Bat Diversity in Cat Ba Biosphere Reserve, Northeastern Vietnam: A Review with New Records from Mangrove Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Bat Capture and Morphological Measurements
2.2. Recording and Analysis of Echolocation Signals
2.3. Tissue Sampling and Genetic Analysis
3. Results
4. Discussion
4.1. Species Recorded over the Surveys
4.1.1. Cynopterus sphinx
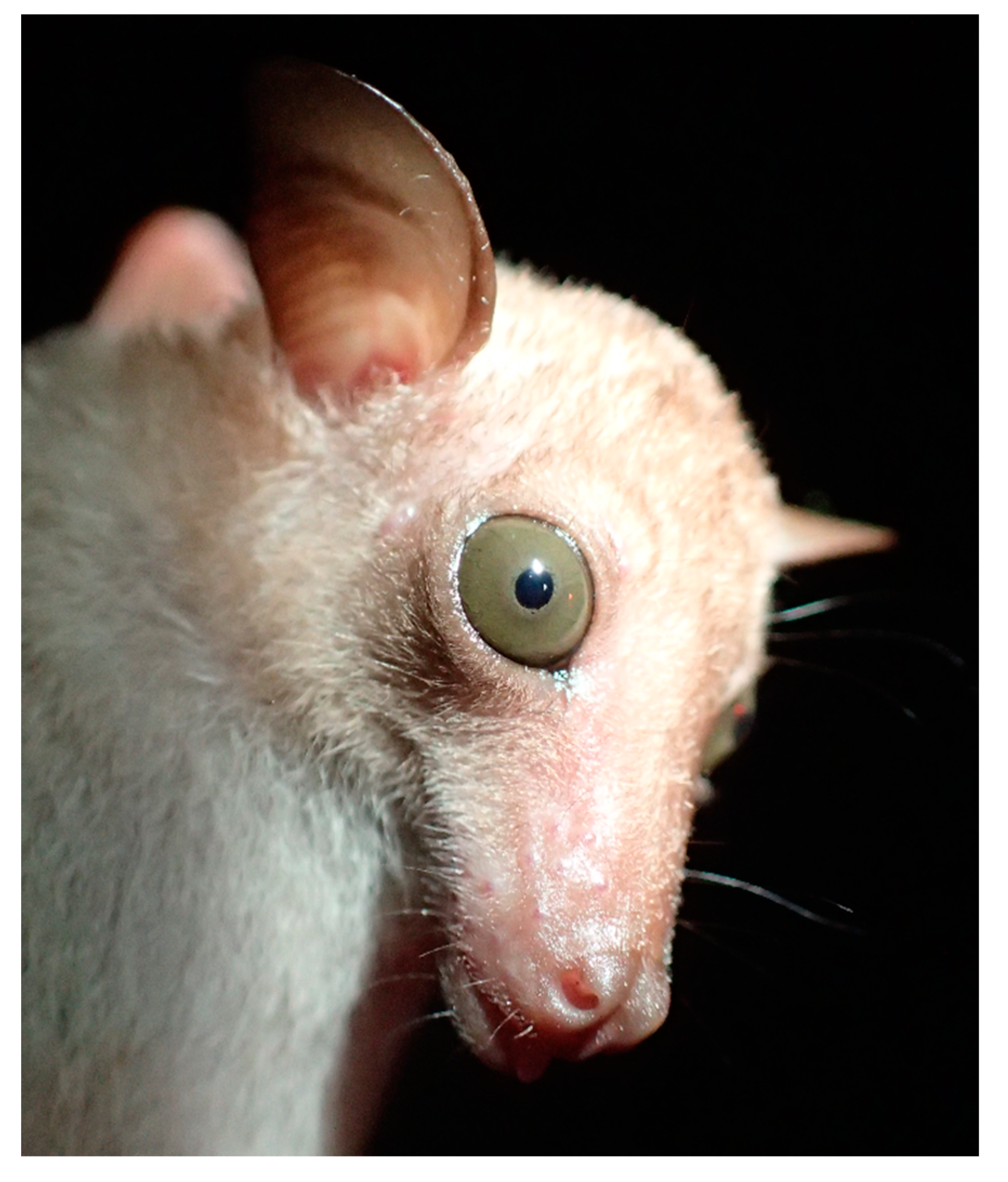
4.1.2. Macroglossus minimus
4.1.3. Taphozous melanopogon
4.1.4. Rhinolophus affinis
4.1.5. Rhinolophus siamensis
4.1.6. Rhinolophus marshalli
4.1.7. Rhinolophus pearsonii
4.1.8. Rhinolophus pusillus
4.1.9. Aselliscus cf. stoliczkanus
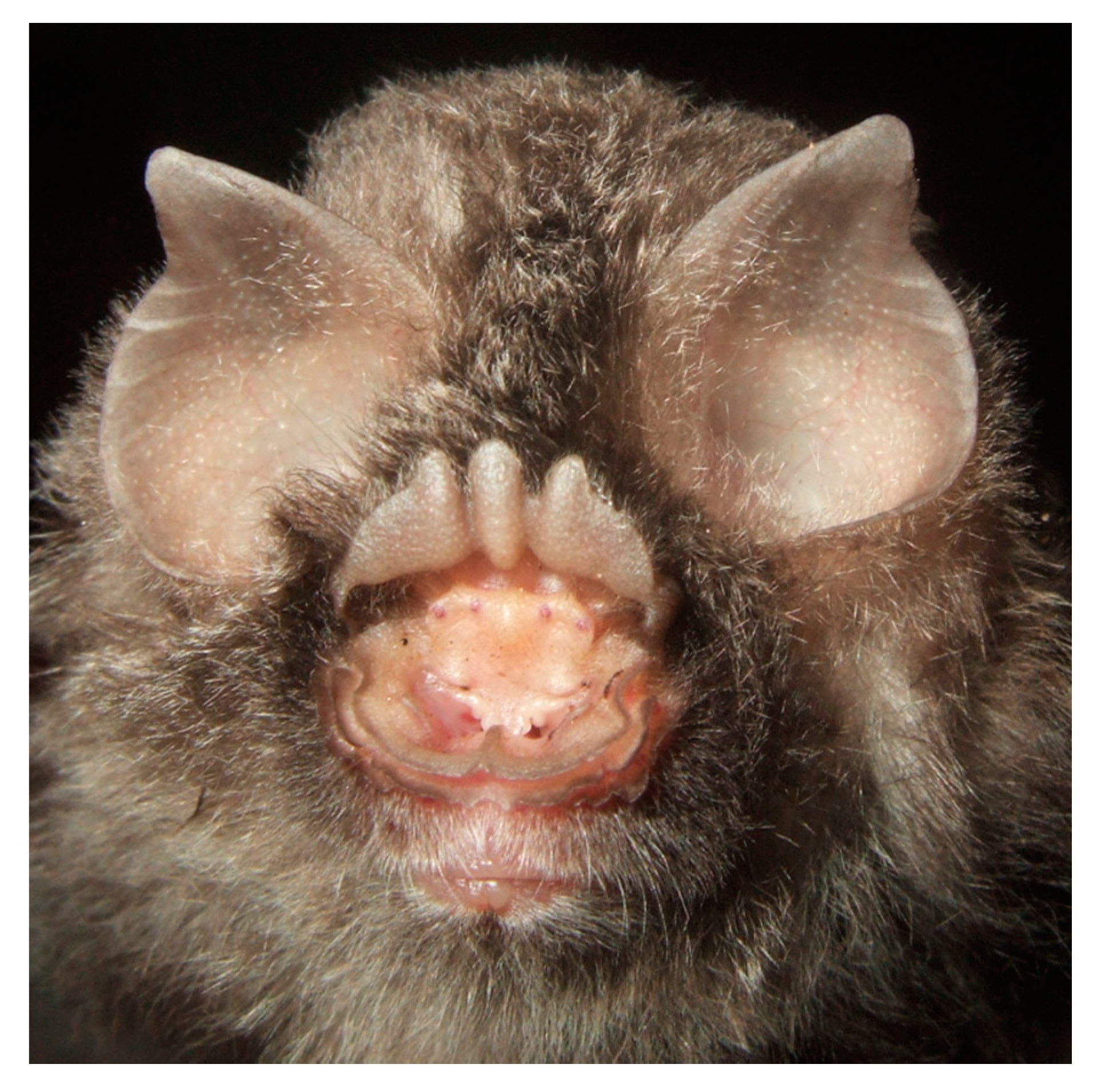
4.1.10. Hipposideros alongensis
4.1.11. Hipposideros armiger
4.1.12. Hipposideros gentilis
4.1.13. Hipposideros larvatus
4.1.14. Hipposideros khaokhouayensis
4.1.15. Hypsugo pulveratus
4.1.16. Murina cyclotis
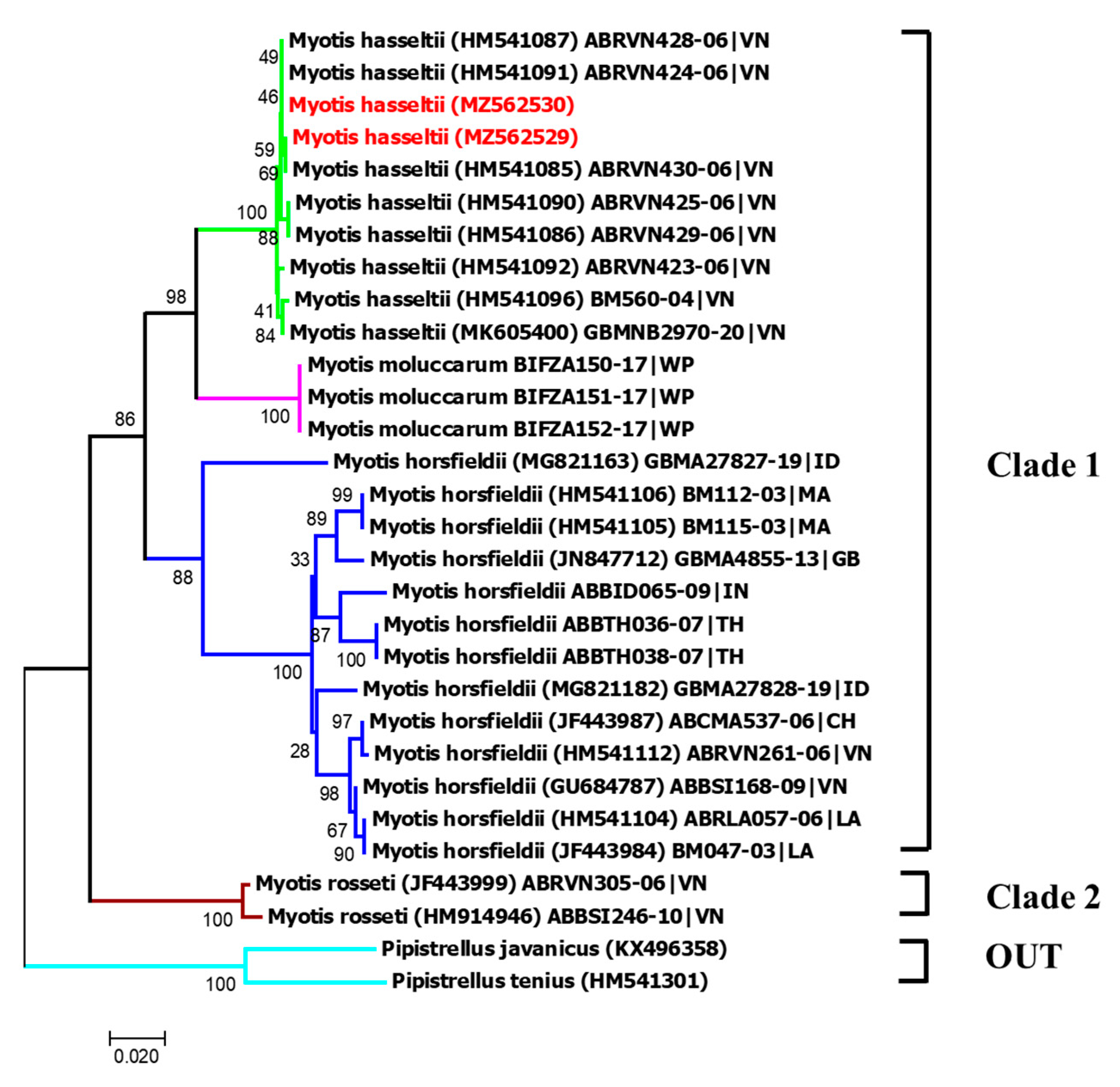
4.1.17. Myotis hasseltii
4.1.18. Myotis pilosus
4.1.19. Myotis alticraniatus
4.1.20. Phoniscus jagorii
4.1.21. Pipistrellus abramus
4.1.22. Tylonycteris fulvida
4.1.23. Miniopterus fuliginosus
4.2. Species Recorded by 2014
4.2.1. Cynopterus horsfieldii
4.2.2. Rousettus amplexicaudatus
4.2.3. Coelops frithii
4.2.4. Hipposideros griffini
4.2.5. Harpiocephalus harpia
4.2.6. Murina harrisoni
4.2.7. Pipistrellus javanicus
4.2.8. Pipistrellus tenuis
4.3. Notes on Unconfirmed Historical Records
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuyen, L.T.; Tri, N.H. Cat Ba Archipelago Biosphere Reserve: Community participation and quality economy development. In Proceedings of the 7th Southeast Asian Biosphere Reserve Network Meeting, Puerto Princesa, Palawan, Phillippines, 24–26 October 2013. [Google Scholar]
- Bourret, R. Sur quelques petits Mammifères du Tonkin et du Laos. In Comptes Rendus du Conseil de Recherches Scientifiques de L’indochine 2ème Semester; Université Indochinoise: Hanoi, Vietnam, 1942; pp. 27–30. [Google Scholar]
- Bourret, R. Les Mammifères de la Collection du Laboratoire de Zoologie de l’École Supérieure des Sciences; Université Indochinoise: Hanoi, Vietnam, 1942; pp. 1–13. [Google Scholar]
- Topál, G. Taxonomic status of Hipposideros larvatus alongensis Bourret, 1942 and the occurrence of H. turpis Bangs, 1901 in Vietnam (Mammalia, Chiroptera). Acta Zool. Hung. 1993, 39, 267–288. [Google Scholar]
- Thong, V.D.; Tu, V.T.; Tien, P.D.; Chu, C.-W.; Senawi, J.; Bates, P.J.J.; Furey, N.M. Echolocation call frequency of Marshall’s horseshoe bat Rhinolophus marshalli from Cat Ba National Park and its current status in Vietnam. In Proceedings of the 2nd National Scientific Conference on Ecology and Biological Resources, Hanoi, Vietnam, 26 October 2007; pp. 274–277. [Google Scholar]
