulate chondrogenesis primarily through β-catenin, which has been demonstrated to inhibit differentiation in non-articular cartilage largely through modulating cyclin D1 [74,75,76]. While col2a1 and acan are the main structural basis of gnathostome cartilage once it differentiates, ECM proteins like type IX and type XI collagen also have important roles in stabilizing the cartilage matrix and its various proteins and macromolecules [77]. Taken together, gnathostome cartilage has a core cassette of genes which have been largely conserved, from the transcription factors and signaling ligands that regulate each step of its development to the collagens and proteoglycans involved in cartilage homeostasis.
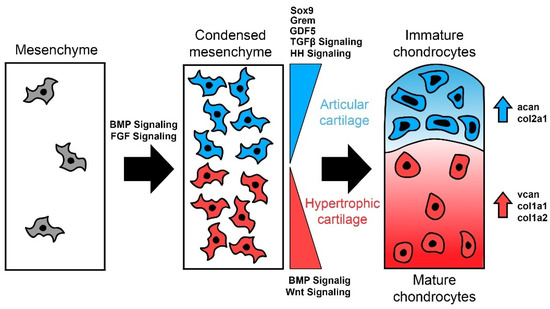
Figure 3.
Differentiation of cartilage along the joint interface in osteichthyans. BMP signaling and FGF signaling contribute to initial mesenchymal condensation while genetic factors proportionally contribute to either articular and hypertrophic cartilage development. Increased TGFβ and hedgehog (HH)signaling along with grem, gdf5, and sox9 activity drive prechondrogenic mesenchyme towards the articular cartilage fate (blue), with increased expression of archetypal cartilage extracellular matrix (ECM) genes like acan and col2a1. Conversely, increased WNT and BMP signaling drive these cells towards hypertrophy (red) and expression of bone-related ECM genes like vcan, col1a1, col1a2.
Articular cartilage is the main cartilaginous tissue in adult Osteichthyes, and is maintained in distinct zones to separate permanent cartilages from those that are transient in the developing bony skeleton, otherwise known as hypertrophic cartilage (Figure 2). Articular cartilage comprises four specialized zones—the superficial zone, the middle zone, the deep zone, and the calcified zone—and these layers differentially regulate chondrogenic genes [78]. There are several transcription factors and signaling ligands involved in delineating the articular cartilage and its various zones from the more transient hypertrophic cartilages. TGFβ signaling and gdf5 have been implicated in establishing the area of future articular cartilage from mesenchyme and maintaining this area, otherwise known as the interzone [79,80,81,82]. Wnt signaling is thought to direct the pre-cartilaginous anlage away from the interzone and towards hypertrophy [74,75,76], and BMP signaling likewise has a similar role in this differentiation after mesenchymal condensation and initial chondrogenesis [62,67,83]. Because of this connection with genes like bmp2 and bmp4, the BMP antagonist gremlin is thought to be involved in maintaining articular cartilage [84,85]. The middle and deep zones, although largely similar to one another, have histological differences which can further distinguish them. While articular cartilages maintain expression of more archetypal cartilage ECM proteins like aggrecan and type II collagen [86], it also uses specialized proteins like lubricin for the function of articulating joints by reducing the friction between elements [87,88,89,90]. Hypertrophic cartilage, conversely, shares expression of genes more attributed to bone like vcan, col1a1, and col1a2 [84]. Because of this, hypertrophic cartilage complicates our understanding of cartilage types in gnathostomes, as they are associated with osteogenesis and mineralization, and it has been shown that some of these hypertrophic cells directly transdifferentiate into osteoblasts [91,92,93,94]. Whether hypertrophy necessarily ends with osteogenesis, however, is still not clear, as hypertrophic cartilages have been observed in both lampreys and hagfishes [95,96]. This histology has not been observed in chondrichthyan chondrocytes, even in mineralizing tissues that have type X collagen, a diagnostic marker for mature/hypertrophic cartilage [97,98]. Because of these discrepancies in hypertrophic cartilage across vertebrates, it is unclear if this cartilaginous tissue is a distinct skeletal type or a convergent cell phenotype. It remains possible that cyclostome and osteichthyan hypertrophic chondrocytes are homologous, but a more thorough understanding of these cells in lampreys and hagfishes is necessary before this could be supported. There are certainly distinct differences between gnathostome articular and hypertrophic cartilages, but it remains a challenge to uncouple features of osteogenesis from the maturation of these tissues.
Our understanding of vertebrate chondrogenesis is complicated by vast differences in morphology between taxa, but there is a cartilage developmental program that is conserved across all, nonetheless. As a cell type, there are a number of transcription factors, signaling ligands, and extracellular matrix proteins that are active in the developing cartilages of all species examined. From this reference, it is possible to compare gene regulation in homologous tissues across species, and different cartilages within the same species or group.
3.2. Gnathostomes Have a Diversity of Cartilage Types
A spectrum of cartilaginous cell types has been previously suggested in gnathostomes [10,99], but there are differences in this diversity amongst jawed vertebrates. It is commonly accepted that there are three cartilage types in tetrapods (hyaline, elastic, and fibrocartilage), but there have been as many as five to ten different types proposed in teleost fishes [10,99]. The extent to which these teleost cartilage types are distinct from one another, however, is unclear, as many of these types are restricted to a small subset of species or structures. For the purpose of this review, we will avoid the distinction between matrix-rich and cell-rich cartilages in gnathostomes. Although there is some evidence that this may be due in part to differences in the expression of catabolic enzymes like aggrecanases and collagenases [100,101,102,103,104], these distinctions between matrix-rich and cell-rich cartilages are largely subjective and only further complicate efforts to group these skeletal tissues. Additionally, we will avoid calcified cartilages in our analysis, as it becomes difficult to separate cartilage and bone development in osteichthyans, or they are associated with lineage-specific synapomorphies as is the case with the calcified tesserae of chondrichthyans [105]. With these considerations in mind, we can group non-mineralizing extant gnathostome cartilages based on the presence and absence of certain ECM proteins.
The most common vertebrate cartilage is hyaline (Figure 4A), which has the archetypal features of cartilage like an ECM containing type II collagen, aggrecan, chondroitin sulfate, and hyaluronan. The cells have a discoidal morphology and are embedded in clusters in the ECM known as lacunae, and they are surrounded by a fibrous perichondrium where immature chondroblasts differentiate and emerge into the lacunae. Mature hyaline cartilage is avascular and is not connected to adjacent cells through cell processes. As the most studied cartilaginous tissue, hyaline provides us a baseline to compare with other tissues. The differences between these cartilages and hyaline would therefore be indicative that they are distinct cell types.
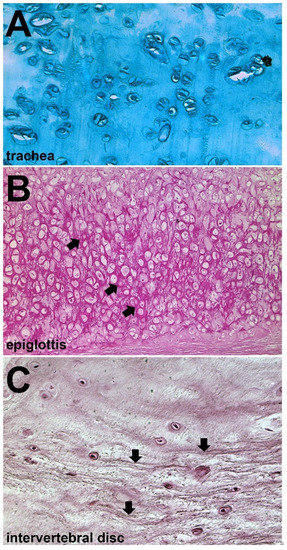
Figure 4.
Common variants of cartilage in tetrapods. (A) Alcian stain of hyaline cartilage from human trachea. Chondrocytes are embedded in a deep extracellular matrix rich in acidic proteoglycans. (B) Orcein stain of elastic cartilage from human epiglottis. Chondrocytes are similar to hyaline, but elastic fibers are more prevalent. Arrows indicate elastic fibers. (C) H&E stain of fibrocartilage from human intervertebral disc. Chondrocytes are more interspersed, and collagenous fibers, indicated by arrows, run mostly parallel to one another. All three photographs depict tissue sections from the histological collection of the Department of Zoology, Comenius University in Bratislava.
Another cartilage shared by teleosts and tetrapods is elastic cartilage (Figure 4B). The histology of hyaline and elastic cartilages is very similar, with the main distinction being the prevalence of elastin fibers in the latter [106,107,108]. It is found in the laryngeal/tracheal cartilages of mammals as well as the ears, while it can be found in the barbels of some teleosts [10,99]. Although the genetic factors involved in both of these skeletal tissues are similar, comparative expression shows that elastic cartilage downregulates important chondrogenic genes like alx1 and col2a1 [109]. Elastic cartilage as a skeletal type is poorly characterized outside of mammalian clades, so its unique mechanical properties as a cartilage and its resistance to resorption warrant closer inspection.
Both tetrapods and teleosts have a chondrogenic cell type which has larger amounts of collagens and fibrous proteins, categorized as “fibrocartilage” (Figure 4C). It is most frequently found along articulating surfaces like menisci, intervertebral discs, tendons, and the temporomandibular joint of mammals. The fibers of fibrocartilage are distinct from those in hyaline in that they are readily visible in histological sectioning and staining. The histogenesis of fibrocartilage is unique compared to other cartilaginous tissues in that it originates from either chondroprogenitors or dense fibrous connective tissues like tendons [55,110,111,112] Fibrocartilage and hyaline share expression of normal ECM proteins like type II collagen and aggrecan in lesser amounts, but fibrocartilage also has high levels of non-cartilaginous proteins like type I and type III collagen [55]. This cartilage is unique compared to other gnathostome skeletal tissues, and its features partially resemble those seen in the cyclostome cartilage-like tissues like hagfish pseudo-cartilage.
The diversity of cartilage types has been thoroughly explored in teleosts [10,99], and there are several connective tissue types in this group which share characteristics with cartilage. One of these groups of tissues is known as muco-chondroid, and its morphology is largely like that of lamprey mucocartilage. These mucous connective tissues have a variety of morphologies ranging from fibroblast-like cells to hyaline-like tissue and are embedded in a pale matrix [99,113,114]. Muco-chondroid is primarily found in the cranial region surrounding sensory organs like the olfactory and stato-acoustic structures as well as connective tissues beneath the skin. While muco-chondroid tissue has also been identified in the gills of a freshwater stingray [115], suggesting that this skeletal type is not necessarily a teleost-specific innovation. Furthermore, fibroblast-like muco-chondroid shares similarities with myxoid, an avascular connective tissue observed in mammals like cats and horses with a chondrocyte-like ECM that lies in close proximity to elastic cartilage and fibrocartilage [116]. Muco-chondroid and myxoid may not be homologous cell types, but they nonetheless represent variations of cartilage-like connective tissues which should be considered in our analysis of gnathostome cartilages.
