Calcium Transport along the Axial Canal in Acropora
Abstract
1. Introduction
2. Methods and Materials
2.1. Sample Collection
2.2. Coral Culture System
2.3. HRCT Test
2.4. Internal Canal Reconstruction
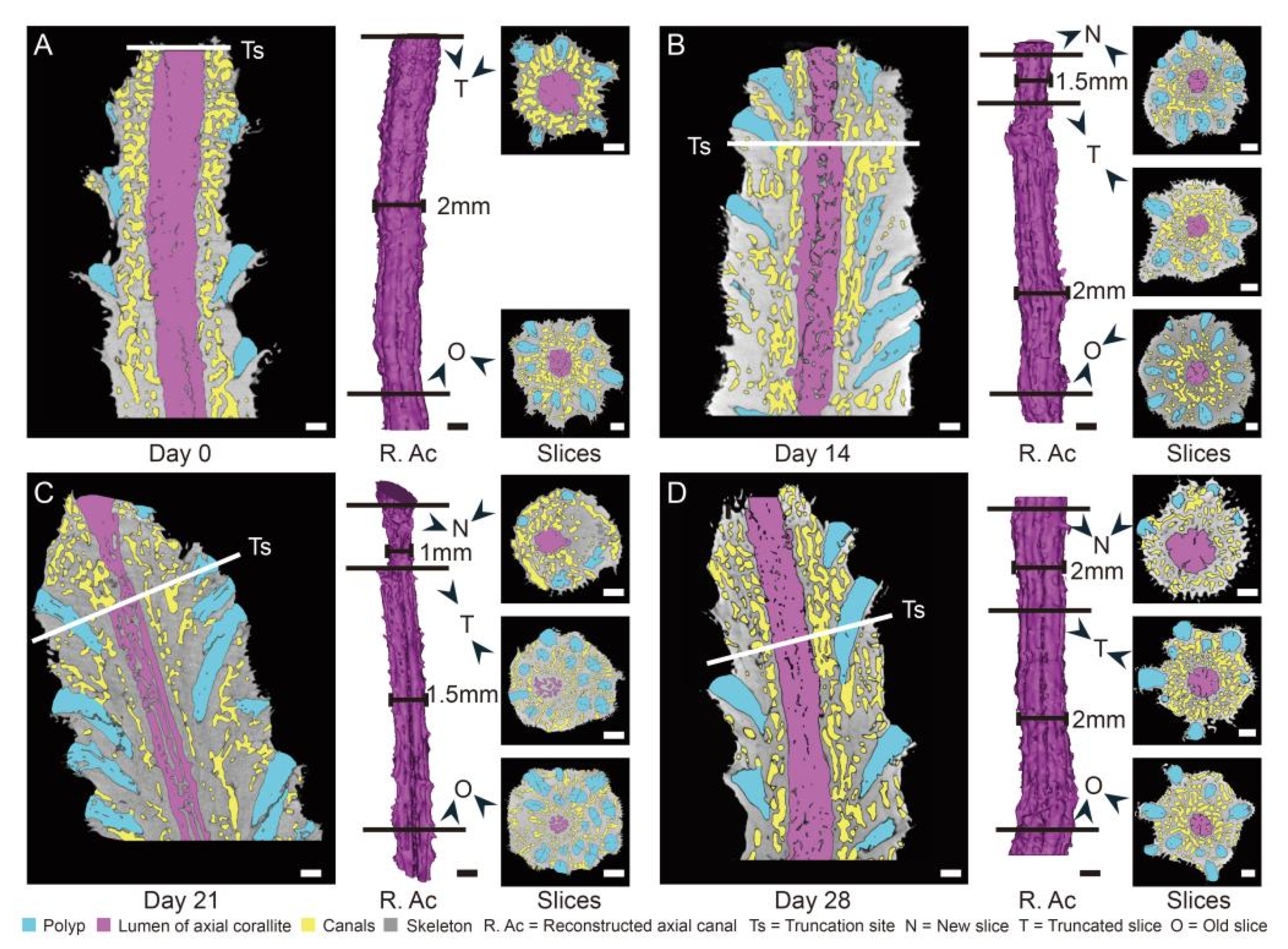
2.5. Truncation Experiment
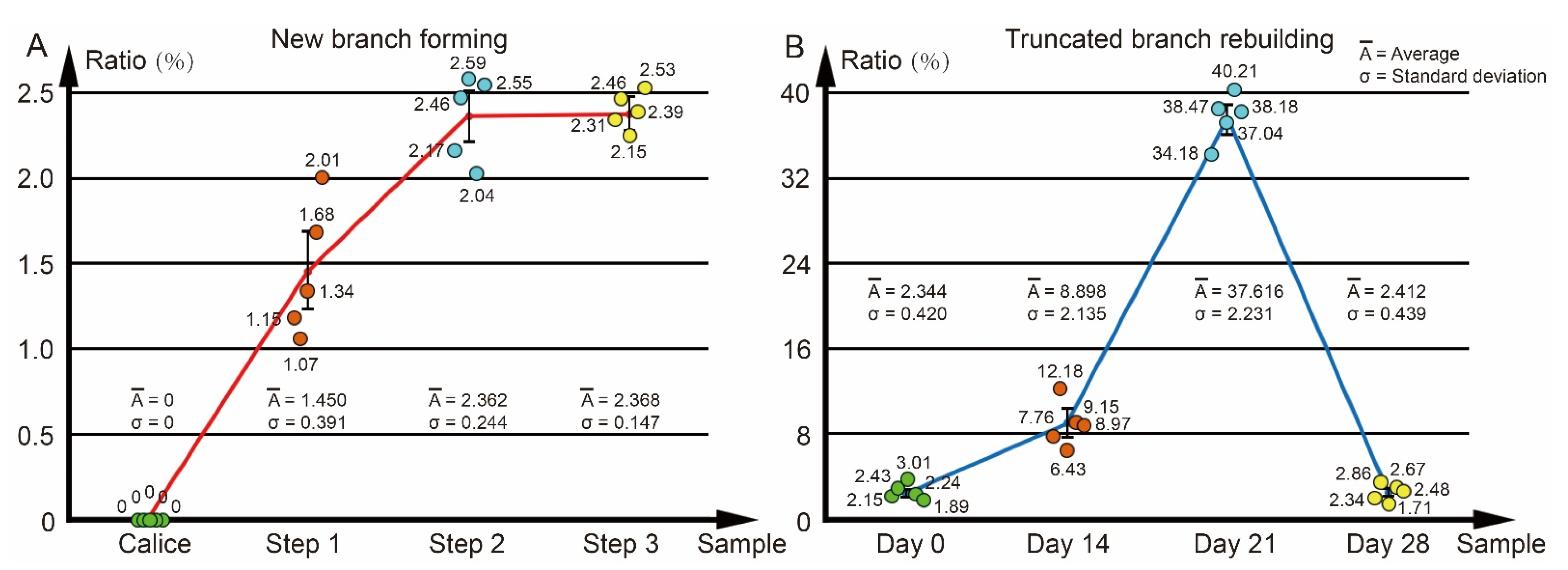
2.6. Calculation of the Calcareous Deposit Volume Ratio in the Axial Canal
3. Results
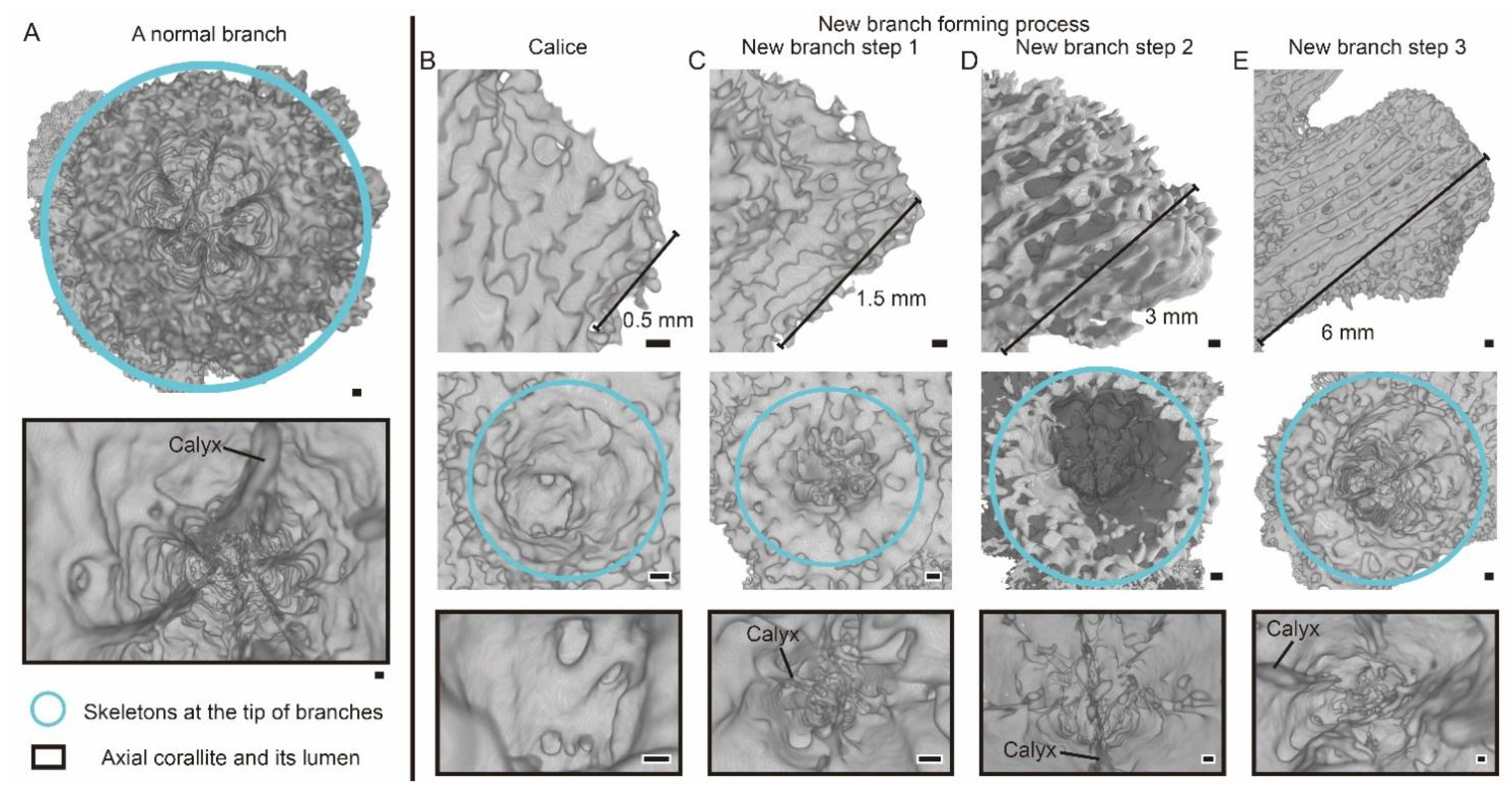
3.1. The Morphology and Internal Structure of Corallite in A. muricata
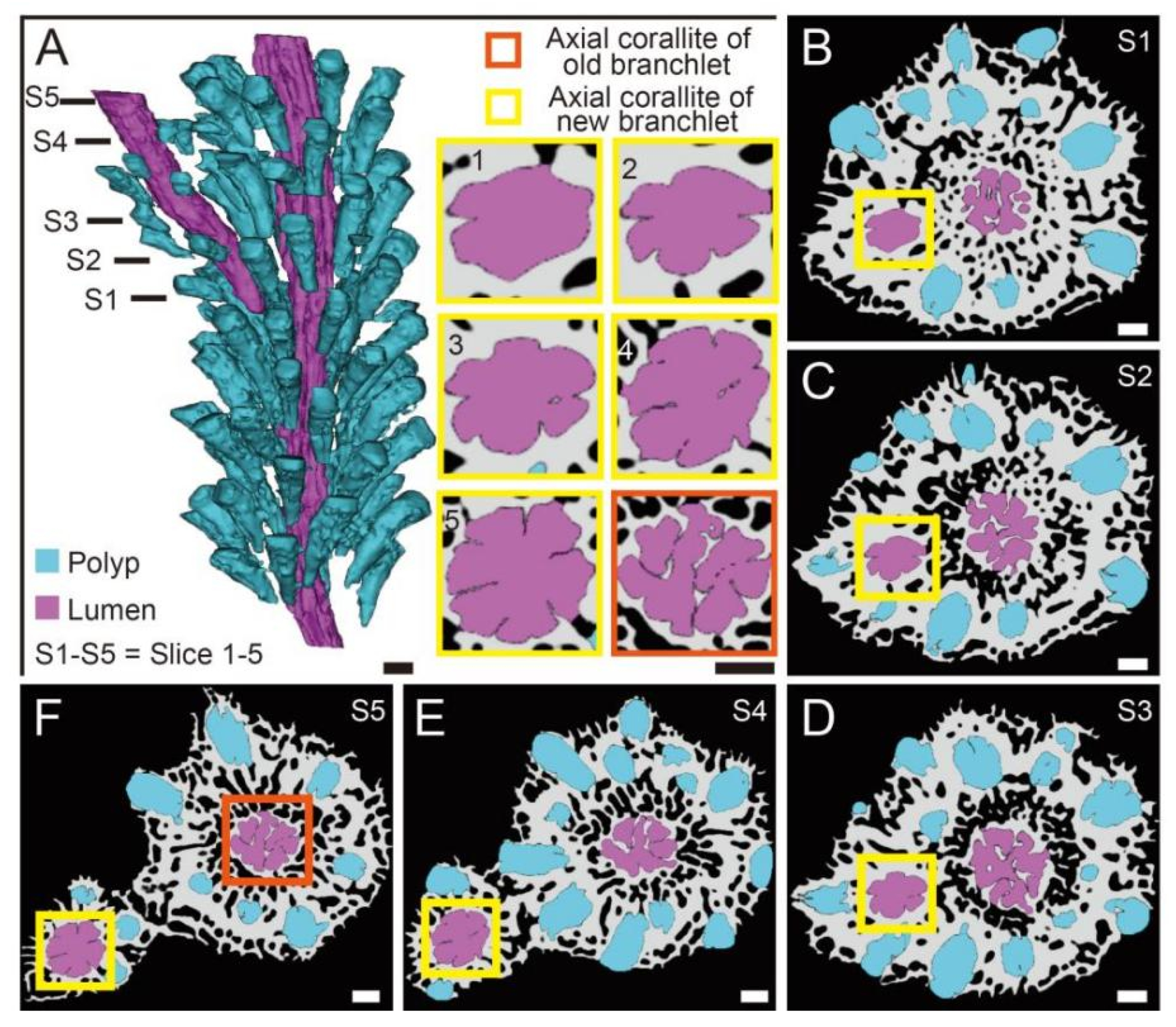
3.2. The Changes of Calyx in Axial Corallite Reveal Calcium Transport Patterns
4. Discussion
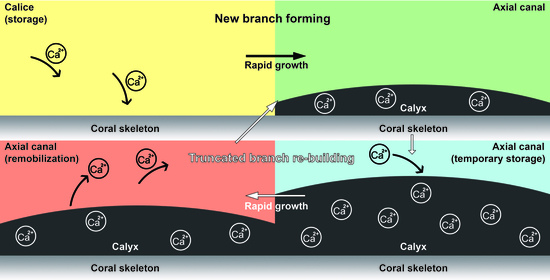
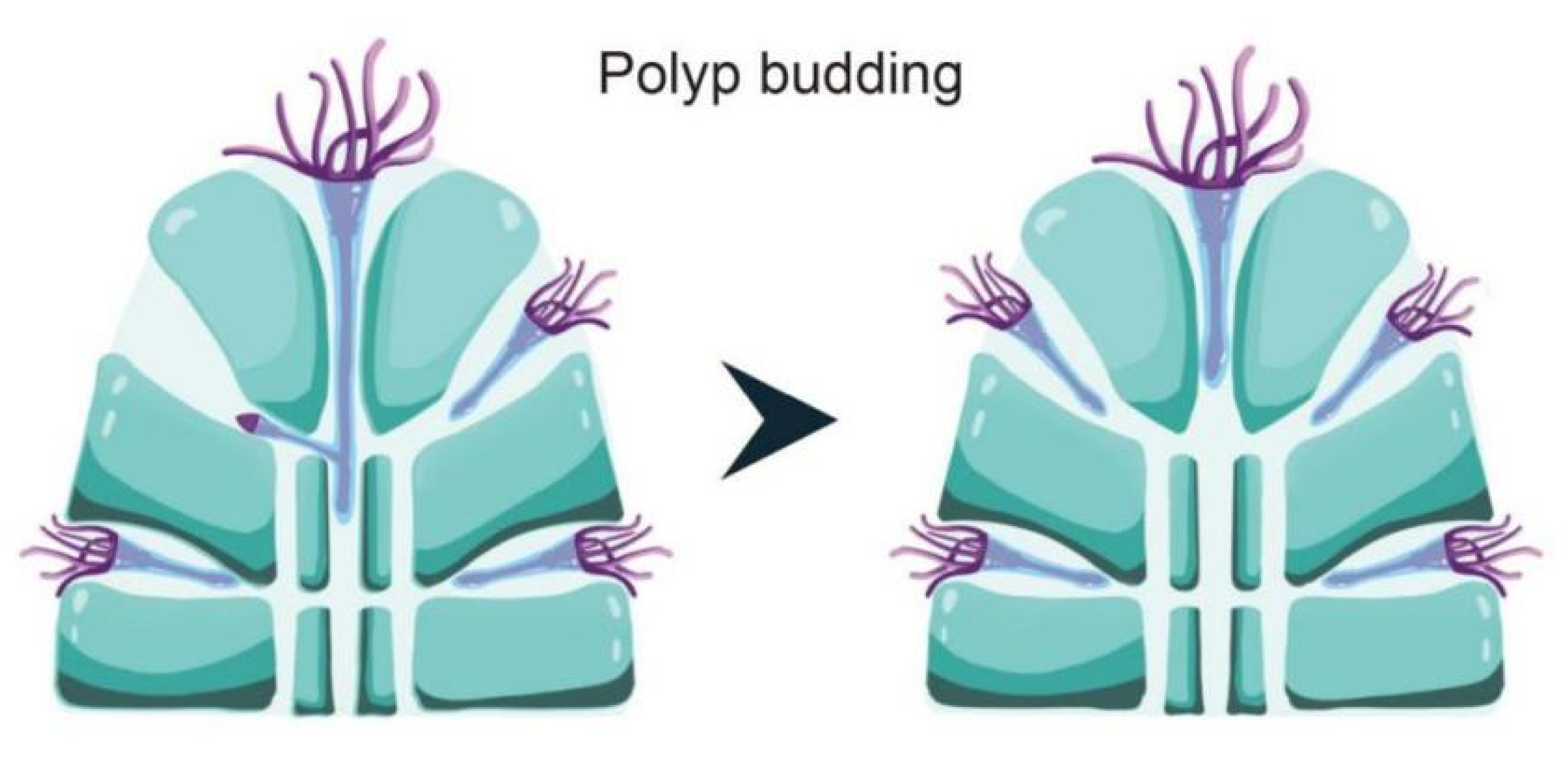
4.1. The Gastrovascular Canal System Regulates the Budding and Branching Process
4.2. Calcium Transport along the Axial Canal during Rapid Growth
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, N.; Ferse, S.C.A.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; Smith, A.; Suggett, D.; Stewart-Sinclair, P.J.; Vardi, T.; et al. Coral restoration—A systematic review of current methods, successes, failures and future directions. PLoS ONE 2020, 15, e0226631. [Google Scholar]
- Costanza, R.; Groot, R.; Sutton, P.; der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; KerryTurner, R. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Malik, A.; Einbinder, S.; Martinez, S.; Tchernov, D.; Haviv, S.; Almuly, R.; Zaslansky, P.; Polishchuk, I.; Pokroy, B.; Stolarski, J.; et al. Molecular and skeletal fingerprints of scleractinian coral biomineralization: From the sea surface to mesophotic depths. Acta. Biomater. 