- Thong, V.D.; Dietz, C.; Schnitzler, H.-U.; Denzinger, A.; Furey, N.M.; Borissenko, A.; Bates, P.J.J. First record of Hipposideros khaokhouayensis (Chiroptera: Hipposideridae) from Vietnam. HNUE J. Sci. 2008, 53, 138–143. [Google Scholar]
- Thong, V.D. Systematics and Echolocation of Rhinolophoid Bats (Mammalia: Chiroptera) in Vietnam. Ph.D. Thesis, University of Tuebingen, Tuebingen, Germany, 26 March 2011. [Google Scholar]
- Thong, V.D.; Dietz, C.; Denzinger, A.; Bates, P.J.J.; Furey, N.M.; Csorba, G.; Hoye, G.; Thuy, L.D.; Schnitzler, H.-U. Further records of Murina tiensa from Vietnam with first information on its echolocation calls. Hystrix Ital. J. Mammal. 2011, 22, 129–138. [Google Scholar]
- Abramov, A.V.; Kruskop, S.V. The mammal fauna of Cat Ba Island, northern Vietnam. Russ. J. Theriol. 2012, 11, 57–72. [Google Scholar] [CrossRef]
- Thong, V.D. New findings and an extensive description of Rhinolophus marshalli Thonglongya, 1973 in Vietnam. HNUE J. Sci. 2012, 57, 3–10. [Google Scholar]
- Thong, V.D.; Puechmaille, S.J.; Denginger, A.; Bates, P.J.J.; Dietz, C.; Csorba, G.; Soisook, P.; Teeling, E.C.; Matsumura, S.; Furey, N.M.; et al. Systematics of the Hipposideros turpis complex and a description of a new subspecies from Vietnam. Mammal Rev. 2012, 42, 166–192. [Google Scholar] [CrossRef]
- Thong, V.D.; Puechmaille, S.J.; Denzinger, A.; Dietz, C.; Csorba, G.; Bates, P.J.J.; Teeling, E.C.; Schnitzler, H.-U. A new species of Hipposideros (Chiroptera: Hipposideridae) from Vietnam. J. Mammal. 2012, 93, 1–11. [Google Scholar] [CrossRef]
- Thong, V.D.; Dietz, C.; Denzinger, A.; Bates, P.J.J.; Puechmaille, S.J.; Callou, C.; Schnitzler, H.-U. Resolving a mammal mystery: The identity of Paracoelops megalotis (Chiroptera: Hipposideridae). Zootaxa 2012, 3505, 75–85. [Google Scholar] [CrossRef]
- Kruskop, S.V. Bats of Vietnam: Checklist and an Identification Manual, 2nd ed.; KMK Ltd.: Moscow, Russia, 2013; pp. 1–300. [Google Scholar]
- Thong, V.D. An updated list of leaf-nosed bats (Hipposideridae) from Vietnam and key features of Hipposideros alongensis. Tap Chi Sinh Hoc (Vietnam. J. Biol.) 2013, 35, 178–184. [Google Scholar] [CrossRef][Green Version]
- Thong, V.D. Acoustic identification and taxonomic remarks of horseshoe bats (Chiroptera: Rhinolophidae) in Cat Ba National Park, north-eastern Vietnam. In Proceedings of the First International VAST-BAS Conference, Ha Long City, Vietnam, 20–21 November 2014; pp. 323–328. [Google Scholar] [CrossRef]
- Thong, V.D. Taxonomy and ecology of Cynopterus horsfieldii (Chiroptera: Pteropodidae) from Vietnam. In Proceedings of the First International VAST-BAS Conference, Ha Long City, Vietnam, 20–21 November 2014; pp. 329–334. [Google Scholar] [CrossRef]
- Thong, V.D.; Dung, D.T.; Thanh, N.V. An overview of bat research in Cat Ba Biosphere Reserve with remarks on previous records. In Proceedings of the 2nd National Scientific Conference on Biological Research and Teaching in Vietnam, Da Nang, Vietnam, 20 May 2016; pp. 737–744. [Google Scholar]