Although the majority of the gnathostome cartilaginous skeleton is composed of hyaline, there are other cartilage tissues which should be considered in the broader context of vertebrate skeletal evolution. These cartilages deviate in their morphology and the extracellular matrix composition, likely due to differences in their core GRN. Several groups have posited that gnathostome cartilages should be considered a complex spectrum rather than a discrete set of cell types [10,99], and the differences and similarities between hyaline and elastic cartilage as well as fibrocartilage and other similar connective tissues prompt a closer look at this spectrum. When comparing cartilaginous tissues between gnathostomes and cyclostomes, we should look at the entirety of these tissues present in both groups to compare features.
4. Current Understanding of Cyclostome Cartilage Development
While there has been more considerable work on the patterning of the head skeleton in both lampreys and hagfishes, the GRN which directs condensed mesenchyme into chondrocytes is less understood. Modern molecular methods allow us to further understand the genetic mechanisms associated with cartilage development, but the vast difference in knowledge between gnathostomes and cyclostomes limits our insights into the latter. While the ideal scenario would be to characterize the expression of every gene known to be involved in gnathostome chondrogenesis for lampreys and test their function, we must prioritize the genes which have more conserved roles in the process. It is in this aspect, therefore, that we have explored parts of the GRN of chondrogenesis in cyclostomes, particularly in lampreys, but there is much more to be done.
4.1. Identification of Unique Proteins in Cyclostome Skeletons
Early biochemical studies in lampreys and hagfishes were often inconclusive in determining the composition of cyclostome cartilages, the best cases of which being the debate as to whether lamprey cartilage contained true elastin or collagen [54,96,117]. The use of cyanogen bromide for protein cleavage [54] helped resolve this issue by degrading methionine-rich proteins like collagens and isolating novel proteins in the cartilage of hagfishes and lampreys. These studies suggested that the cyclostome cartilaginous ECM does not contain significant amounts of collagen, a finding which changes our understanding of the ancestral vertebrate chondrocyte and its ECM. However, the discovery of these novel cyclostome-unique proteins also shows the extent that lampreys and hagfishes have an independently evolved skeleton.
The first unique cyclostome cartilage protein identified was lamprin, a hydrophobic protein with tandem repeats similar to those seen in elastin and invertebrate silk proteins [54,117,118]. Despite lamprin’s reactivity to elastin antibodies, its sequence is unlike anything else seen in gnathostomes. It is distributed throughout the entirety of the adult lamprey skeleton and is even located in the notochord sheath [119]. Another lamprin-like protein was identified using similar methods, its distribution mostly in the notochord sheath and the fin skeleton, but its exact molecular structure is unknown [54]. More recently, an additional lamprin-like protein was identified in the transcriptome of the Arctic lamprey L. camtschaticum, a single exon gene known as pharymprin with distinctive repeat domains from lamprin and is expressed primarily in the ammocoete branchial cartilages [120]. Cyanogen bromide studies in hagfish likewise revealed a similar protein to lamprin, which was subsequently called myxinin [95,121]. The composition and general properties of lamprin and myxinin are thought to be like one another, but their relationship remains unclear, as transcripts for myxinin have never been found. Interestingly, the distribution of myxinin is more heterogeneous than lamprin, as certain cartilage types contain large proportions of the protein while other cartilages contain none.
The nature of these cyclostome cartilaginous proteins complicates early vertebrate skeletal evolution. Despite the general absence of collagens in the cartilage ECM of lampreys and hagfishes, collagens have been identified in the cartilages of horseshoe crabs, cephalopods, and invertebrate chordates like amphioxus [6,7,9]. While it is possible that the last common ancestor of vertebrates had a skeleton of non-collagenous cartilage, it also remains plausible that cyclostomes have an independently modified skeleton which no longer requires it, but the larger implications of this will be discussed later. The existence of lamprin, myxinin, pharymprin, and a third potential lamprey protein are likely not symplesiomorphic for vertebrates but rather a cyclostome-unique change to cartilage. These proteins all contain similar repeating motifs, but it is not known whether these genes are rapidly evolving paralogs of an ancestral gene or are all independently-evolved ECM proteins. Despite the potentially independent origins of these genes, they are very useful to our understanding of cyclostome cartilage evolution, as they can help us further demarcate lamprey and hagfish cartilage types from one another.
4.2. Aspects of the Skeletal GRN of Hagfish Appear Similar to That in Gnathostomes
Our understanding of hagfish cartilage gene regulation and cartilage evolution has been lacking in part to the paucity of embryos which can be found for appropriate stages. Other developmental and evolutionary questions regarding cyclostomes further divide what few embryos are obtained. Despite these obstacles, progress has been made in the previous decade in understanding the development of the hagfish head skeleton and vertebrae.
The identification of vertebra-like elements in the ventral portion of the inshore hagfish E. burgeri in the post-anal tail was an important discovery and contribution to the idea that hagfish morphology was largely degenerate from the ancestral vertebrate condition rather than a primitive trait [38,39,40]. These vertebral elements share some characteristics with gnathostome vertebrae. They are derived from twist and pax1/9-positive sclerotome which migrates ventrally to encompass the aorta, and they also express transcripts for the cartilage ECM proteins decorin and biglycan [40].
As previously mentioned, type II collagen does not comprise a major portion of the hagfish cartilaginous skeleton. in situ hybridizations and immunohistochemical analyses provide conflicting evidence for this observation. Type II collagen antibodies showed reactivity throughout the “soft” cartilages of the head as well as the fin [122], but transcripts for col2a1 could only be identified in portions of this soft cartilage like the dental plate and interior lateral bar [123]. There are multiple explanations that could account for these differences, but the most likely is that the mammalian antibodies used did not target type II collagen specifically but rather another type of fibrillar collagen.
Even though a more thorough understanding of hagfish chondrogenesis and its gene regulation is necessary, we can make some inferences about hagfish cartilage. Perhaps the most important is that there is a molecular basis for the skeletal heterogeneity which had been observed. Paired with histological analyses, we can confidently say that the “hard” and “soft” cartilages as well as the pseudo-cartilages of hagfishes are distinct cell types with different ECM compositions, at least with respect to type II collagen. Although the extent to which these cell types are different remains unknown, we can expect to find a core cassette of ECM proteins and transcription factors involved in differentiation and maintenance. The limiting factor in our understanding of these processes is the lack of available embryos for analysis, but this is likely to change as our techniques for hagfish husbandry improve.
4.3. The Core Skeletal GRN of Lamprey Is Similar to That in Gnathostomes
In contrast to hagfish, lamprey embryos have been readily available for centuries, and it is from these species that much of our understanding of cyclostome biology and evolution has come. The similarities between lamprey branchial cartilage and that seen in gnathostomes provides a more direct comparison of chondrogenesis between the groups, while mucocartilage and the adult cranial cartilages allow us to explore ways in which the cyclostome skeleton has diverged and created new features. As discussed in the previous chapter, the main driving components of the gnathostome cartilage GRN are transcription factors from the soxE family and ligands from the BMP/GDF/FGF families while its principal ECM proteins are fibrillar collagens and proteoglycans. There have been several studies to characterize the expression and function of these conserved genes, but this GRN has not been further elaborated.
Lampreys have three duplicates of the soxE gene, and they are all expressed in the developing branchial chondrocytes [124]. Interestingly, soxE3 is the only gene which is expressed in mucocartilage. Loss-of-function experiments with soxE1 and soxE2 show greatly reduced chondrogenesis in the branchial arches while soxE3 morpholinos only caused differences in branchial chondrocyte morphology [125]. soxE expression has also been identified in the sclerotome [126], supporting its role in cartilage development outside of the neural crest-derived head skeleton. These findings together support the connection between soxE activity and chondrogenesis as a core feature to vertebrate cartilage.
The relationship between BMP/FGF signaling and lamprey chondrogenesis, however, is less clear. Lampreys have three duplicates of a BMP2/4 proortholog, and they have considerable differences in expression across time, with BMP2/4a being the only gene present in the early skeleton [127]. Later in development, however, all three genes can be found expressed in tissues adjacent to but not directly in branchial and annular cartilages. Considering that these genes are ligands, it is probable that they are still influencing chondrogenesis, but analysis of BMPr genes would be necessary to confirm this. Lampreys also have multiple duplicates of a GDF5/6/7 proortholog, but there are considerable differences in their expression. Of concern to us is GDF5/6/7b, which has been identified in the ventral pharynx and anterior head, both prominent locations and sources of mucocartilage [20]. Lastly, among the well-established ligands involved in chondrogenesis that have been studied in lampreys are the FGFs. These genes have both direct and indirect roles in the development of lamprey cartilage. Both FGF3 and FGF8 ligands as well as FGFRa are expressed in the nascent pharyngeal pouches adjacent to future cartilage, and inhibition of this signaling leads to the loss of branchial cartilage [128]. Additionally, FGFRa is expressed in the anterior mucocartilage while FGF8 can be identified in the adjacent pharyngeal ectoderm. Whereas inhibition of FGF signaling results in a dramatic loss of branchial chondrocytes, its effects on mucocartilage is much less pronounced, suggesting that FGFs are not necessary for mucocartilage differentiation but rather have an important role later in development. Taken together, BMP2/4, GDF5/6/7, and FGF3/8 homologs are important to chondrogenesis in both gnathostomes and lamprey, but the mechanisms and downstream targets are largely unknown in the latter group. Furthermore, the differences in signaling between lamprey branchial cartilage and mucocartilage are unclear and need to be elaborated more.