2021, 120, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Gratwicke, B.; Speight, M.R. The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J. Fish. Biol. 2005, 66, 650–667. [Google Scholar] [CrossRef]
- Fontaneto, D.; Sanciangco, J.C.; Carpenter, K.E.; Etnoyer, P.J.; Moretzsohn, F. Habitat Availability and Heterogeneity and the Indo-Pacific Warm Pool as Predictors of Marine Species Richness in the Tropical Indo-Pacific. PLoS ONE 2013, 8, e56245. [Google Scholar]
- Hatcher, B.G.; Johannes, R.E.; Robertson, A.J. Conservation of Shallow-water Marine Ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 1989, 27, 320. [Google Scholar]
- Wallace, C.C.; Rosen, B.R. Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: Implications for the evolution of modern diversity patterns of reef corals. Proc. R. Soc. B Biol. Sci. 2006, 273, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.W. The nomenclature and type species of some genera of recent and fossil corals. Am. J. Sci. 1936, 31, 97–134. [Google Scholar] [CrossRef]
- Madin, J.S.; Anderson, K.D.; Andreasen, M.H.; Bridge, T.C.L.; Cairns, S.D.; Connolly, S.R.; Darling, E.S.; Diaz, M.; Falster, D.S.; Franklin, E.C.; et al. The Coral Trait Database, a curated database of trait information for coral species from the global oceans. Sci. Data 2016, 3, 160017. [Google Scholar] [CrossRef]