- Yuzefovich, A.P.; Artyushin, I.A.; Kruskop, S.V. Not the Cryptic Species: Diversity of Hipposideros gentilis (Chiroptera: Hipposideridae) in Indochina. Diversity 2021, 13, 218. [Google Scholar] [CrossRef]
- Furey, N. Fauna. Cat Ba National Park: Biodiversity Survey 1999; Furey, N., Canh, L.X., Fanning, E., Eds.; Frontier Vietnam Environmental Research Report 20; Society for Environmental Exploration, UK and Institute of Ecology and Biological Resources: Hanoi, Vietnam, 2002; pp. 18–30. [Google Scholar]
- Canh, L.X.; Sung, C.V.; Lee, S.D. Mammal resources of Cat Ba and surrounding areas in Vietnam. In Ecosystem and Biodiversity of Cat Ba National Park and Ha Long Bay, Vietnam; Annals of Nature Conservation, The Korean National Council for Conservation of Nature, Korea, and Institute of Ecology and Biological Resources: Hanoi, Vietnam, 1997; Volume 12, pp. 147–159. [Google Scholar]
- Borissenko, A.V.; Kruskop, S.V. Bats of Vietnam and Adjacent Territories: An Identification Manual; Joint Russian-Vietnamese Science and Technological Tropical Centre, Moscow and Hanoi, Russia and Vietnam: Moscow, Russia, 2003; pp. 1–212. [Google Scholar]
- Thong, V.D. Bat Conservation at Cat Ba Biosphere Reserve, North-east Vietnam, Conservation Leadership Programme, 2008. Available online: www.conservationleadershipprogramme.org (accessed on 19 May 2021).
- Thong, V.D.; Furey, N.M. The bat fauna of Cat Ba Biosphere. Tap Chi Sinh Hoc (Vietnam. J. Biol.) 2008, 30, 73–77. [Google Scholar]
- Can, D.N.; Endo, H.; Son, N.T.; Oshida, T.; Canh, L.X.; Phuong, D.H.; Lunde, D.P.; Kawada, S.-I.; Hayashida, A.; Sasaki, M. Checklist of Wild Mammal Species of Vietnam; Kyoto University, Japan, and Institute of Ecology and Biological Resources, Vietnam, Shoukadoh Book Sellers: Kyoto, Japan, 2008; pp. 68–182. [Google Scholar]
- Nga, C.T.T.; Tung, N.S. Biodiversity research and conservation in Cat Ba National Park with updated records from recent field surveys. J. Viet. Environ. 2018, 9, 285–290. [Google Scholar] [CrossRef]
- Thong, V.D.; Southaphan, S.; Nha, P.V. The conservation status of bats (Mammalia: Chiroptera) in Cat Ba National Park, Northern Vietnam. HNUE J. Sci. 2020, 65, 92–98. [Google Scholar]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Shingh, A.; Loveland, T. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Dat, P.T.; Yoshino, K.; Kaida, N. Monitoring Mangrove Forest changes in Cat Ba Biosphere Reserve using ALOS PALSAR Imagery and a GIS-based Support Vector Machine Algorithm. In Advances and Applications in Geospatial Technology and Earth Resources; Tien, B.D., Ed.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 103–118. [Google Scholar]