While some aspects of the lamprey cartilaginous extracellular matrix have been studied, most components remain unclear. Whether type II collagen is present in lamprey cartilage has been an area of great interest. Similar to hagfish, various studies have demonstrated that this protein does not comprise a major portion of the cartilaginous ECM [54,96]. Both in situ hybridization and antibody analyses confirm type II collagen throughout the skeleton later in development [126,129], but its presence in early ammocoetes is debated. While some results have suggested its presence [126], multiple studies were unable to find type II collagen before 32dpf in both the sea lamprey Petromyzon marinus and the Arctic lamprey Lethenteron camtschaticum [129,130]. The timing of this change is relevant for our understanding of cartilage evolution, as it shows that early chondrocytes in lamprey may not require type II collagen. Additional work has explored the deployment of lectican proteins in the cartilaginous extracellular matrix. While gnathostome cartilage primarily has the lectican protein aggrecan and occasionally versican, lamprey utilize an independently-duplicated set of four different lectican genes that are spatially scattered throughout its skeleton [16]. Overall, comparisons of cartilaginous ECM proteins between cyclostomes and gnathostomes, even if not comprehensive at this point, are valuable for our understanding of the ancestral cartilage GRN, as it lets us determine what features of cartilage morphology and histology are conserved or divergent in both lineages. lectican proteins were likely present in the cartilage of the last common ancestor of vertebrates, and it remains unclear whether type II collagen comprised part of the ancestral cartilaginous ECM, a finding which would change our understanding of vertebrate cartilage if true.
4.4. Larval Lampreys Have a Diversity of Cartilage Types
Gene expression studies in previous decades have further elucidated the development of the lamprey larval head skeleton, dividing it into various subpopulations (Figure 5). Paired with differences in cellular morphology, it provides the strongest evidence for these cartilages being distinct cell types. This diversity of cartilages shows us that, at the developmental level, these mesenchymal populations are receiving distinct signaling and are modifying the base chondrocyte gene regulatory network. Essential to this base GRN is the presence of soxE paralogs, which have been identified in all cartilages types in the lamprey head skeleton [124]. Furthermore, all of these cell types will stain for alcian blue and contain type II collagen later in development [19,124,129].
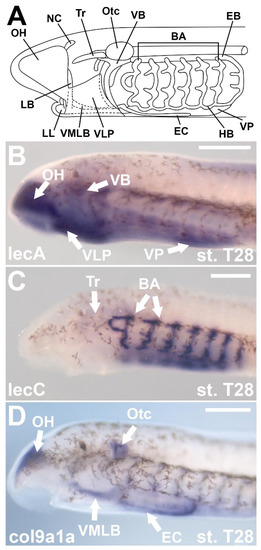
Figure 5.
Molecular heterogeneity in larval lamprey cartilages during development. (A) Diagram of the larval lamprey skeleton at Tahara [131] stage T28–30. (B) Expression of lecA at stage T28. (C) Expression of lecC at T28. (D) Expression of col9a1a at stage T28. Certain ECM proteins are restricted to different regions of the larval skeleton. Scale bar approximately 250 μm. Keywords: BA: branchial arches; EB: epibranchial bar; EC: endostylic cartilages (also known as ventromedial lateral bars); HB: hypobranchial bar; LB: lateral bar; LL: lower lip; NC: nasal capsule; OH: oral hood; Otc: otic capsule; Tr: trabeculae; VB: velar bar; VMLB: ventromedial longitudinal bar; VLP: ventrolateral plate; VP: ventral pharynx mucocartilage.
Non-mucocartilage skeletal tissue can be found in the branchial bars as well as the dorsal and ventral bars which run perpendicular to them and connect them, all containing discoidal or polygonal chondrocytes (Figure 6E,G) [129]. Interestingly, these gnathostome-like cartilages also express homologs of soxD. Conversely, there are two main subpopulations of mucocartilage based on cellular morphology alone. The dominant mucocartilage is composed of mesenchymal-like cells, and are distributed throughout the velum, upper and lower lip, and well as the lateral bars (Figure 6D,F). The minor mucocartilage has a much looser composition and can be found in the ventral pharynx and in the oral hood (Figure 6D,E). Taken together, larval lamprey likely have five morphologically distinct cartilage types, three being variants of a gnathostome-like chondrocyte and two being mucocartilage based.
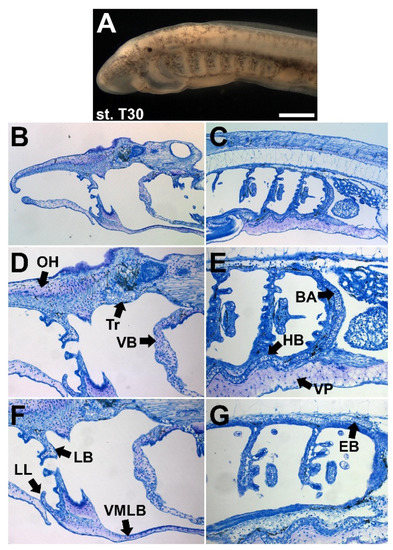
Figure 6.
Histological differences in larval lamprey cartilages. (A) Left lateral view of stage T30 lamprey. Scale bar approximately 100 μm. (B,D,F) Toluidine blue staining of T30 sagittal sections of the anterior head skeleton. (C,E,G) Toluidine blue staining of T30 sagittal sections of the posterior pharyngeal skeleton. Lamprey cartilages can be divided between mucocartilage (pale purple matrix) and hyaline-like tissues (discoidal morphology, thin blue matrix), but there are further differences in morphology and histology within these groups. Keywords: BA: branchial arches; EB: epibranchial bar; HB: hypobranchial bar; LB: lateral bar; LL: lower lip; OH: oral hood; Tr: trabeculae; VMLB: ventromedial longitudinal bar; VP: ventral pharynx mucocartilage.
5. Future Areas of Interest in Cyclostome Cartilage Research
While modern research has made great efforts in the previous decades to elucidate the cartilage gene regulatory network in cyclostomes, there is considerably more work to be done. There is a large disparity in our understanding of these processes between lampreys and hagfishes which must be reconciled. It is largely accepted that cyclostome morphology has diverged significantly since the lamprey and hagfish lineages diverged, and cyclostomes split from the lineage leading to gnathostomes, meaning that our understanding of vertebrate skeletal evolution would be incomplete without data from both lampreys and hagfishes. Furthermore, any comprehensive understanding of the chondrocyte gene regulatory network will need more information about the downstream targets of those core genes like soxE as well as BMP/GDF/FGF signaling. We have made great progress in elaborating more on the skeletal heterogeneity that has been observed in cyclostomes, but continued work on cartilaginous ECM proteins will make this much easier, providing a more robust way to characterize cell morphology and histology beyond visual comparisons. Lastly, comparisons of cartilage populations across different life stages will be valuable, particularly with lamprey. The low fecundity of hagfishes means that, even if methods improve to acquire more embryos, they will almost certainly still be eclipsed by the total number of lamprey embryos, placing heavy emphasis on ammocoete development. If we could homologize regions of the larval lamprey head skeleton with a metamorphosed adult, we can further understand cyclostome and vertebrate skeletal evolution by connecting lamprey larval and adult cartilage and subsequently lamprey and hagfish adult cartilages.
Molecular evidence suggests that lampreys and hagfishes may have diverged as early as 400 million years ago [30], meaning that we cannot assume the gene expression in one group reflects all cyclostomes. Furthermore, the dramatic change in lamprey ammocoete morphology during metamorphosis means that we cannot assume the homology of pre-metamorphic cartilage elements. It will therefore be important to compare early hagfish and lamprey development to determine whether lamprey pre-metamorphic chondrogenesis resembles that of hagfish. While some recent fossil evidence suggests that the lamprey ammocoete is a derived form [32], both the pre-metamorphic and adult lamprey skeleton remain important for larger evolutionary inferences with a non-metamorphic cyclostome. Advances in single cell RNA sequencing (scRNA-seq) can help elucidate what gross histology alone cannot, as we can create entire gene expression atlases of certain cell populations using a smaller number of specimens [132,133], which will be useful for cyclostomes whose embryos are hard to obtain like hagfishes. Furthermore, loss-of-function experiments like CRISPR/cas9 have been effectively developed in lampreys [1,134], meaning we can more thoroughly test the interaction of genes involved in the cyclostome cartilage GRN. Further work on both hagfish and lamprey embryos is still necessary to reconcile their differences for our understanding of vertebrate cartilage evolution, but new genomic and transcriptomic resources as well as genetic tools are making this increasingly feasible.
While there are a suite of specific transcription factors and signaling ligands which have been studied in jawed vertebrates, we should emphasize those genes which have been characterized across multiple taxonomic classes, as it is more likely that they are conserved rather than divergent elements of cartilage development. Among these likely conserved genes are those involved in hedgehog signaling as well as TGFβ signaling, as their roles in cartilage differentiation and proliferation have been well-documented [135,136,137,138,139]. While previous work has demonstrated the role of hedgehog signaling in lampreys during brain and somite patterning and development [140,141,142], its role in chondrogenesis is uncertain. TGFβ signaling is even less understood in lamprey, despite its important role in cartilage development. A thorough understanding of these signaling pathways in cyclostomes will provide insights into the conserved pathway of chondrogenesis and how these morphogenetic signals contribute to it. Our knowledge of transcription factors involved in cartilage development across gnathostomes is relatively narrow, with most work focusing on soxE/D homologs as well as genes involved in skeletal patterning. Of potential interest in cyclostomes are members of the Iroquois (irx) gene family, which are thought to be involved in both the patterning and differentiation of cartilage [143,144,145]. Additionally, the zinc finger transcription factor trps1 and homeobox barx1 should be of interest, as their roles in delineating and maintaining articular cartilage by balancing proliferation and differentiation are well known in gnathostomes [70,72,146,147,148,149]. Lastly, although previous work has already demonstrated the spatiotemporal expression of soxE/D homologs in lamprey, their piecemeal contribution to the head skeleton should be of interest, as it may provide insights into a base modularity of chondrogenesis in vertebrates by explaining cyclostome-specific modifications amongst these genes. If large-scale comparisons of cartilage development are to be ever taken between cyclostomes and gnathostomes, a deeper understanding of the genes involved and their respective functions will be necessary.
