Soil Microbial Community Based on PLFA Profiles in an Age Sequence of Pomegranate Plantation in the Middle Yellow River Floodplain
Abstract
:1. Introduction
2. Materials and Methods
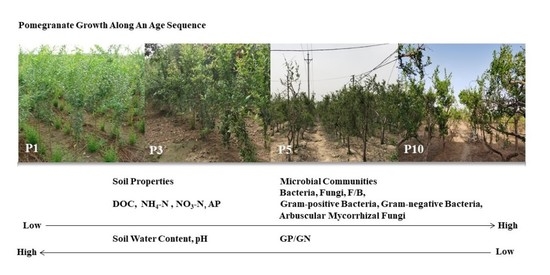
2.1. Research Site
2.2. Sample Collection and Processing
2.3. Phospholipid Fatty Acid Analysis
2.4. Statistical Analysis
3. Results
3.1. PLFAs for Microbial Community
3.2. Soil Physicochemical Properties and Relationships with Soil Microbial PLFAs
4. Discussion
4.1. Soil Properties in Pomegranate Plantation
4.2. Soil Microbial Communities and Influential Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okhovatian-Ardakani, A.R.; Mehrabanian, M.; Dehghani, F.; Akbarzadeh, A. Salt tolerance evaluation and relative comparison in cuttings of different pomegranate cultivar. Plant Soil Environ. 2010, 56, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Aseri, G.K.; Jain, N.; Panwar, J.; Rao, A.V.; Meghwal, P.R. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum, L.) in Indian Thar Desert. Sci. Hortic. 2008, 117, 130–135. [Google Scholar] [CrossRef]
- Volschenk, T. Water use and irrigation management of pomegranate trees—A review. Agric. Water Manag. 2020, 241, 106375. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Yang, F.; Lei, Y.; Zhang, Q.; Cheng, X. Alterations in soil microbial community composition and biomass following agricultural land use change. Sci. Rep. 2016, 6, 36587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Bowman, D.; Shi, W. Soil microbial community structure and diversity in a turfgrass chronosequence: Land-use Change versus turfgrass management. Appl. Soil Ecol. 2006, 34, 209–218. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G.E.D. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Seiwa, K.; Negishi, Y.; Eto, Y.; Hishita, M.; Masaka, K.; Fukasawa, Y.; Matsukura, K.; Suzuki, M. Successful seedling establishment of arbuscular mycorrhizal-compared to ectomycorrhizal-associated hardwoods in arbuscular cedar plantations. For. Ecol. Manag. 2020, 468, 118155. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, X.; Duan, Y.; Fu, X.; Wu, F. Wheat cover crop promoted cucumber seedling growth through regulating soil nutrient resources or soil microbial communities? Plant Soil 2017, 418, 1–17. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Dennis, P.G.; Paungfoo-Lonhienne, C.; Weber, L.; Brackin, R.; Ragan, M.A.; Schmidt, S.; Hugenholtz, P. Evolutionary Conservation of a Core Root Microbiome across Plant Phyla along a Tropical Soil Chronosequence. Nat. Commun. 2017, 8, 215. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, B.; Treves, D.S.; Wu, L.-Y.; Marsh, T.L.; O’Neill, R.V.; Palumbo, A.V.; Tiedje, J.M. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 2002, 68, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Liu, J.; Wu, F. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; van Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R.W. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, P.; Forge, T.A.; Jones, M.D.; Nelson, L.M. Soil biota from newly established orchards are more beneficial to early growth of cherry trees than biota from older orchards. Appl. Soil Ecol. 2020, 155, 103658. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, J.; Zhou, X.; Cao, J.; Wang, R.W.; Wang, Y.; Shelton, S.; Jin, Z.; Walker, L.M.; et al. Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob. Chang. Biol. 2017, 23, 1328–1337. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kumar, P.; Bhardwaj, S.K.; Chandel, A. Agronomic performance, nutrient cycling and microbial biomass in soil as affected by pomegranate based multiple crop sequencing. Sci. Hortic. 2015, 197, 504–515. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Spring, A.M.; Domingue, K.D.; Kerber, T.V.; Mooney, M.M.; Hale, R.L.; Lemmer, K.M.; Docherty, K.M. Land use effects on airborne bacterial communities are evident in both near-surface and higher-altitude air. Diversity 2021, 13, 85. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.; Qiao, N.; Geng, Q.; Liu, Y.; Song, H.; Yang, Z.; Liu, C.; Wang, G. Effects of mowing and fertilization on soil quality in a semiarid grassland of North China. Land Degrad. Dev. 2021, 32, 1656–1666. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.; Bååth, E. Investigating the mechanisms for the opposing PH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Zhong, Z.; Huang, X.; Feng, D.; Xing, S.; Weng, B. Long-term effects of legume mulching on soil chemical properties and bacterial community composition and structure. Agric. Ecosyst. Environ. 2018, 268, 24–33. [Google Scholar] [CrossRef]
- Yu, Q.; Hanif, A.; Rao, X.; He, J.; Sun, D.; Liu, S.; He, D.; Shen, W. Long-term restoration altered edaphic properties and soil microbial communities in forests: Evidence from four plantations of southern China. Restor. Ecol. 2021, 4, e13354. [Google Scholar]
- Zheng, J.; Chen, J.; Pan, G.; Wang, G.; Liu, X.; Zhang, X.; Li, L.; Bian, R.; Cheng, K.; Zheng, J. A long-term hybrid poplar plantation on cropland reduces soil organic carbon mineralization and shifts microbial community abundance and composition. Appl. Soil Ecol. 2017, 111, 94–104. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Ramsey, P.W.; Rillig, M.C.; Feris, K.P.; Holben, W.E.; Gannon, J.E. Choice of methods for soil microbial community analysis: PLFA maximizes power compared to CLPP and PCR-based approaches. Pedobiologia 2006, 50, 275–280. [Google Scholar] [CrossRef]
- Galindo, A.; Rodríguez, P.; Collado-González, J.; Cruz, Z.N.; Torrecillas, E.; Ondoño, S.; Corell, M.; Moriana, A.; Torrecillas, A. Rainfall intensifies fruit peel cracking in water stressed pomegranate trees. Agric. For. Meteorol. 2014, 194, 29–35. [Google Scholar] [CrossRef]
- An, S.; Mentler, A.; Mayer, H.; Blum, W.E.H. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the loess plateau, China. CATENA 2010, 81, 226–233. [Google Scholar] [CrossRef]
- Deurer, M.; Grinev, D.; Young, I.; Clothier, B.E.; Müller, K. The impact of soil carbon management on soil macropore structure: A comparison of two apple orchard systems in New Zealand. Eur. J. Soil Sci. 2009, 60, 945–955. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Zelles, L.; Bai, Q.Y.; Beck, T.; Beese, F. Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol. Biochem. UK 1992, 24, 317–323. [Google Scholar] [CrossRef]
- Abaye, D.A.; Lawlor, K.; Hirsch, P.R.; Brookes, P.C. Changes in the microbial community of an arable soil caused by long-term metal contamination. Eur. J. Soil Sci. 2005, 56, 93–102. [Google Scholar] [CrossRef]