- Sikes, R.S.; Gannon, W.L.; The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Sikes, R.S.; The Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Francis, C.M. A comparison of mist nets and two types of harp traps for capturing bats. J. Mammal. 1989, 70, 865–870. [Google Scholar] [CrossRef]
- Bates, P.J.J.; Harrison, D.L. Bats of the Indian Subcontinent; Harrison Zoological Museum: Sevenoaks, UK, 1997; pp. 1–258. [Google Scholar]
- Csorba, G.; Ujhelyi, P.; Thomas, N. Horseshoe Bats of the World (Chiroptera: Rhinolophidae); Alana Books: Shropshire, UK, 2003; pp. 1–160. [Google Scholar]
- Racey, P.A. Reproductive assessment in bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, ML, USA, 2009; pp. 249–264. [Google Scholar]
- Brunet-Rossinni, A.K.; Wilkinson, G.S. Methods for age estimation and the study of senescence in bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, ML, USA, 2009; pp. 315–325. [Google Scholar]
- Ivanova, N.V.; Dewaard, J.R.; Hebert, P.D. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes 2006, 6, 998–1002. [Google Scholar] [CrossRef]
- Hall, T.; BioEdit. A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K.; MEGA7. Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Tamura, K.; Đuley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Wilson, D.E.; Mittermerier, R.A.; Martinez-Vilalta, A.; Leslie, D.M.; Olive, M.; Elliott, A.; Velikov, I.; Mascarell, A.; Sogorb, L.; Marti, B.; et al. Handbook of the Mammals of the World; Lynx Edicions: Barcelona, Spain, 2019; pp. 1–1008. [Google Scholar]
- Francis, C. A Guide to the Mammals of South-East Asia, 2nd ed.; Bloomsbury Publishing Plc: London, UK, 2019; pp. 1–416. [Google Scholar]
- Thong, V.D. First record of bats (Mammalia: Chiroptera) from mangrove in Dam Nai area, Ninh Thuan province, Central Vietnam. Acad. J. Biol. 2021, 43, 135–139. [Google Scholar]
- Thanh, H.T.; Son, N.T.; Duong, V.T.; Luong, N.T.; Loi, D.N.; Thong, V.D. New records and morphological assessments of long-nosed fruit bats (chiroptera: Pteropodidae: Macroglossus spp.) from Vietnam. Acad. J. Biol. 2019, 41, 117–124. [Google Scholar] [CrossRef]
- Thong, V.D.; Nha, P.V.; Tien, P.D.; Luong, N.T.; Dang, N.X.; Nghia, N.X.; Southaphanh, S.; Thongphachanh, L.; Dung, D.T.; Khu, N.X.; et al. Horseshoe bat species recorded in the mangrove ecosystem of the Cat Ba National Park, Northern Vietnam. Acad. J. Biol. 2021, 43, 127–133. [Google Scholar]
- Thong, V.D. New records of griffin’s leaf-nosed bat (Hipposideros griffini Thong et al. 2012) from Vietnam. J. Biol. 2012, 34, 323–327. [Google Scholar] [CrossRef][Green Version]
- Douangboubpha, B. Hipposideros khaokhouayensis (Amended Version of 2019 Assessment). The IUCN Red List of Threatened Species 2020: E.T136819A166602959. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-1.RLTS.T136819A166602959.en (accessed on 10 July 2021).