While aspects of the cartilaginous ECM have been elaborated in cyclostomes, they are but a portion of the total proteins involved. The extracellular matrix of cartilage contains other structures beyond collagens and lecticans like fibronectin, laminin, elastin, and other non-fibrillar collagens [88,106,107,150,151,152,153,154]. Additionally, the possible absence of type II collagen in cyclostome cartilage during early development is intriguing as well for our understanding of cartilage development and homeostasis. To further expand on the shared vertebrate cartilaginous ECM, exploration into these other components is necessary. These proteins may be able to explain more of the cellular heterogeneity in cyclostomes than transcription factors or signaling ligands and receptors. While the differences in chondrogenesis between lamprey mucocartilage populations remain largely unclear, the spatial differences in lectican homolog expression help support distinct subpopulations of cartilage [16]. If additional extracellular matrix genes could be studied in this detail, we could build a more comprehensive portrait of cyclostome skeletal heterogeneity from the bottom up. This heterogeneity at the cellular level is essential for our understanding of the vertebrate chondrocyte as a diverse cell type.
The diversity of cartilage types in lampreys and hagfishes is of great interest not only to our understanding of chondrogenesis but of connective tissue development entirely. Even if these cartilages are cyclostome-specific cell types, this developmental flexibility may have been present in the last common ancestor of vertebrates. The presence of multiple cartilage types in gnathostomes supports this intrinsic malleability of the chondrocyte as well as their similarities with other non-cartilaginous tissues. Mesenchyme forms not only skeletal cells but also adipocytes, myocytes, tenocytes, and various other connective tissues, so all of these cell types share a myriad of genes by default that are involved in differentiation [73,155,156,157,158,159,160,161]. The common ancestry of these cell types makes it possible that skeletal tissues are further influenced by signaling pathways which are associated with non-skeletogenic connective tissues. A more thorough review of the similarities between the development of cartilage and non-skeletogenic tissues is necessary if we are to understand the evolution of vertebrate connective tissues and its distinct lineages.
From a common mesenchymal progenitor, tendons and cartilage share many similarities during development compared to these other tissues, as several key regulatory genes are involved in the differentiation of both. Chondrogenic master genes like sox9 and gdf5 are important in the development of most tendons [162,163,164,165,166] while tenogenic master genes like scx and egr1 are also thought to play a role in the early development of chondroprogenitors [69,167]. The cell lineages are close enough that “fate-splitting” has been observed during early differentiation in the sclerotome. These ventral and dorsal components, destined as cartilage and tendons, respectively, share expression of both sox9 and scx are earlier stages but are selectively repressed by SHH and FGF signaling pathways which directs the sclerotome to one fate or the other [168]. The balance between these cell types is critical during early development, and heterotopic issues like tendon ossification are a common pathological condition which further shows the closeness of these cell types. Although the role of tenogenic genes like scx in this process is largely unknown, recent studies have shown a connection between tendon ossification and deficiency of mkx [169], a recently-discovered transcription factor heavily involved in tenogenesis [170,171]. Even normal tenocytes are capable of transdifferentiation into cartilage and bone, as the cells are highly responsive to mechanical stimuli and changes in mechanical force on tendons can also induce differences in gene expression similar to those seen during ectopic ossification [172,173,174,175,176,177]. This interplay between tendon and skeletal tissues is critical for the development of regular fibrocartilage and sesamoid bones. These bones protect tendon tissues by absorbing potentially damaging mechanical force and stabilizing the rotation and flexing of the tendon that it is associated with, depending on the type of sesamoid. Cells of the sesamoid tissue come from the same lineage that gives rise to bone superstructures, which form the zone where tendons attach to the bone [178]. These sesamoid bones have distinct gene expression during early development, with scx activity segregating early sox9-positive cells of the cartilaginous anlage towards future sesamoid tissues [178,179]. These sesamoids are still largely dependent on other genes involved in skeletal differentiation, and TGFβ and BMP signaling are maintained for this process. The differences between chondrogenic and tenogenic signals become more pronounced later in development, as the morphology and histology of these cells begin to differ more. Tendons are arranged in a linear fashion and have an elongated morphology, with type I collagen as its predominant ECM protein [180]. Archetypal cartilage, in contrast, has a variety of orientations and morphologies, and type II collagen is the most common ECM protein. There are several ECM genes that are uniquely shared by connective tissues, however, specifically the small leucine-rich proteoglycan (SLRP) gene family, and the similarities in expression of genes like biglycan, decorin, lumican, and fibromodulin in both tendons and cartilage have been observed [180,181]. These features together suggest that, although tendons and traditional cartilage have many differences later in development, they use several of the same genes involved in early differentiation and ECM composition. Cell types like gnathostome fibrocartilage and cyclostome “vorknorpel” have tendon-like features which distinguish them from more archetypal cartilages, and these similarities may be partially explained by the similarities in cartilage and tendon gene expression.
The diversity of cartilage types in both cyclostomes and gnathostomes supports the idea that cartilages exist on a spectrum with continuous features, and the applications of these extend beyond this to the cells that they are distantly related to. Gene expression data in cyclostomes still lag behind that of jawed vertebrates, but we have begun to compare genes that are relevant to that core chondrogenic GRN as well as its resting extracellular matrix. Understanding this gene regulatory network has important implications not only for homologizing cartilage types between vertebrates but also connective tissues as a whole, as they descend from common cell types and thus share gene expression during early development.
6. Conclusions
The evolution of the vertebrate lineage coincides with several innovations to the chordate skeletal system. Because most jawless vertebrates have been extinct since the Late Devonian, it has been difficult to determine the stepwise acquisition and development of this cartilaginous skeleton. Cyclostomes are therefore an important model for addressing this question, as they are the most distantly related extant vertebrate taxa from all jawed species. Lampreys and hagfishes have diverged considerably in their respective morphologies, but they retain shared characteristics that, when compared with their gnathostome counterparts, provide insights into the evolution of vertebrate cartilage. Modern molecular analyses allow us to further explore this process and expand upon what 19th-century morphologists noticed in initial comparative studies of lampreys and hagfishes, but our understanding of cyclostome biology continues to lag behind that of jawed vertebrates.
Despite these shortcomings, we have begun to discern the genetic factors involved in cyclostome cartilage development. The “hard” and “soft” cartilages of cyclostomes have unique characteristics that set them apart on the molecular level and confirm that they are distinct tissue types, even if the differences between them are still not fully understood. Likewise, the cartilage-like tissues like the pseudo-cartilages of hagfishes and the mucocartilage of lampreys have chondrocyte characteristics but a morphology partially resembling fibroblasts, akin to but more dramatic than the fibrocartilage of gnathostomes. Comparisons of the chondrogenic GRN between cyclostomes and jawed vertebrates are limited mostly by the paucity of data in the former, but these findings so far greatly help our understanding of vertebrate chondrogenesis by finding conserved factors like soxEs, GDF5, and BMP2/4 among others. There are multiple ECM proteins that are likely unique to lampreys and hagfishes, but their origins and importance for cartilage remain unknown. The unique morphology of pseudo-cartilage and mucocartilage also prompts an investigation into whether or how these cell types are influenced by non-chondrogenic signaling like fibroblasts or tenocytes. From a common mesenchymal progenitor, it stands to reason that there are many differentiation signals that are shared by these cell types. We posit, therefore, that the diversities of cartilages we see across vertebrates, even if not strictly homologous to one another, exist along a spectrum of signaling pathways wherein distinct cell morphologies arise (Figure 7). This spectrum is also shared by non-chondrogenic cells and may exist as a larger continuum. By treating these connective tissues as non-binary cell types with subtle differences, these differences can be further elaborated and made as useful markers in the diagnostic and treatment of musculoskeletal disorders in general. A key issue in comparative studies between cyclostomes and gnathostomes is the accessibility of embryos for the former, but new sequencing methods and functional studies now allow us to further understand and manipulate lampreys and hagfishes in spite of this. The evolution of vertebrate cartilages has an important intersection with other connective tissues, and cyclostomes have a role in understanding the ways in which these cell types have diverged in feature but also the ways in which they have conserved the ancestral connective tissue layout.
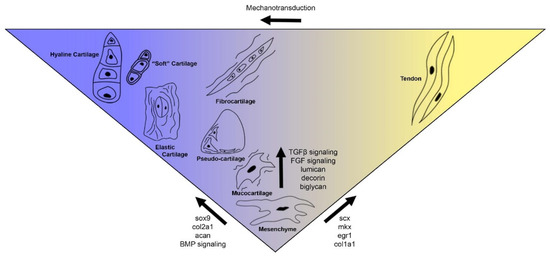
Figure 7.
Proposed spectrum of vertebrate cartilage types and their relatedness with one another as well as cell types like tendon. From a common mesenchymal precursor, cells receive continuous input from chondrogenic genes like sox9 or tenogenic genes like scx or mkx and are driven towards an archetypal cell fate. These fates share expression of SLRP genes like lumican, biglycan, and decorin, and are also similarly affected by TGFβ and FGF signaling. Even after cells begin displaying tendon-like features, they may transdifferentiate to a cartilage-like fate through mechanotransduction. As a spectrum, there would be quantifiable differences in gene expression between individual cartilage types, both regulatory transcription factors and signaling ligands and receptors as well as ECM proteins.
Author Contributions
Z.D.R., C.G. and M.B. wrote the manuscript, with Z.D.R., D.J. and D.M.M. editing subsequent drafts. Z.D.R. prepared the figures and legends. Z.D.R., D.J. and D.M.M. approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of and approved by the Institutional Animal Care and Use Committee (IACUC) Protocol Number 2392.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated and analyzed for this study are not available online, but can be personally requested from the authors.