- Tunlid, A.; Hoitink, H.A.; Low, C.; White, D.C. Characterization of bacteria that suppress rhizoctonia damping-off in bark compost media by analysis of fatty acid biomarkers. Appl. Environ. Microbiol. 1989, 55, 1368–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.P.; Shen, W.J.; Li, Y.E.; Hui, D.F. Interactive effects of temperature and moisture on composition of the soil microbial community. Eur. J. Soil Sci. 2017, 68, 909–918. [Google Scholar] [CrossRef]
- Zelles, L.; Rackwitz, R.; Bai, Q.; Beck, T.; Beese, F. Discrimination of microbial diversity by fatty acid profiles of phospholipids and lipo-polysaccharids in differently cultivated soils. Plant Soil 1995, 170, 115–122. [Google Scholar] [CrossRef]
- Kaiser, C.; Frank, A.; Wild, B.; Koranda, M.; Richter, A. Negligible contribution from roots to soil-borne phospholipid fatty acid fungal biomarkers 18:2ω6,9 and 18:1ω9. Soil Biol. Biochem. 2010, 42, 1650–1652. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.Y.; Zhou, D.-M.; Cang, L. Microbial and Enzyme properties of apple orchard soil as affected by long-term application of copper fungicide. Soil Biol. Biochem. 2009, 41, 1504–1509. [Google Scholar] [CrossRef]
- He, S.; Zheng, Z.; Zhu, R. Long-term tea plantation effects on composition and stabilization of soil organic matter in southwest China. CATENA 2021, 199, 105132. [Google Scholar] [CrossRef]
- Wang, D.; Chi, Z.; Yue, B.; Huang, X.; Zhao, J.; Song, H.; Yang, Z.; Miao, R.; Liu, Y.; Zhang, Y.; et al. Effects of mowing and nitrogen addition on the ecosystem c and n pools in a temperate steppe: A case study from northern China. CATENA 2020, 185, 104332. [Google Scholar] [CrossRef]
- Yang, K.; Wang, K.; Zhang, X.; Chang, X.; Bai, G.; Zheng, J.; Wu, G.-L. Change in soil water deficit and soil organic matter consumption over time in rain-fed apricot orchards on the semi-arid loess plateau, China. Agric. Ecosyst. Environ. 2021, 314, 107381. [Google Scholar] [CrossRef]
- Huo, G.; Zhao, X.; Gao, X.; Wang, S.; Pan, Y. Seasonal water use patterns of rainfed jujube trees in stands of different ages under semiarid plantations in China. Agric. Ecosyst. Environ. 2018, 265, 392–401. [Google Scholar] [CrossRef]
- Gao, X.; Li, H.; Zhao, X.; Ma, W.; Wu, P. Identifying a suitable revegetation technique for soil restoration on water-limited and degraded land: Considering both deep soil moisture deficit and soil organic carbon sequestration. Geoderma 2018, 319, 61–69. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Felton, A.J.; Xia, L.; Zhang, Y.; Luo, Y.; Cheng, X.; Cao, J. Post-fire co-stimulation of gross primary production and ecosystem respiration in a meadow grassland on the tibetan plateau. Agric. For. Meteorol. 2021, 303, 108388. [Google Scholar] [CrossRef]
- Degens, B. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 2000, 32, 189–196. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Katsalirou, E.; Deng, S.; Gerakis, A.; Nofziger, D.L. Long-term management effects on soil P, microbial biomass P, and phosphatase activities in prairie soils. Eur. J. Soil Biol. 2016, 76, 61–69. [Google Scholar] [CrossRef]