- Tu, V.T.; Arai, A.; Kikuchi, F.; Hang, C.T.; Tuan, T.A.; Csorba, G.; Gorfol, T. Rediscovery of Van Hasselt’s mouse-eared bat Myotis hasseltii (Temmink, 1840) and its first genetic data from Hanoi, northern Vietnam. J. Threat. Taxa 2019, 11, 13915–13919. [Google Scholar] [CrossRef]
- Jiang, T.L.; Feng, J.; Csorba, G.; Bates, P. Myotis pilosus. The IUCN Red List of Threatened Species 2019: E.T14193A22062554. 2019. Available online: http://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T14193A22062554.en (accessed on 10 July 2021).
- Thong, V.D. New records of Hipposideros griffini from lava caves and the threats to its conservation in Vietnam. Acad. J. Biol. 2019, 41, 31–36. [Google Scholar] [CrossRef]
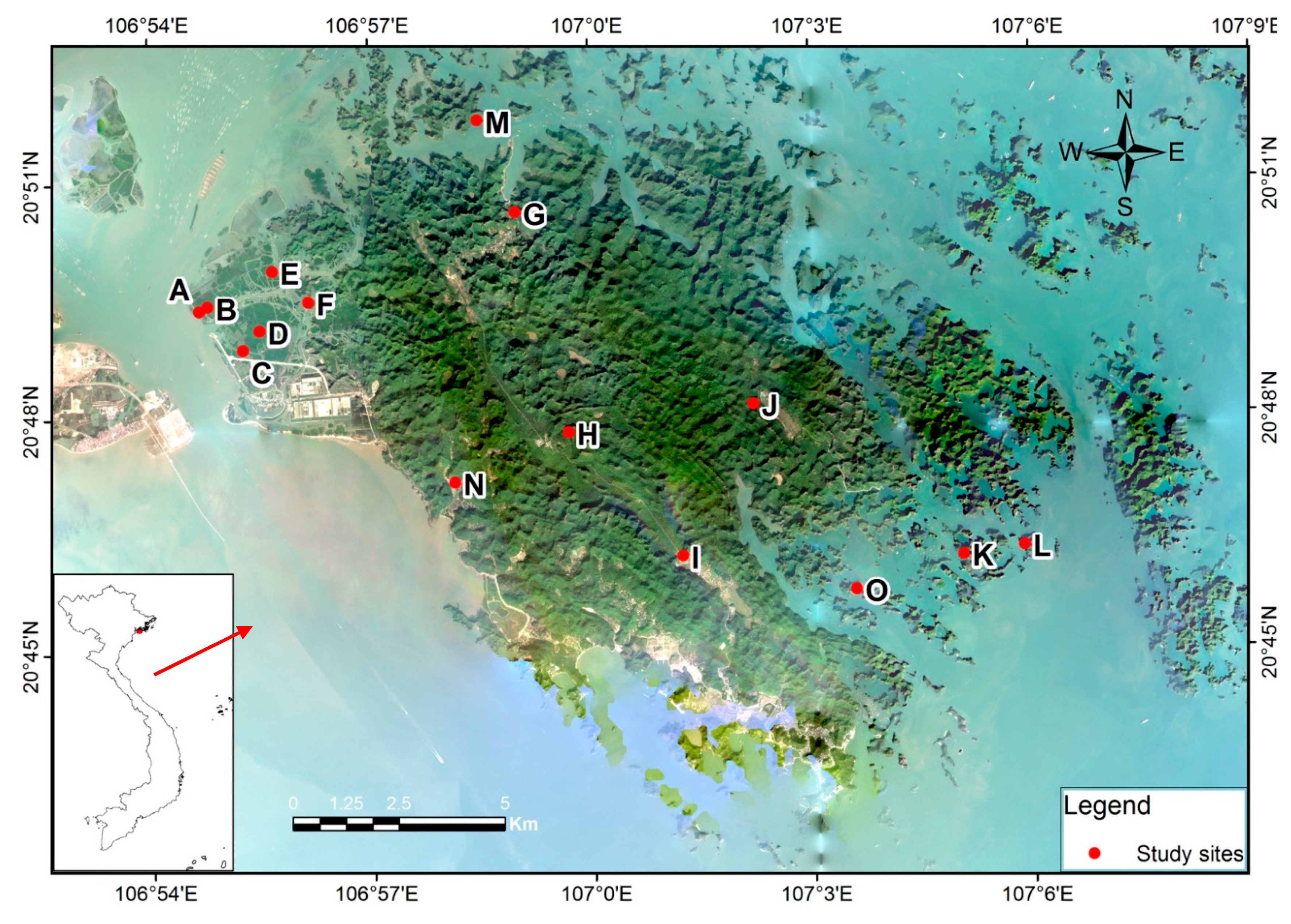




















| Study Sites | Localities | Coordinates | Habitats | Captured Species (Individuals) |
|---|---|---|---|---|
| A | Cai Vieng area, Phu Long Commune, Cat Ba Island | 20°49′22.9″ N; 106°54′40.1″ E | Mangrove | C. sphinx (1♂), M. minimus (1♀), T. melanopogon (2♂), R. marshalli (3♂, 3♀), H. larvatus (2♂, 3♀) |
| B | Cai Vieng area, Phu Long Commune, Cat Ba Island | 20°49′26.4″ N; 106°54′47.2″ E | Mangrove | R. affinis (1♂), R. siamensis (1♂, 1♀), H. gentilis (1♂, 2♀) |
| C | Cai Vieng area, Phu Long Commune, Cat Ba Island | 20°48′52.9″ N; 106°55′15.7″ E | Mangrove | R. affinis (2♀); M. hasseltii (1♂), T. melanopogon (1♂, 2♀), |
| D | Cai Vieng area, Phu Long Commune, Cat Ba Island | 20°49′07.4″ N; 106°55′29.6″ E | Mangrove | R. pearsonii (1♂, 2♀), H. khaokhouayensis (1♂), H. larvatus (1♀) |
| E | Cai Vieng area, Phu Long Commune, Cat Ba Island | 20°49′53.2″ N; 106°55′40.2″ E | Mangrove | M. minimus (1♂), R. marshalli (1♂, 2♀), R. pearsonii (1♂, 1♀), H. gentilis(2♀), M. hasseltii (1♀), M. pilosus (1♂, 1♀) |
| F | Cai Vieng area, Phu Long Commune, Cat Ba Island | 20°49′29.4″ N; 106°56′09.5″ E | Mangrove | C. sphinx (1♀), R. pusillus (1♂, 1♀), M. alticraniatus (1♀) |
| G | Hoa Cuong Cave area, Gia Luan Commune, Cat Ba Island | 20°50′36.8″ N; 106°58′59.0″ E | Cave and forests | R. marshalli (1♂, 1♀), R. pusillus (1♀), A. cf. stoliczkanus (1♂, 2♀), H. armiger (1♂, 1♀), H. gentilis (1♂, 2♀), H. larvatus (2♂, 2♀) |
| H | Freshwater lake, Headquarter area, Cat Ba National Park, Cat Ba Island | 20°47′47.5″N; 106°59′41.5″E | Forests and plantation | R. pearsonii (2♂, 1♀), H. larvatus (1♂, 2♀), M. cyclotis (1♀), M. pilosus (1♂), M. alticraniatus (1♂, 1♀), P. jagorii (1♀), T. fulvida (1♀) |
| I | Hospital Cave area, Cat Ba Island | 20°46′11.8″ N; 107°01′13.7″ E | Cave and forests | T. melanopogon (2♂), A. cf. stoliczkanus (3♀), H. alongensis (1♀), H. pulveratus (1♂, 1♀), P. abramus (1♂) |