Acknowledgments
The authors would like to thank Brian Eames at the University of Saskatchewan for comments and suggestions. Zachary Root, David Jandzik, and Daniel Medeiros were supported by National Science Foundation grants IOS 1656843 and IOS 1257040 to Daniel Medeiros. Zachary Root, Claire Gould, and Margaux Brewer were also supported by the Beverly Sears, EBIO, and UROP grants through the University of Colorado Boulder (Grant 13414815, 11060912, and 13410919). David Jandzik was additionally supported by the Scientific Grant Agency of the Slovak Republic VEGA 1/0450/21.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Square, T.A. Evolution of the endothelin pathway drove neural crest cell diversification. Nature 2020, 585, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Martik, M.L. Evolution of the new head by gradual acquisition of neural crest regulatory circuits. Nature 2019, 574, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Kuratani, S. Cephalic neural crest cells and the evolution of craniofacial structures in vertebrates: Morphological and embryological significance of the premandibular–mandibular boundary. Zoology 2005, 108, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Trainor, P.A. The development, patterning and evolution of neural crest cell differentiation into cartilage and bone. Bone 2020, 137, 115409. [Google Scholar] [CrossRef]
- York, J.R.; McCauley, D.W. The origin and evolution of vertebrate neural crest cells. Open Biol. 2020, 10, 190285. [Google Scholar] [CrossRef] [PubMed]
- Jandzik, D. Evolution of the new vertebrate head by co-option of an ancient chordate skeletal tissue. Nature 2015, 518, 534–537. [Google Scholar] [CrossRef]
- Rychel, A.L. Evolution and development of the chordates: Collagen and pharyngeal cartilage. Mol. Biol. Evol. 2006, 23, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, S.; Wada, H. Regeneration of amphioxus oral cirri and its skeletal rods: Implications for the origin of the vertebrate skeleton. J. Exp. Zool. Part B Mol. Dev. Evol. 2011, 316, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, O.A. The genetic program for cartilage development has deep homology within Bilateria. Nature 2016, 533, 86–89. [Google Scholar] [CrossRef]
- Witten, P.; Huysseune, A.; Hall, B. A practical approach for the identification of the many cartilaginous tissues in teleost fish. J. Appl. Ichthyol. 2010, 26, 257–262. [Google Scholar] [CrossRef]
- Panopoulou, G.; Poustka, A.J. Timing and mechanism of ancient vertebrate genome duplications–the adventure of a hypothesis. Trends Genet. 2005, 21, 559–567. [Google Scholar] [CrossRef]
- Holland, L.Z.; Daza, D.O. A new look at an old question: When did the second whole genome duplication occur in vertebrate evolution? Genom. Biol. 2018, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, Y.; Gu, J. Age distribution of human gene families shows significant roles of both large-and small-scale duplications in vertebrate evolution. Nat. Genet. 2002, 31, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Simakov, O. Deeply conserved synteny resolves early events in vertebrate evolution. Nat. Ecol. Evol. 2020, 4, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Arora, J.; Isambert, H. Identification of ohnolog genes originating from whole genome duplication in early vertebrates, based on synteny comparison across multiple genomes. PLoS Comput. Biol. 2015, 11, e1004394. [Google Scholar] [CrossRef]
- Root, Z.D. Lamprey lecticans link new vertebrate genes to the origin and elaboration of vertebrate tissues. Dev. Biol. 2021, 476, 282–293. [Google Scholar] [CrossRef]
- Square, T. The origin and diversification of the developmental mechanisms that pattern the vertebrate head skeleton. Dev. Biol. 2017, 427, 219–229. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Crump, J.G. New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev. Biol. 2012, 371, 121–135. [Google Scholar] [CrossRef]
- Yao, T. Development of lamprey mucocartilage and its dorsal–ventral patterning by endothelin signaling, with insight into vertebrate jaw evolution. J. Exp. Zool. Part B Mol. Dev. Evol. 2011, 316, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Cerny, R. Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proc. Nat. Acad. Sci. USA 2010, 107, 17262–17267. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.T. sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development 2000, 127, 3815–3828. [Google Scholar] [CrossRef]
- Janvier, P. The dawn of the vertebrates: Characters versus common ascent in the rise of current vertebrate phylogenies. Palaeontology 1996, 39, 259–287. [Google Scholar]
- Donoghue, P.C.; Forey, P.L.; Aldridge, R.J. Conodont affinity and chordate phylogeny. Biol. Rev. 2000, 75, 191–251. [Google Scholar] [CrossRef] [PubMed]
- Stock, D.W.; Whitt, G.S. Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group. Science 1992, 257, 787–789. [Google Scholar] [CrossRef]
- Heimberg, A.M. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc. Nat. Acad. Sci. USA 2010, 107, 19379–19383. [Google Scholar] [CrossRef] [PubMed]
- Kuraku, S. Monophyly of lampreys and hagfishes supported by nuclear DNA–coded genes. J. Mol. Evol. 1999, 49, 729–735. [Google Scholar] [CrossRef]
- Mallatt, J.; Sullivan, J. 28S and 18S rDNA sequences support the monophyly of lampreys and hagfishes. Mol. Biol. Evol. 1998, 15, 1706–1718. [Google Scholar] [CrossRef]
- Yalden, D.W. Feeding mechanisms as evidence for cyclostome monophyly. Zool. J. Linnean Soc. 1985, 84, 291–300. [Google Scholar] [CrossRef]
- Miyashita, T. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny. Proc. Nat. Acad. Sci. USA 2019, 116, 2146–2151. [Google Scholar]
- Kuraku, S.; Kuratani, S. Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zool. Sci. 2006, 23, 1053–1064. [Google Scholar] [CrossRef]
- Gess, R.W.; Coates, M.I.; Rubidge, B.S. A lamprey from the Devonian period of South Africa. Nature 2006, 443, 981–984. [Google Scholar] [CrossRef]
- Miyashita, T. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 2021, 591, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Bardack, D. First fossil hagfish (Myxinoidea): A record from the Pennsylvanian of Illinois. Science 1991, 254, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T. A Paleozoic stem hagfish Myxinikela siroka—revised anatomy and implications for evolution of the living jawless vertebrate lineages. Can. J. Zool. 2020, 98, 850–865. [Google Scholar] [CrossRef]
- Kuratani, S.; Oisi, Y.; Ota, K.G. Evolution of the vertebrate cranium: Viewed from hagfish developmental studies. Zool. Sci. 2016, 33, 229–238. [Google Scholar] [CrossRef]
- Oisi, Y. Development of the chondrocranium in hagfishes, with special reference to the early evolution of vertebrates. Zool. Sci. 2013, 30, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Kuratani, S. Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: Revising the heterotopy scenario of gnathostome jaw evolution. J. Anatom. 2013, 222, 41–55. [Google Scholar] [CrossRef]
- Ota, K.G. Late development of hagfish vertebral elements. J. Exp. Zool. Part B Mol. Dev. Evol. 2013, 320, 129–139. [Google Scholar] [CrossRef]
- Ota, K.G. The origin of developmental mechanisms underlying vertebral elements: Implications from hagfish evo-devo. Zoology 2014, 117, 77–80. [Google Scholar] [CrossRef]
- Ota, K.G. Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish. Nat. Commun. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Mallatt, J. The suspension feeding mechanism of the larval lamprey Petromyzon marinus. J. Zool. 1981, 194, 103–142. [Google Scholar] [CrossRef]
- Mallatt, J. Pumping rates and particle retention efficiencies of the larval lamprey, an unusual suspension feeder. Biol. Bull. 1982, 163, 197–210. [Google Scholar] [CrossRef]
- Rovainen, C.M. Feeding and breathing in lampreys. Brain Behav. Evol. 1996, 48, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Wright, G.M.; Youson, J. Transformation of mucocartilage to a definitive cartilage during metamorphosis in the sea lamprey, Petromyzon marinus. J. Morphol. 1987, 194, 1–21. [Google Scholar] [CrossRef]
- Müller, J. Vergleichende Anatomie der Myxinoiden; Königlichen Akademie der Wissenschaften: Berlin, Germany, 1834. [Google Scholar]
- Parker, W.K. X. On the Skeleton of the Marsipohranch Fishes.—Part II. Petromyzon; Philosophical Transactions of the Royal Society of London: London, UK, 1883; Volume 174, pp. 411–457. [Google Scholar]
- Parker, W.K. On the Skeleton of the Marsipobranch Fishes. Part I. The Myxinoids (Myxine, and Bdellostoma); Philosophical Transactions of the Royal Society of London: London, UK, 1883; Volume 174, pp. 373–409. [Google Scholar]
- Cole, F.J. XXX.—A Monograph on the General Morphology of the Myxinoid Fishes, Based on a Study of Myxine. Part I. The Anatomy of the Skeleton; Earth and Environmental Science Transactions of The Royal Society of Edinburgh: Edinburgh, UK, 1906; Volume 41, pp. 749–788. [Google Scholar]