- Li, Y.; Han, T.; Bi, K.; Liang, K.; Chen, J.; Lu, J.; He, C.; Lu, Z. The 3D reconstruction of Pocillopora colony sheds light on the growth pattern of this reef-building coral. Iscience 2020, 23, 101069. [Google Scholar] [CrossRef]
- Moura, R.L.; Amado-Filho, G.M.; Moraes, F.C.; Brasileiro, P.S.; Salomon, P.S.; Mahiques, M.M.; Bastos, A.C.; Almeida, M.G.; Silva, J.M.; Araujo, B.F.; et al. An extensive reef system at the Amazon River mouth. Sci. Adv. 2016, 2, e1501252. [Google Scholar] [CrossRef] [PubMed]
- Pablo, J. Life and Death of Coral Reefs. Fish. Res. 2004, 48, 53–74. [Google Scholar]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Tambutte, S.; Holcomb, M.; Ferrier-Pages, C.; Reynaud, S.; Tambutte, E.; Zoccola, D.; Allemand, D. Coral biomineralization: From the gene to the environment. J. Exp. Mar. Biol. Ecol. 2011, 408, 58–78. [Google Scholar] [CrossRef]
- Conci, N.; Vargas, S.; Worheide, G. The Biology and Evolution of Calcite and Aragonite Mineralization in Octocorallia. Front. Ecol. Evol. 2021, 9, 623774. [Google Scholar] [CrossRef]
- Levy, S.; Elek, A.; Grau-Bové, X.; Menéndez-Bravo, S.; Iglesias, M.; Tanay, A.; Mass, T.; Sebé-Pedrós, A. A stony coral cell atlas illuminates the molecular and cellular basis of coral symbiosis, calcification, and immunity. Cell 2021, 184, 2973. [Google Scholar] [CrossRef]
- Dzakula, B.N.; Fermani, S.; Dubinsky, Z.; Goffredo, S.; Falini, G.; Kralj, D. In Vitro Coral Biomineralization under Relevant Aragonite Supersaturation Conditions. Chem. A Eur. J. 2019, 25, 10616–10624. [Google Scholar] [CrossRef]