| J | Viet Hai Cave area, Viet Hai Commune, Cat Ba Island | 20°48′08.0″ N; 107°02′12.5″ E | Cave and forests | H. alongensis(1♂), H. larvatus (1♂, 1♀), H. pulveratus (2♀) |
| K | Monkey Island, Cat Ba National Park | 20°46′11.1″ N; 107°05′03.2″ E | Forests and shrubs | H. larvatus (1♂, 1♀), H. pulveratus (2♂, 2♀) |
| L | An unnamed island, Cat Ba Biosphere Reserve | 20°46′17.7″ N; 107°05′52.7″ E | Forests and shrubs | R. affinis (1♂, 2♀), R. marshalli (1♀), R. pusillus (1♂); A. cf. stoliczkanus (1♀), H. larvatus (1♀), M. fuliginosus (1♀) |
| M | An unnamed island, Cat Ba Biosphere Reserve | 20°51′47.6″ N; 106°58′29.0″ E | Mangrove and forests | H. larvatus (1♀), H. pulveratus(2♂, 2♀), M. cyclotis (1♂), M. pilosus (1♀) |
| N | Hien Hao Commune, Cat Ba Island | 20°47′10.1″ N; 106°58′08.0″ E | Forests and plantation | T. melanopogon (1♂, 1♀), R. pearsonii (1♀), A. cf. stoliczkanus (1♀), H. gentilis (1♂, 1♀), H. khaokhouayensis (1♀), M. alticraniatus (1♀) |
| O | An unnamed island, Cat Ba Biosphere Reserve | 20°45′45.4″ N; 107°03′35.2″ E | Forests and shrubs | R. siamensis (2♂, 2♀), H. armiger (1♂), H. pulveratus (1♂) |
| Species | n | FA | EH | EW | NL | Tail | TIB | HF |
|---|---|---|---|---|---|---|---|---|
| Cynopterus sphinx | 2 | 82.3; 83.6 | 16.8; 19.5 | – | – | 6.8; 8.3 | 26.6; 36.2 | 14.5; 16.5 |
| Macroglossus minimus | 2 | 42.2; 44.2 | 12.7; 13.1 | 8.6; 9.9 | – | – | 16.4; 17.6 | 10.0; 10.5 |
| Taphozous melanopogon | 9 | 65.7 ± 1.4 63.7–67.3 | 16.7 ± 4.4 11.5–21.3 | 11.7 ± 1.0 10.5–13.3 | – | 22.9 ± 1.2 20.7–24.6 | 25.1 ± 0.8 23.1–25.9 | 10.4 ± 0.3 10.0–10.8 |
| Rhinolophus affinis | 6 | 51.4 ± 0.9 50.4–52.5 | 21.3 ± 0.8 20.0–22.0 | 16.3 ± 0.9 15.0–17.5 | – | – | – | – |
| R. siamensis | 6 | 38.8 ± 0.7 37.7–39.6 | 20.2 ± 0.5 19.9–21.0 | 14.3 ± 1.3 12.5–15.6 | – | – | – | – |
| R. marshalli | 12 | 46.5 ± 1.5 44.4–48.6 | 24.9 ± 1.4 23.1–26.8 | 17.3 ± 0.8 15.8–18.3 | 6.6 ± 2.7 4.7–8.5 (2) | 19.6 ± 0.5 19.4–20.2 (3) | 18.8 ± 1.1 17.6–19.8 (3) | 6.5 ± 1.2 5.1–7.2 (3) |
| R. pearsonii | 9 | 51.9 ± 1.3 49.0–53.4 | 23.4 ± 1.5 21.5–26.0 | 16.9, 1.1 14.1–18.0 | 11.9 ± 0.3 11.7–12.3(3) | 20.1 ± 2.1 17.7–21.8 (3) | 25.9 ± 1.2 24.5–26.9 (3) | 10.5 ± 0.3 10.3–10.9 (3) |
| R. pusillus | 4 | 36.1 ± 1.5 34.0–37.5 | 16.1 ± 0.8 15.0–16.8 | 11.3 ± 1.0 9.8–12.0 | 7.2; 7.5 | 18.0; 20.1 (2) | 15.0; 15.8 (2) | 4.1; 6.0 (2) |
| Aselliscus cf. stoliczkanus | 8 | 44,6 ± 1.5 41.3–44.6 | 9.5 ± 0.6 8.5–9.5 | 8.7 ± 0.4 8.4–8.7 (3) | 5.5 ± 0.2 5.4–5.5 (3) | 35.8 ± 4.1 33.3–35.8 (4) | 19.5 ± 0.5 19.1–19.5 (4) | 4.7 ± 0.5 4.3–4.7 (4) |
| Hipposideros alongensis | 2 | 69.68; 70.48 | 23.36; 23.84 | – | – | – | – | – |