- Schaffer, J. Bemerkungen über die Histologie und Histogenese des Knorpels der Cyclostomen. Archiv. Mikroskopische Anatomie 1897, 50, 170–188. [Google Scholar] [CrossRef]
- Schneider, A. Anatomie und Entwickelungsgeschichte von Petromyzon und Ammocoetes; Beitrage zur vergleichenden. Anatomie und Entwickelungsgeschichte der Wirbeltiere; Reimer Publishing: Berlin, Germany, 1879; pp. 85–92. [Google Scholar]
- Johnels, A.G. On the development and morphology of the skeleton of the head of Petromyzon. Acta Zool. 1948, 29, 139–279. [Google Scholar] [CrossRef]
- Miyashita, T. Comparative Analysis of the Anatomy of the Myxinoidea and the Ancestry of Early Vertebrate Lineages. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2012. [Google Scholar]
- Huxley, T.H. The nature of the craniofacial apparatus of Petromyzon. J. Anatomy Physiol. 1876, 10, 412. [Google Scholar]
- Wright, G.M.; Keeley, F.; Youson, J. Lamprin: A new vertebrate protein comprising the major structural protein of adult lamprey cartilage. Experientia 1983, 39, 495–497. [Google Scholar] [CrossRef]
- Benjamin, M.; Ralphs, J. Biology of fibrocartilage cells. Int. Rev. Cytol. 2004, 233, 1–46. [Google Scholar]
- Eames, B.F.; Medeiros, D.M.; Adameyko, I. On the Evolution of Skeletal Cells befire Abd after Neural Crest Evolving Neural Crest Cells; CRC Press: Boca Raton, NJ, USA, 2020. [Google Scholar]
- Zhang, P.; Jimenez, S.A.; Stokes, D.G. Regulation of human COL9A1 gene expression: Activation of the proximal promoter region by SOX9. J. Biol. Chem. 2003, 278, 117–123. [Google Scholar] [CrossRef]
- Bridgewater, L.C. Adjacent DNA sequences modulate Sox9 transcriptional activation at paired Sox sites in three chondrocyte-specific enhancer elements. Nucl. Acids Res. 2003, 31, 1541–1553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akiyama, H. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Hojo, H.; Ohba, S. Insights into gene regulatory networks in chondrocytes. Int. J. Mol. Sci. 2019, 20, 6324. [Google Scholar] [CrossRef]
- Schmidt, A.; Rossi, P.; De Crombrugghe, B. Transcriptional control of the mouse alpha 2 (I) collagen gene: Functional deletion analysis of the promoter and evidence for cell-specific expression. Mol. Cell. Biol. 1986, 6, 347–354. [Google Scholar] [CrossRef]
- Thielen, N.G.; van der Kraan, P.M.; van Caam, A.P. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells 2019, 8, 969. [Google Scholar]
- Hatakeyama, Y.; Tuan, R.S.; Shum, L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J. Cell. Biochem. 2004, 91, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef]
- Ray, A. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development 2015, 142, 1169–1179. [Google Scholar] [CrossRef]
- Rahman, M.S. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Li, T.F. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J. Bone Mineral Res. 2006, 21, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Lim, J. BMP–Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Developmental Biol. 2015, 400, 132–138. [Google Scholar] [CrossRef]
- Dalcq, J. RUNX3, EGR1 and SOX9B form a regulatory cascade required to modulate BMP-signaling during cranial cartilage development in zebrafish. PLoS ONE 2012, 7, e50140. [Google Scholar] [CrossRef]
- Barlow, A.J. Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Dev. Dynam. Off. Publ. Am. Assoc. Anatom. 1999, 214, 291–302. [Google Scholar]
- Tan, Z. Synergistic co-regulation and competition by a SOX9-GLI-FOXA phasic transcriptional network coordinate chondrocyte differentiation transitions. PLoS Genet. 2018, 14, e1007346. [Google Scholar] [CrossRef]
- Wuelling, M. Trps1, a regulator of chondrocyte proliferation and differentiation, interacts with the activator form of Gli3. Dev. Biol. 2009, 328, 40–53. [Google Scholar] [CrossRef]
- Muraglia, A.; Cancedda, R.; Quarto, R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J. Cell Sci. 2000, 113, 1161–1166. [Google Scholar] [CrossRef]
- Tamamura, Y. Developmental regulation of Wnt/β-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 2005, 280, 19185–19195. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Kim, S.J.; Kin, S.H.; Oh, C.D.; Hwang, S.G.; Chun, C.H.; Oh, S.H.; Seong, J.K.; Huh, T.L.; Chun, J.S. Regulation of the chondrocyte phenotype by β-catenin. J. Dev. 2002, 129, 5541–5550. [Google Scholar] [CrossRef] [PubMed]
- Eames, B.F.; De La Fuente, L.; Helms, J.A. Molecular ontogeny of the skeleton. Birth Defects Res. Part C Embryo Today Rev. 2003, 69, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S.; Koyama, E.; Pacifici, M. Articular cartilage: Structural and developmental intricacies and questions. Curr. Osteoporosis Rep. 2015, 13, 407–414. [Google Scholar] [CrossRef]
- Grogan, S.P. Zone-specific gene expression patterns in articular cartilage. Arthritis Rheumat. 2013, 65, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, A. TGF-β signaling is essential for joint morphogenesis. J. Cell Biol. 2007, 177, 1105–1117. [Google Scholar] [CrossRef]
- Longobardi, L.; Li, T.; Myers, T.J.; O’Rear, L.; Ozkan, H.; Li, Y.; Contaldo, C.; Spagnoli, A. TGF-β Type II Receptor/MCP-5 Axis: At the Crossroad between Joint and Growth Plate Development. Dev. Cell 2012, 23, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Longobardi, L.; Myers, T.J.; Temple, J.D.; Chandler, R.L.; Özkan, H.; Contaldo, C.; Spagnoli, A. Joint TGF-β Type II Receptor-Expressing Cells: Ontogeny and Characterization as Joint Progenitors. Stem Cells Dev. 2013, 22, 1342–1359. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Hodges, P.T.; Aguilera, X.M.; Missana, L.; Moylan, P.E. Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J. Histochem. Cytochem. 2000, 48, 1493–1502. [Google Scholar] [CrossRef]
- Gelse, K.; Ekici, A.; Cipa, F.; Swoboda, B.; Carl, H.; Olk, A.; Hennig, F.; Klinger, P. Molecular differentiation between osteophytic and articular cartilage—Clues for a transient and permanent chondrocyte phenotype. Osteoarthr. Cartil. 2012, 20, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.C.H. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheumat. 2012, 64, 3302–3312. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Maenohara, Y.; Chijimatsu, R.; Tachibana, N.; Uehara, K.; Xuan, F.; Mori, D.; Murahashi, Y.; Nakamoto, H.; Oichi, T.; Chang, S.H.; et al. Lubricin Contributes to Homeostasis of Articular Cartilage by Modulating Differentiation of Superficial Zone Cells. J. Bone Miner. Res. 2020, 36, 792–802. [Google Scholar] [CrossRef]
- Flowers, S.; Zieba, A.; Örnros, J.; Jin, C.; Rolfson, O.; Björkman, L.I.; Eisler, T.; Kalamajski, S.; Kamali-Moghaddam, M.; Karlsson, N.G. Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Jones, A.R.; Gleghorn, J.; Hughes, C.; Fitz, L.J.; Zollner, R.; Wainwright, S.D.; Caterson, B.; Morris, E.A.; Bonassar, L.J.; Flannery, C.R. Binding and localization of recombinant lubricin to articular cartilage surfaces. J. Orthop. Res. 2007, 25, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Askary, A.; Smeeton, J.; Paul, S.; Schindler, S.; Braasch, I.; Ellis, N.A.; Postlethwait, J.; Miller, C.T.; Crump, J.G. Ancient origin of lubricated joints in bony vertebrates. eLife 2016, 5, e16415. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Nagano, K.; Moriishi, T.; Miyazaki, T.; Komori, H.; Ito, K.; Von Der Mark, K.; Sakane, C.; Kaneko, H.; et al. Runx2 is essential for the transdifferentiation of chondrocytes into osteoblasts. PLoS Genet. 2020, 16, e1009169. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Yue, S.; Lee, A.; Kikani, B.; Temnycky, A.; Barnes, K.M.; Baron, J. Persistent Sox9 expression in hypertrophic chondrocytes suppresses transdifferentiation into osteoblasts. Bone 2019, 125, 169–177. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Gómez-Picos, P.; Eames, B.F. On the evolutionary relationship between chondrocytes and osteoblasts. Front. Genet. 2015, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.M.; Keeley, F.W.; Youson, J.H.; Babineau, D.L. Cartilage in the atlantic hagfish, Myxine glutinosa. Am. J. Anat. 1984, 169, 407–424. [Google Scholar] [CrossRef]
- Wright, G.M.; Youson, J.H. Ultrastructure of cartilage from young adult sea lamprey, Petromyzon marinus L: A new type of vertebrate cartilage. Am. J. Anat. 1983, 167, 59–70. [Google Scholar] [CrossRef]
- Debiais-Thibaud, M.; Simion, P.; Ventéo, S.; Muñoz, D.; Marcellini, S.; Mazan, S.; Haitina, T. Skeletal Mineralization in Association with Type X Collagen Expression Is an Ancestral Feature for Jawed Vertebrates. Mol. Biol. Evol. 2019, 36, 2265–2276. [Google Scholar] [CrossRef]
- Seidel, R.; Blumer, M.; Chaumel, J.; Amini, S.; Dean, M.N. Endoskeletal mineralization in chimaera and a comparative guide to tessellated cartilage in chondrichthyan fishes (sharks, rays and chimaera). J. R. Soc. Interface 2020, 17, 20200474. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M. The cranial cartilages of teleosts and their classification. J. Anat. 1990, 169, 153–172. [Google Scholar]