- Sugiura, M.; Yasumoto, K.; Iijima, M.; Oaki, Y.; Imai, H. Morphological study of fibrous aragonite in the skeletal framework of a stony coral. Crystengcomm 2021, 23, 3693–3700. [Google Scholar] [CrossRef]
- Chalker, B.E. Calcium-transport during skeletogenesis in hermatypic corals. Comp. Biochem. Physiol. Part A Physiol. 1976, 54, 455–459. [Google Scholar] [CrossRef]
- Marfenin, N.N. Non-radial symmetry of the transport system of Acropora corals. Invertebr. Zool. 2015, 12, 53–59. [Google Scholar] [CrossRef]
- Gladfelter, E.H. Skeletal development in Acropora cervicornis I. Patterns of calcium carbonate accretion in the axial corallite. Coral Reefs. 1982, 1, 45–51. [Google Scholar] [CrossRef]
- Pearse, V.B.; Muscatine, L. Role of symbiotic algae (zooxanthellae) in coral calcification. Biol. Bull. 1971, 141, 350–363. [Google Scholar] [CrossRef]
- Gladfelter, E.H. Skeletal development in Acropora cervicornis II. Diel patterns of calcium carbonate accretion. Coral Reefs. 1983, 2, 91–100. [Google Scholar] [CrossRef]
- Gladfelter, E.H. Circulation of fluids in the gastrovascular system of the reef coral Acropora cervicornis. Biol. Bull. 1983, 165, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Marfenin, N.N. Colony morphology and transport system in two species of hermatypic coral genus Acropora. Zool. Zhurnal. 1983, 62, 131983. [Google Scholar]
- Taylor, D.L. Intra-colonial transport of organic compounds and calcium in some Atlantic reef corals. Proc. Third Int. Coral Reef Symp. 1977, 1, 431–436. [Google Scholar]