| H. armiger | 3 | 94.3 ± 2.0 92.1–95.7 | 26.9 ± 4.0 22.5–30.4 | 23.22 (1) | 12.0 9 (1) | 30.11 (1) | 40.5 (1) | 15.16 (1) |
| H. gentilis | 10 | 41.5 ± 1.4 38.2–43.4 | 23.0 ± 2.3 20.1–27.4 | 17.1 ± 1.6 14.5–19.3(9) | 5.4 ± 0.7 4.3–6.6(9) | 29.0 ± 4.4 23.4–36.0(9) | 19.5 ± 0.4 19.0–20.1(9) | 5.2 ± 1.1 3.9–6.6 (9) |
| H. larvatus | 19 | 54.9 ± 2.0 48.7–59.0 | 20.4 ± 2.2 14.4–23.5 | 16.2 ± 1.3 14.4–17.9 (12) | 7.1 ± 0.9 5.3–8.3 (12) | 30.5 ± 3.2 24.8–35.5 (12) | 21.7 ± 0.9 20.2–22.6 (12) | 8.5 ± 0.8 6.8–10.1 (12) |
| H. khaokhouayensis | 2 | 43.1; 42.2 | 22.4; 20.6 | 17.0 (1) | 5.5 (1) | 28.2 (1) | 28.2 (1) | 6.4 (1) |
| Hypsugo pulveratus | 13 | 35.2 ± 0.7 33.7–36.3 | 10.6 ± 1.3 8.9–12.8 | 7.7 ± 0.6 6.7–8.2 | – | 34.2 ± 0.4 33.8–34.5(5) | 14.5 ± 0.2 14.3–14.7(5) | 6.0 ± 0.1 5.8–6.1(5) |
| Murina cyclotis | 2 | 31.3; 34.1 | 14.7; 13.9 | 9.3; 9.9 | 36.0; 38.8 | 16.8; 19.0 | 7.9; 7.8 | |
| Myotis alticraniatus | 4 | 33.6 ± 1.3 32.1–35.1 | 12.9 ± 3.3 10.0–15.9 | 5.4 ± 0.3 5.0–5.8 | – | 18.0 ± 2.2 15.6–20.3 | 14.0 ± 0.5 13.5–14.7 | 5.2 ± 0.5 4.6–5.9 |
| M. hasseltii | 2 | 38.6; 38.5 | 13.5; 14.3 | – | – | 40.0; 38.5 | 16.8; 15.6 | 9.8; 10.5 |
| M. pilosus | 4 | 54.0 ± 3.0 51.1–56.7 | 17.2 ± 0.9 16.2–18.3 | 13.8 ± 2.9 10.5–16.2 | – | – | 20.5 ± 1.1 18.9–21.6 | 16.0 ± 2.3 13.4–18.9 |
| Phoniscus jagorii | 1 | 35.8 | 13.4 | 9.7 | 36.4 | 16.7 | 9.3 | |
| Pipistrellus abramus | 1 | 28.6 | 12.5 | – | – | 38.6 | – | – |
| Tylonycteris fulvida | 1 | 26.8 | 8.8 | 5.8 | 32.3 | 11.2 | 8.8 | |
| Miniopterus fuliginosus | 1 | 52.1 | 8.9 | 8.4 | – | 34.1 | 21.4 | 10.0 |
| Species | n | SL | CCL | IOW | ZW | MW | C1-C1 | M3-M3 | mL | c1-m3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Taphozous melanopogon | 9 | 21.6 ± 0.2 21.3–21.8 | 20.0 ± 0.4 19.4–20.6 | 4.9 ± 0.2 4.5–5.2 | 12.4 ± 0.3 11.8–12.6 | 11.1 ± 0.1 11.0–11.3 | 3.8 ± 0.2 3.5–4.1 | 8.8 ± 0.1 8.6–8.9 | 16.0 ± 0.2 15.6–16.3 | 9.8 ± 0.2 9.6–10.1 |
| Rhinolophus marshalli | 3 | 19.1 ± 0.5 18.7–19.6 | 17.3 ± 0.6 16.7–17.9 | 2.4 ± 0 2.4–2.5 | 8.3 ± 0.1 8.2–8.4 | 9.1 ± 0.3 8.8–9.4 | 3.7 ± 0.3 3.3–3.9 | 5.4 ± 0,1 5.4–5.5 | 11.3 ± 0.3 11.0–11.6 | 6.6 ± 0.2 6.4–6.8 |
| R. pearsonii | 3 | 24.2 ± 0.2 24.0–24.3 | 21.9 ± 0.3 21.6–22.1 | 2.3 ± 0.1 2.2–2.4 | 11.1 ± 0.1 11.0–11.3 | 10.4 ± 0.2 10.2–10.6 | 5.9 ± 0.1 5.8–6.1 | 8.3 ± 0.1 8.2–8.3 | 16.0 ± 0.3 15.7–16.4 | 9.9 ± 0.2 9.7–10.1 |
| R. pusillus | 2 | 15.6; 15.7 | 14.2; 14.6 | 2.06; 2.1 | 7.1; 7.4 | 7.2; 7.3 | 3.2; 3.7 | 5.2; 5.3 | 9.6; 10.0 | 5.67 |
| Aselliscus cf. stoliczkanus | 4 | 15.5 ± 0.3 15.1–16.0 | 13.9 ± 0.3 13.6–14.2 | 2.0 ± 0.1 1.9–2.1 | 7.6 ± 0.1 7.5–7.7 | 7.3 ± 0.1 7.3–7.4 | 3.3 ± 0.3 3.1–3.7 | 5.4 ± 0.1 5.3–5.5 | 9.5 ± 0.3 9.3–9.8 | 5.5 ± 0.4 5.0–5.8 |