- Enochson, L.; Stenberg, J.; Brittberg, M.; Lindahl, A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthr. Cartil. 2014, 22, 566–577. [Google Scholar] [CrossRef]
- Inada, M.; Wang, Y.; Byrne, M.H.; Rahman, M.U.; Miyaura, C.; López-Otín, C.; Krane, S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 2004, 101, 17192–17197. [Google Scholar] [CrossRef]
- Fosang, A.; Last, K.; Knauper, V.; Murphy, G.; Neame, P.J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 1996, 380, 17–20. [Google Scholar] [CrossRef]
- Flannery, C.R.; Little, C.; Hughes, C.; Caterson, B. Expression of ADAMTS Homologues in Articular Cartilage. Biochem. Biophys. Res. Commun. 1999, 260, 318–322. [Google Scholar] [CrossRef]
- Collins-Racie, L.A.; Flannery, C.R.; Zeng, W.; Corcoran, C.; Annis-Freeman, B.; Agostino, M.J.; Arai, M.; DiBlasio-Smith, E.; Dorner, A.J.; Georgiadis, K.E.; et al. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004, 23, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Grogan, E.; Lund, R.; Greenfest-Allen, E. The Origin and Relationships of Early Chondrichthyans. Biol. Sharks Relat. 2012, 2, 3–29. [Google Scholar] [CrossRef]
- Keith, D.A.; Paz, A.; Gallop, P.M.; Glimcher, M.J. Histologic and biochemical identification and characterization of an elastin in cartilage. J. Histochem. Cytochem. 1977, 25, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wu, J.-P.; Chen, H.H.; Kirk, T.; Xu, J. Elastin fibers display a versatile microfibril network in articular cartilage depending on the mechanical microenvironments. J. Orthop. Res. 2013, 31, 1345–1353. [Google Scholar] [CrossRef]
- Field, J.; Rodger, G.; Hunter, J.; Serafini-Fracassini, A.; Spina, M. Isolation of elastin from bovine auricular cartilage. Arch. Biochem. Biophys. 1978, 191, 705–713. [Google Scholar] [CrossRef]
- Hellingman, C.A.; Verwiel, E.T.P.; Slagt, I.; Koevoet, W.; Poublon, R.M.L.; Nolst-Trenité, G.J.; De Jong, R.J.B.; Jahr, H.; Van Osch, G.J.V.M. Differences in Cartilage-Forming Capacity of Expanded Human Chondrocytes from Ear and Nose and Their Gene Expression Profiles. Cell Transplant. 2011, 20, 925–940. [Google Scholar] [CrossRef]
- Qin, S.; Wang, W.; Liu, Z.; Hua, X.; Fu, S.; Dong, F.; Li, A.; Liu, Z.; Wang, P.; Dai, L.; et al. Fibrochondrogenic differentiation potential of tendon-derived stem/progenitor cells from human patellar tendon. J. Orthop. Transl. 2019, 22, 101–108. [Google Scholar] [CrossRef]
- Ruscitto, A.; Scarpa, V.; Morel, M.; Pylawka, S.; Shawber, C.; Embree, M. Notch Regulates Fibrocartilage Stem Cell Fate and Is Upregulated in Inflammatory TMJ Arthritis. J. Dent. Res. 2020, 99, 1174–1181. [Google Scholar] [CrossRef]
- Dyment, N.A.; Breidenbach, A.P.; Schwartz, A.G.; Russell, R.P.; Aschbacher-Smith, L.; Liu, H.; Hagiwara, Y.; Jiang, R.; Thomopoulos, S.; Butler, D.L.; et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 2015, 405, 96–107. [Google Scholar] [CrossRef]
- Benjamin, M.; Ralphs, J. Extracellular matrix of connective tissues in the heads of teleosts. J. Anat. 1991, 179, 137–148. [Google Scholar]
- Benjamin, M. Mucochondroid (mucous connective) tissues in the heads of teleosts. Anat. Embryol. 1988, 178, 461–474. [Google Scholar] [CrossRef]
- Duncan, W.P.; Da Costa, O.T.F.; Sakuragui, M.M.; Fernandes, M.N. Functional Morphology of the Gill in Amazonian Freshwater Stingrays (Chondrichthyes: Potamotrygonidae): Implications for Adaptation to Freshwater. Physiol. Biochem. Zool. 2010, 83, 19–32. [Google Scholar] [CrossRef]
- Egerbacher, M.; Böck, P. Myxoid Tissue: Its Morphology, Histochemistry, and Relationship with Other Supporting Tissues. Arch. Histol. Cytol. 1997, 60, 121–131. [Google Scholar] [CrossRef]
- Robson, P.; Wright, G.; Sitarz, E.; Maiti, A.; Rawat, M.; Youson, J.; Keeley, F. Characterization of lamprin, an unusual matrix protein from lamprey cartilage. Implications for evolution, structure, and assembly of elastin and other fibrillar proteins. J. Biol. Chem. 1993, 268, 1440–1447. [Google Scholar] [CrossRef]
- Bochicchio, B.; Pepe, A.; Tamburro, A. On (GGLGY) synthetic repeating sequences of lamprin and analogous sequences. Matrix Biol. 2001, 20, 243–250. [Google Scholar] [CrossRef]
- McBurney, K.; Keeley, F.; Kibenge, F.; Wright, G. Spatial and temporal distribution of lamprin mRNA during chondrogenesis of trabecular cartilage in the sea lamprey. Anat. Embryol. 1996, 193, 419–426. [Google Scholar] [CrossRef]
- Yokoyama, H.; Morino, Y.; Wada, H. Identification of a unique lamprey gene with tandemly repeated sequences and pharyngeal chondrocyte-specific expression. Gene 2019, 701, 9–14. [Google Scholar] [CrossRef]
- Robson, P.; Wright, G.M.; Keeley, F.W. Distinct non-collagen based cartilages comprising the endoskeleton of the Atlantic hagfish, Myxine glutinosa. Anat. Embryol. 2000, 202, 281–290. [Google Scholar] [CrossRef]
- Zhang, G.; Cohn, M.J. Hagfish and lancelet fibrillar collagens reveal that type II collagen-based cartilage evolved in stem vertebrates. Proc. Natl. Acad. Sci. USA 2006, 103, 16829–16833. [Google Scholar] [CrossRef]
- Ota, K.G.; Kuratani, S. Expression pattern of two collagen type 2 α1 genes in the Japanese inshore hagfish (Eptatretus burgeri) with special reference to the evolution of cartilaginous tissue. J. Exp. Zool. Part B Mol. Dev. Evol. 2009, 9999B, 157–165. [Google Scholar] [CrossRef]
- Lakiza, O.; Miller, S.; Bunce, A.; Lee, E.M.-J.; McCauley, D.W. SoxE gene duplication and development of the lamprey branchial skeleton: Insights into development and evolution of the neural crest. Dev. Biol. 2011, 359, 149–161. [Google Scholar] [CrossRef]
- McCauley, D.W.; Bronner-Fraser, M. Importance of SoxE in neural crest development and the evolution of the pharynx. Nature 2006, 441, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Miyamoto, M.M.; Cohn, M.J. Lamprey type II collagen and Sox9 reveal an ancient origin of the vertebrate collagenous skeleton. Proc. Natl. Acad. Sci. USA 2006, 103, 3180–3185. [Google Scholar] [CrossRef]
- McCauley, D.W.; Bronner-Fraser, M. Conservation and divergence of BMP2/4 genes in the lamprey: Expression and phylogenetic analysis suggest a single ancestral vertebrate gene. Evol. Dev. 2004, 6, 411–422. [Google Scholar] [CrossRef]
- Jandzik, D.; Hawkins, M.; Cattell, M.V.; Cerny, R.; Square, T.; Medeiros, D. Roles for FGF in lamprey pharyngeal pouch formation and skeletogenesis highlight ancestral functions in the vertebrate head. Development 2014, 141, 629–638. [Google Scholar] [CrossRef]
- Cattell, M.; Lai, S.; Cerny, R.; Medeiros, D.M. A New Mechanistic Scenario for the Origin and Evolution of Vertebrate Cartilage. PLoS ONE 2011, 6, e22474. [Google Scholar] [CrossRef]
- Ohtani, K.; Yao, T.; Kobayashi, M.; Kusakabe, R.; Kuratani, S.; Wada, H. Expression of Sox and fibrillar collagen genes in lamprey larval chondrogenesis with implications for the evolution of vertebrate cartilage. J. Exp. Zool. Part B Mol. Dev. Evol. 2008, 310, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Yutaka, T. Normal Stages of Development in the Lamprey, Lampetra reissued (Dybowski). Zool. Sci. 1988, 5, 109–118. [Google Scholar]
- Xia, J.; Kang, Z.; Xue, Y.; Ding, Y.; Gao, S.; Zhang, Y.; Lv, P.; Wang, X.; Ma, D.; Wang, L. A single-cell resolution developmental atlas of hematopoietic stem and progenitor cell expansion in zebrafish. Proc. Natl. Acad. Sci. USA 2021, 118, e2015748118. [Google Scholar] [CrossRef]
- Moreno-Ayala, R.; Junker, J.P. Single-cell genomics to study developmental cell fate decisions in zebrafish. Briefings Funct. Genom. 2021. [Google Scholar] [CrossRef]
- Square, T.; Romášek, M.; Jandzik, D.; Cattell, M.V.; Klymkowsky, M.; Medeiros, D.M. CRISPR/Cas9-mediated mutagenesis in the sea lamprey, Petromyzon marinus: A powerful tool for understanding ancestral gene functions in vertebrates. Development 2015, 142, 4180–4187. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef]
- Long, F.; Zhang, X.M.; Karp, S.; Yang, Y.; McMahon, A.P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 2001, 128, 5099–5108. [Google Scholar] [CrossRef]
- Kim, E.-J.; Cho, S.-W.; Shin, J.-O.; Lee, M.-J.; Kim, K.-S.; Jung, H.-S. Ihh and Runx2/Runx3 Signaling Interact to Coordinate Early Chondrogenesis: A Mouse Model. PLoS ONE 2013, 8, e55296. [Google Scholar] [CrossRef]
- Keller, B.; Yang, T.; Chen, Y.; Munivez, E.; Bertin, T.; Zabel, B.; Lee, B. Interaction of TGFβ and BMP Signaling Pathways during Chondrogenesis. PLoS ONE 2011, 6, e16421. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.L. Expression of patched, prdm1 and engrailed in the lamprey somite reveals conserved responses to Hedgehog signaling. Evol. Dev. 2009, 11, 27–40. [Google Scholar] [CrossRef]