- Huang, J.; Hu, B.; Qi, K.; Chen, W.; Pang, X.; Bao, W.; Tian, G. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol. 2016, 72, 35–41. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; Zhou, J.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021, 230, 304–315. [Google Scholar] [CrossRef]
- Sheng, M.; Lalande, R.; Hamel, C.; Ziadi, N. Effect of long-term tillage and mineral phosphorus fertilization on arbuscular mycorrhizal fungi in a humid continental zone of eastern Canada. Plant Soil 2013, 369, 599–613. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H. Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Bååth, E.; Frostegard, A.; Pennanen, T.; Fritze, H. Microbial community structure and PH response in relation to soil organic matter quality in wood-ash fertilized, clear-cut or burned coniferous forest soils. Soil Biol. Biochem. 1995, 27, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Helgason, B.L.; Walley, F.L.; Germida, J.J. Fungal and bacterial abundance in long-term no-till and intensive-till soils of the northern great plains. Soil Sci. Soc. Am. J. 2009, 73, 120–127. [Google Scholar] [CrossRef]
- Holland, E.A.; Coleman, D.C. Litter placement effects on microbial and organic matter dynamics in an agroecosystem. Ecology 1987, 68, 425–433. [Google Scholar] [CrossRef]
- Stromberger, M.; Shah, Z.; Westfall, D. Soil microbial communities of no-till dryland agroecosystems across an evapotranspiration gradient. Appl. Soil Ecol. 2007, 35, 94–106. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Rousk, J.; Brookes, P.C.; Bååth, E. Bacterial PH-optima for growth track soil PH, but are higher than expected at low PH. Soil Biol. Biochem. 2011, 43, 1569–1575. [Google Scholar] [CrossRef]
- Bååth, E.; Anderson, T.-H. Comparison of Soil Fungal/Bacterial Ratios in a PH Gradient Using Physiological and PLFA-Based Techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40, 425–433. [Google Scholar] [CrossRef]
- Sekhohola-Dlamini, L.; Dlamini, P.; Selvarajan, R.; Ogola, H.J.O.; Tekere, M. Influences of geochemical factors and substrate availability on gram-positive and gram-negative bacterial distribution and bio-processes in ageing municipal landfills. Int. Microbiol. 2021, 24, 311–332. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. The ratio of gram-positive to gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Orwin, K.H.; Dickie, I.A.; Wood, J.R.; Bonner, K.I.; Holdaway, R.J. Soil microbial community structure explains the resistance of respiration to a dry–rewet cycle, but not soil functioning under static conditions. Funct. Ecol. 2016, 30, 1430–1439. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]





| Stand Age | Tree Growth Condition (cm) | Particle Size Analysis (%) | |||||
|---|---|---|---|---|---|---|---|
| Basal Diameter | Height | Crown Height | CrownWidth | Clay | Silt | Sand | |
| P1 | 1.43 | 128.90 | 93.62 | 33.02 | 0.44 | 70.10 | 29.46 |
| P3 | 2.74 | 234.85 | 175.84 | 76.04 | 0.02 | 67.39 | 32.58 |
| P5 | 7.57 | 215.87 | 154.04 | 101.01 | 0.05 | 71.91 | 28.04 |
| P10 | 10.13 | 303.89 | 243.52 | 186.87 | 0.12 | 63.34 | 36.54 |
| Stand Age (S) | Depth (D) | S × D | |
|---|---|---|---|
| BD | NS | - | - |
| SWC | 10.179 *** | NS | NS |
| pH | 17.218 *** | 10.360 *** | 8.956 *** |
| DOC | 7.835 *** | 15.172 *** | 3.872 ** |
| NH4-N | 62.039 *** | NS | NS |
| NO3-N | 8.572 *** | NS | NS |
| AP | 124.783 *** | 101.777 *** | 41.197 *** |