- Parrin, A.P.; Netherton, S.E.; Bross, L.S.; McFadden, C.S.; Blackstone, N.W. Circulation of fluids in the gastrovascular system of a stoloniferan octocoral. Biol. Bull. 2010, 219, 112–121. [Google Scholar] [CrossRef]
- DeCarlo, T.M.; Ross, C.L.; McCulloch, M.T. Diurnal cycles of coral calcifying fluid aragonite saturation state. Mar. Biol. 2019, 166, 28. [Google Scholar] [CrossRef]
- Knebel, O.; Carvajal, C.; Standish, C.D.; de la Vega, E.; Chalk, T.B.; Ryan, E.J.; Guo, W.; Ford, M.; Foster, G.L.; Kench, P. Porites Calcifying Fluid pH on Seasonal to Diurnal Scales. J. Geophys. Res. Ocean 2021, 126, e2020JC016889. [Google Scholar] [CrossRef]
- Crossland, C.J.; Barnes, D.J. Calcification in the staghorn coral Acropora acuminata: Variations in apparent skeletal incorporation of radioisotopes due to different methods of processing. Mar. Biol. 1977, 43, 57–62. [Google Scholar] [CrossRef]
- Bonesso, J.L.; Leggat, W.; Ainsworth, T.D. Exposure to elevated sea-surface temperatures below the bleaching threshold impairs coral recovery and regeneration following injury. PeerJ 2017, 5, e3719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chindapol, N.; Kaandorp, J.A.; Cronemberger, C.; Mass, T.; Genin, A. Modelling growth and form of the scleractinian coral Pocillopora verrucosa and the influence of hydrodynamics. PLoS Comput. Biol. 2013, 9, e1002849. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Rosales, S.M.; Zaneveld, J.R.; Payet, J.P.; McMinds, R.; Hubbs, S.L.; Thurber, R.L.V. Alien vs. predator: Bacterial challenge alters coral microbiomes unless controlled by Halobacteriovorax predators. PeerJ 2017, 5, e3315. [Google Scholar] [CrossRef]