- Kano, S.; Xiao, J.-H.; Osório, J.; Ekker, M.; Hadzhiev, Y.; Müller, F.; Casane, D.; Magdelenat, G.; Rétaux, S. Two Lamprey Hedgehog Genes Share Non-Coding Regulatory Sequences and Expression Patterns with Gnathostome Hedgehogs. PLoS ONE 2010, 5, e13332. [Google Scholar] [CrossRef]
- Sugahara, F.; Aota, S.-I.; Kuraku, S.; Murakami, Y.; Takio-Ogawa, Y.; Hirano, S.; Kuratani, S. Involvement of Hedgehog and FGF signalling in the lamprey telencephalon: Evolution of regionalization and dorsoventral patterning of the vertebrate forebrain. Development 2011, 138, 1217–1226. [Google Scholar] [CrossRef]
- Díaz-Hernández, M.E.; Bustamante, M.; Galván-Hernández, C.I.; Chimal-Monroy, J. Irx1 and Irx2 Are Coordinately Expressed and Regulated by Retinoic Acid, TGFβ and FGF Signaling during Chick Hindlimb Development. PLoS ONE 2013, 8, e58549. [Google Scholar] [CrossRef]
- Zülch, A.; Becker, M.-B.; Gruss, P. Expression pattern of Irx1 and Irx2 during mouse digit development. Mech. Dev. 2001, 106, 159–162. [Google Scholar] [CrossRef][Green Version]
- Askary, A.; Mork, L.; Paul, S.; He, X.; Izuhara, A.K.; Gopalakrishnan, S.; Ichida, J.K.; McMahon, A.P.; Dabizljevic, S.; Dale, R.; et al. Iroquois Proteins Promote Skeletal Joint Formation by Maintaining Chondrocytes in an Immature State. Dev. Cell 2015, 35, 358–365. [Google Scholar] [CrossRef]
- Square, T.; Jandzik, D.; Cattell, M.; Coe, A.; Doherty, J.; Medeiros, D.M. A gene expression map of the larval Xenopus laevis head reveals developmental changes underlying the evolution of new skeletal elements. Dev. Biol. 2015, 397, 293–304. [Google Scholar] [CrossRef]
- Nichols, J.T.; Pan, L.; Moens, C.; Kimmel, C.B. barx1 represses joints and promotes cartilage in the craniofacial skeleton. Development 2013, 140, 2765–2775. [Google Scholar] [CrossRef]
- Michikami, I.; Fukushi, T.; Honma, S.; Yoshioka, S.; Itoh, S.; Muragaki, Y.; Kurisu, K.; Ooshima, T.; Wakisaka, S.; Abe, M. Trps1 is necessary for normal temporomandibular joint development. Cell Tissue Res. 2012, 348, 131–140. [Google Scholar] [CrossRef]
- Itoh, S.; Kanno, S.; Gai, Z.; Suemoto, H.; Kawakatsu, M.; Tanishima, H.; Morimoto, Y.; Nishioka, K.; Hatamura, I.; Yoshida, M. Trps1 plays a pivotal role downstream of Gdf5 signaling in promoting chondrogenesis and apoptosis of ATDC5 cells. Genes Cells 2008, 13, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Li, Y.; Gui, C.; Ma, Y.; Ge, Y.; Dai, H.; Zhang, K.; Du, J.; Guo, Y.; Jiang, Y.; et al. Fibronectin Enhances Cartilage Repair by Activating Progenitor Cells Through Integrin α5β1 Receptor. Tissue Eng. Part A 2018, 24, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Burton-Wurster, N.; Lust, G. Fibronectin in cartilage. In Fibronectin in Health and Disease; CRC Press: Boca Raton, FL, USA, 2018; pp. 243–254. [Google Scholar]
- Sun, Y.; Wang, T.; Toh, W.S.; Pei, M. The role of laminins in cartilaginous tissues: From development to regeneration. Eur. Cells Mater. 2017, 34, 40–54. [Google Scholar] [CrossRef]
- Dürr, J.; Lammi, P.; Goodman, S.L.; Aigner, T.; Von Der Mark, K. Identification and Immunolocalization of Laminin in Cartilage. Exp. Cell Res. 1996, 222, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Quintarelli, G.; Starcher, B.C.; Vocaturo, A.; Di Gianfilippo, F.; Gotte, L.; Mecham, R.P. Fibrogenesis and Biosynthesis of Elastin in Cartilage. Connect. Tissue Res. 1979, 7, 1–19. [Google Scholar] [CrossRef]
- Silva Jr, W.A. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells 2003, 21, 661–669. [Google Scholar] [CrossRef]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and Expression Analysis of Mesenchymal Stem Cells from Human Bone Marrow and Adipose Tissue. Cell. Physiol. Biochem. 2004, 14, 311–324. [Google Scholar] [CrossRef]
- Liu, T.M.; Martina, M.; Hutmacher, D.W.; Hui, J.H.P.; Lee, E.H.; Lim, B. Identification of common pathways mediating differentiation of bone marrow-and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 2007, 25, 750–760. [Google Scholar] [CrossRef]
- Covas, D.T.; Panepucci, R.; Fontes, A.M.; Silva, W.A.; Orellana, M.D.; Freitas, M.C.; Neder, L.; Santos, A.R.; Peres, L.C.; Jamur, M.C.; et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008, 36, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Muneta, T.; Makino, H.; Nimura, A.; Mochizuki, T.; Ju, Y.-J.; Ezura, Y.; Umezawa, A.; Sekiya, I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J. Orthop. Res. 2009, 27, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Shenaq, D.S.; Rastegar, F.; Petkovic, D.; Zhang, B.-Q.; He, B.-C.; Chen, L.; Zuo, G.-W.; Luo, Q.; Shi, Q.; Wagner, E.R.; et al. Mesenchymal Progenitor Cells and Their Orthopedic Applications: Forging a Path towards Clinical Trials. Stem Cells Int. 2010, 2010, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Parham, A.; Mehrjerdi, H.K.; Dehghani, H. Equine Adipose-Derived Mesenchymal Stem Cells: Phenotype and Growth Characteristics, Gene Expression Profile and Differentiation Potentials. Cell J. 2015, 16, 456–465. [Google Scholar] [PubMed]
- Asou, Y. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J. Orthop. Res. 2002, 20, 827–833. [Google Scholar] [CrossRef]
- Soeda, T.; Deng, J.M.; de Crombrugghe, B.; Behringer, R.R.; Nakamura, T.; Akiyama, H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis 2010, 48, 635–644. [Google Scholar] [CrossRef]
- Blitz, E.; Sharir, A.; Akiyama, H.; Zelzer, E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 2013, 140, 2680–2690. [Google Scholar] [CrossRef]
- Tan, S.-L.; Ahmad, R.E.; Ahmad, T.S.; Merican, A.M.; Abbas, A.A.; Ng, W.M.; Kamarul, T. Effect of Growth Differentiation Factor 5 on the Proliferation and Tenogenic Differentiation Potential of Human Mesenchymal Stem Cells in vitro. Cells Tissues Organs 2012, 196, 325–338. [Google Scholar] [CrossRef]
- Tan, S.-L.; Ahmad, T.S.; Ng, W.-M.; Azlina, A.A.; Azhar, M.M.; Selvaratnam, L.; Kamarul, T. Identification of Pathways Mediating Growth Differentiation Factor5-Induced Tenogenic Differentiation in Human Bone Marrow Stromal Cells. PLoS ONE 2015, 10, e0140869. [Google Scholar] [CrossRef]
- Spaapen, F.; Akker, G.G.H.V.D.; Caron, M.M.J.; Prickaerts, P.; Rofel, C.; Dahlmans, V.E.H.; Surtel, D.A.M.; Paulis, Y.; Schweizer, F.; Welting, T.J.M.; et al. The Immediate Early Gene Product EGR1 and Polycomb Group Proteins Interact in Epigenetic Programming during Chondrogenesis. PLoS ONE 2013, 8, e58083. [Google Scholar] [CrossRef]
- Brent, A.E.; Braun, T.; Tabin, C.J. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 2005, 132, 515–528. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Jiang, R. Mkx -Deficient Mice Exhibit Hedgehog Signaling–Dependent Ectopic Ossification in the Achilles Tendons. J. Bone Miner. Res. 2018, 34, 557–569. [Google Scholar] [CrossRef]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef]
- Liu, W.; Watson, S.S.; Lan, Y.; Keene, D.R.; Ovitt, C.E.; Liu, H.; Schweitzer, R.; Jiang, R. The Atypical Homeodomain Transcription Factor Mohawk Controls Tendon Morphogenesis. Mol. Cell. Biol. 2010, 30, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, P.; Chen, L.; Zhou, Y.; Wang, A.; Zheng, Q.; Mitchell, C.A.; Leys, T.; Tuan, R.S.; Zheng, M.H. Reduction of mechanical loading in tendons induces heterotopic ossification and activation of the β-catenin signaling pathway. J. Orthop. Transl. 2021, 29, 42–50. [Google Scholar] [CrossRef]
- Subramanian, A.; Kanzaki, L.F.; Galloway, J.L.; Schilling, T.F. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. eLife 2018, 7, e38069. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Bürgisser, G.M. Biomechanics of Tendons and Ligaments: Tissue Reconstruction and Regeneration; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Wang, Z.; Jing, Y.; Chen, D.; Ma, C.; Feng, J.Q. Tendon Cells Directly Form Bone Ridges via Multiple Cell Transdifferentiation: Tendon-Fibroblast-Bone Cells beyond Simply Connecting Bone And Muscles. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Gaspar, D.; Zeugolis, D. Macromolecular Crowding and Mechanical Stimulation for Tenogenic Phenotype Maintenance and Differentiation/Transdifferentiation. In Proceedings of the Orthopaedic Proceedings, Munich, Germany, 13–15 September 2017; The British Editorial Society of Bone & Joint Surgery: London, UK, 2018. [Google Scholar]
- de Mos, M. Intrinsic differentiation potential of adolescent human tendon tissue: An in-vitro cell differentiation study. BMC Muscul. Disord. 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Eyal, S.; Rubin, S.; Krief, S.; Levin, L.; Zelzer, E. Common cellular origin and diverging developmental programs for different sesamoid bones. Development 2019, 146, dev.167452. [Google Scholar] [CrossRef]
- Eyal, S.; Blitz, E.; Shwartz, Y.; Akiyama, H.; Schweitzer, R.; Zelzer, E. On the development of the patella. Development 2015, 142, 1831–1839. [Google Scholar] [CrossRef]
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon Functional Extracellular Matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).