| Total PLFAs | 10.008 *** | 38.887 *** | 3.214 ** |
| Bacterial PLFAs | 9.579 *** | 36.743 *** | 3.045 * |
| Fungal PLFAs | 12.489 *** | 34.215 *** | 4.403 ** |
| F/B ratio | 5.199 ** | 28.114 *** | 5.532 *** |
| GP bacterial PLFAs | 12.073 *** | 23.820 *** | NS |
| GN bacterial PLFAs | 7.952 *** | 56.704 *** | 4.403 ** |
| GP/GN ratio | 4.701 ** | 40.835 *** | 6.798 *** |
| AMF PLFAs | 10.029 *** | 51.315 *** | 4.875 ** |
| Depth (cm) | Stand Age | BD (kg/m3) | SWC (%) | pH | DOC (mg kg−1) | NH4-N (mg kg−1) | NO3-N (mg kg−1) | AP (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| 0–10 | P1 | 1.21 ± 0.04A | 14.73 ± 2.06Aa | 8.10 ± 0.05Aa | 62.09 ± 6.64BCa | 3.76 ± 0.03Ba | 19.16 ± 4.62ABa | 7.25 ± 0.92Ca |
| P3 | 1.15 ± 0.03A | 13.31 ± 2.14Aa | 7.97 ± 0.03Bb | 54.78 ± 4.27Ca | 3.76 ± 0.03Ba | 22.23 ± 1.94Aa | 5.77 ± 0.65Ca | |
| P5 | 1.21 ± 0.03A | 8.97 ± 0.72Ba | 7.88 ± 0.04Bc | 72.69 ± 4.69Ba | 3.91 ± 0.05Aa | 8.85 ± 2.18Ba | 64.85 ± 5.37Ba | |
| P10 | 1.17 ± 0.04A | 9.06 ± 0.77Ba | 7.87 ± 0.03Bb | 100.40 ± 6.65Aa | 3.99 ± 0.02Aa | 13.61 ± 3.07ABa | 123.53 ± 7.14Aa | |
| 10–20 | P1 | - | 15.11 ± 2.01Aa | 8.20 ± 0.05Aa | 56.05 ± 3.38ABa | 3.72 ± 0.02Ba | 17.40 ± 2.31Aa | 5.55 ± 1.02Ca |
| P3 | - | 14.21 ± 1.35ABa | 8.11 ± 0.04ABab | 49.90 ± 2.50Ba | 3.67 ± 0.01Bb | 20.73 ± 1.60Aab | 6.16 ± 1.02Ca | |
| P5 | - | 10.96 ± 0.74BCa | 8.03 ± 0.03Bb | 67.59 ± 2.81Aa | 3.91 ± 0.03Aa | 9.19 ± 1.64Bab | 31.59 ± 5.66Bb | |
| P10 | - | 9.43 ± 0.73Ca | 8.04 ± 0.04Bab | 66.43 ± 4.92Ab | 3.88 ± 0.06Aa | 13.87 ± 0.48ABa | 46.46 ± 5.35Ab | |
| 20–30 | P1 | - | 17.17 ± 2.18Aa | 8.24 ± 0.04Aa | 47.86 ± 4.55Aa | 3.70 ± 0.03Ba | 17.78 ± 0.92Aa | 4.74 ± 0.46Ba |
| P3 | - | 16.04 ± 0.88Aa | 8.16 ± 0.03Aa | 54.52 ± 1.29Aa | 3.68 ± 0.02Bb | 13.02 ± 2.74ABb | 5.82 ± 0.55Ba | |
| P5 | - | 10.70 ± 1.11Ba | 8.21 ± 0.01Aa | 54.21 ± 1.82Ab | 3.89 ± 0.02Aa | 7.90 ± 1.44Bb | 15.15 ± 1.57Ab | |
| P10 | - | 8.47 ± 0.83Ba | 8.09 ± 0.08Ba | 51.84 ± 2.76Ac | 3.97 ± 0.06Aa | 11.30 ± 0.83ABa | 12.21 ± 1.83Ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yan, X.; Wang, D.; Siddique, I.A.; Chen, J.; Xu, Q.; Zhao, C.; Yang, L.; Miao, Y.; Han, S. Soil Microbial Community Based on PLFA Profiles in an Age Sequence of Pomegranate Plantation in the Middle Yellow River Floodplain. Diversity 2021, 13, 408. https://doi.org/10.3390/d13090408
Wang S, Yan X, Wang D, Siddique IA, Chen J, Xu Q, Zhao C, Yang L, Miao Y, Han S. Soil Microbial Community Based on PLFA Profiles in an Age Sequence of Pomegranate Plantation in the Middle Yellow River Floodplain. Diversity. 2021; 13(9):408. https://doi.org/10.3390/d13090408
Chicago/Turabian StyleWang, Shilin, Xinyu Yan, Dong Wang, Imran Ahammad Siddique, Ji Chen, Qi Xu, Cancan Zhao, Leyun Yang, Yuan Miao, and Shijie Han. 2021. "Soil Microbial Community Based on PLFA Profiles in an Age Sequence of Pomegranate Plantation in the Middle Yellow River Floodplain" Diversity 13, no. 9: 408. https://doi.org/10.3390/d13090408
APA StyleWang, S., Yan, X., Wang, D., Siddique, I. A., Chen, J., Xu, Q., Zhao, C., Yang, L., Miao, Y., & Han, S. (2021). Soil Microbial Community Based on PLFA Profiles in an Age Sequence of Pomegranate Plantation in the Middle Yellow River Floodplain. Diversity, 13(9), 408. https://doi.org/10.3390/d13090408