- Knackstedt, M.A.; Arns, C.H.; Senden, T.J.; Gross, K. Structure and properties of clinical coralline implants measured via 3D imaging and analysis. Biomaterials 2006, 27, 2776–2786. [Google Scholar] [CrossRef]
- Kruszynski, K.; Liere, R.V.; Kaandorp, J.A. An Interactive Visualization System for Quantifying Coral Structures. In Proceedings of the Eurographics/IEEE VGTC Symposium on Visualization EUROVIS 2006, Lisbon, Portugal, 8–10 May 2006; Aire-la-Ville (Switzerland): Eurographics Association, in cooperation with Institute of Computer Graphics & Knowledge Visualization at Graz University of Technology and Institute of Scientific Computing at Technical University at Brunswick, 2006; pp. 283–290. [Google Scholar]
- Beuck, L.; Vertino, A.; Stepina, E.; Karolczak, M.; Pfannkuche, O. Skeletal response of Lophelia pertusa (Scleractinia) to bioeroding sponge infestation visualised with micro-computed tomography. Facies 2007, 53, 157–176. [Google Scholar] [CrossRef]
- Kruszynski, K.; Kaandorp, J.A.; Liere, R.V. A computational method for quantifying morphological variation in scleractinian corals. Coral Reefs. 2007, 26, 831–840. [Google Scholar] [CrossRef]
- Nishikawa, T.; Okamura, T.; Masuno, K.; Tominaga, K.; Wato, M.; Kokubu, M.; Imai, K.; Takeda, S.; Hidaka, M.; Tanaka, A. Physical characteristics and interior structure of coral skeleton as a bone scaffold material. J. Oral. Tissue Eng. 2009, 7, 121–127. [Google Scholar]
- Janiszewska, K.; Stolarski, J.; Benzerara, K.; Meibom, A.; Mazur, M.; Kitahara, M.V.; Cairns, S.D. A unique skeletal microstructure of the deep-sea micrabaciid scleractinian corals. J. Morphol. 2011, 2, 191–203. [Google Scholar] [CrossRef]
- Janiszewska, K.; Jaroszewicz, J.; Stolarski, J. Skeletal ontogeny in basal scleractinian micrabaciid corals. J. Morphol. 2013, 3, 243–257. [Google Scholar] [CrossRef]
- Tambutté, E.; Venn, A.A.; Holcomb, M.; Segonds, N.; Techer, N.; Zoccola, D.; Tambutté, S. Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat. Commun. 2015, 6, 7368. [Google Scholar] [CrossRef] [PubMed]
- Gladfelter, E.H. Skeletal Development in Acropora cervicornis III. A Comparison of Monthly Rates of Linear Extension and Calcium Carbonate Accretion Measured over a Year. Coral Reefs. 1984, 3, 51–57. [Google Scholar] [CrossRef]
- Goreau, T.F.; Bowen, V.T. Calcium uptake by a coral. Sci. N. Y. 1955, 122, 1188–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, T.; Wang, G.; Chen, J.; He, C.; Lu, Z. Coral growth monitoring in 24 weeks with laboratory auto calibration balance system. J. Coast. Res. 2020, 108, 288–293. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Guo, D.; Shi, G. Three-Dimensional Morphology and Stratigraphic Structure of Coral Reef in Xisha and Nasha Islands. Adv. Geosci. 2019, 9, 61–68. [Google Scholar] [CrossRef]
- Barnes, D.J.; Crossland, C.J. Diurnal and seasonal variations in the growth of staghorn coral measured by time-lapse photography. Limnol. Oceanogr. 1980, 25, 1113–1117. [Google Scholar] [CrossRef]

| Sample | Voltage | Current | Voxel Size | Timing | Number of Images | Image Width | Image Height |
|---|---|---|---|---|---|---|---|
| Acropora colony one | 150 kV | 180 μA | 37 μm | 1 s | 2000 | 3990 pixels | 4000 pixels |
| Acropora branch one | 130 kV | 60 μA | 6 μm | 500 ms | 1500 | 2800 pixels | 4000 pixels |
| Acropora branch two | 120 kV | 115 μA | 12 μm | 500 ms | 2400 | 1980 pixels | 2000 pixels |
| Acropora branch three | 130 kV | 60 μA | 6 μm | 500 ms | 1500 | 2800 pixels | 4000 pixels |
| Acropora branch four | 130 kV | 100 μA | 9 μm | 500 ms | 2500 | 1985 pixels | 2000 pixels |
| Acropora branch five | 160 kV | 70 μA | 9 μm | 500 ms | 1600 | 1500 pixels | 4000 pixels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liao, X.; He, C.; Lu, Z. Calcium Transport along the Axial Canal in Acropora. Diversity 2021, 13, 407. https://doi.org/10.3390/d13090407
Li Y, Liao X, He C, Lu Z. Calcium Transport along the Axial Canal in Acropora. Diversity. 2021; 13(9):407. https://doi.org/10.3390/d13090407
Chicago/Turabian StyleLi, Yixin, Xin Liao, Chunpeng He, and Zuhong Lu. 2021. "Calcium Transport along the Axial Canal in Acropora" Diversity 13, no. 9: 407. https://doi.org/10.3390/d13090407
APA StyleLi, Y., Liao, X., He, C., & Lu, Z. (2021). Calcium Transport along the Axial Canal in Acropora. Diversity, 13(9), 407. https://doi.org/10.3390/d13090407