| Hipposideros armiger | 1 | 32.33 | 28.48 | 4.22 | 17.82 | 15.34 | 8.8 | 12.24 | 22.42 | 13.6 |
| H. gentilis | 9 | 18.4 ± 0.6 17.7–19.2 | 15.9 ± 0.3 5.6–16.4 | 2.7 ± 0.1 2.6–2.9 | 8.9 ± 0.4 8.4–9.9 | 9.5, 0.7 8.9–10.5 | 3.5 ± 0.1 3.3–3.7 | 6.5 ± 0.5 5.7, 7.0 | 10.9 ± 0.2 10.7, 11.0 | 6.6 ± 0.2 6.4–6.8 |
| H. larvatus | 12 | 21.6 ± 0.3 21.2–21.8 | 19.5 ± 0.7 18.4–2.0 | 3.1 ± 0.2 2.9–3.3 | 12.2 ± 0.4 12.0–12.8 | 10.3 ± 0.2 10.1–10.5 | 4.7 ± 0.2 4.5–4.9 | 8.2 ± 0.2 8.0–8.4 | 14.4 ± 0.3 14.2–14.7 | 8.8 ± 0.1 8.7–9.0 |
| H. khaokhouayensis | 1 | 17.88 | 16.22 | 2.55 | 8.5 | 9.36 | 3.62 | 5.6 | 10.77 | 6.18 |
| Myotis alticraniatus | 4 | 12.2 ± 0.3 11.8–12.5 | 11.0 ± 0.6 10.5–11.7 | 3.1 ± 0.2 3.0–3.3 | 7.3 ± 0.3 7.0–7.7 | 6.5, 0.1 6.4–6.7 | 2.2 ± 0.1 2.2–2.3 | 4.9 ± 0.1 4.7–5.0 | 8.5 ± 0.1 8.4–8.7 | 4.2 4.2–4.3 |
| M. pilosus | 1 | 20.09 | 19.04 | 4.9 | 12.5 | 9.66 | 5.44 | 8.18 | 15.13 | 8.15 |
| Miniopterus fuliginosus | 1 | 16.92 | 16.08 | 4.08 | 9.58 | 9.17 | 3.35 | 7.32 | 11.68 | 7.2 |
| Scientific Name | Common Name | Records with Reference to Material(s) | Recorded without Reference to Material(s) |
|---|---|---|---|
| Pteropodidae | Fruit bats | ||
| Cynopterus sphinx | Greater short-nosed fruit bat | Abramov & Kruskop 2012 [9]; Furey 2002 [20]; This study | Canh et al., 1997 [21]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| C. horsfieldii | Horsfield’s fruit bat | Thong 2014b [17] | Thong et al., 2020 [27] |
| Macroglossus minimus | Dagger-toothed long-nosed fruit bat | This study | |
| Rousettus amplexicaudatus | Geoffroy’s rousette | Abramov & Kruskop 2012 [9]; | Kruskop 2013 [14]; Thong 2008 [23]; Thong et al., 2020 [27] |
| Emballonuridae | Sheath-tailed and tomb bats | ||
| Taphozous melanopogon | Black-bearded tomb bat | Thong et al., 2016 [18]; This study | Abramov & Kruskop 2012 [9]; Kruskop 2013 [14]; Borrisenko&Kruskop 2003 [22]; Can et al., 2008 [25]; Nga & Tung 2018 [26]; Thong et al., 2020 [27] |
| Rhinolophidae | Horseshoe bats | ||
| Rhinolophus affinis | Intermediate horseshoe bat | Thong 2011 [7]; Thong, 2014a [16]; This study | Abramov & Kruskop 2012 [9]; Thong 2008 [23]; Thong & Furey 2008 [24]; Thong et al., 2020 [27] |
| R. siamensis | Thai horseshoe bat | Thong 2011 [7] (=R. macrotis); Thong, 2014a [16] (=R. macrotis); This study | Abramov & Kruskop 2012 [9] (=R. macrotis); Thong 2008 [23] (=R. macrotis); Thong et al., 2020 [27] (=R. macrotis) |
| R. marshalli | Marshall’s horseshoe bat | Thong et al., 2007 [5]; Thong 2011 [7]; Abramov & Kruskop 2012 [9]; Thong 2012 [10]; Kruskop 2013 [14]; Thong, 2014a [16]; Thong et al., 2016 [18]; Furey 2002 [20]; This study | Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27]; Thong et al., 2021 [43] |
| R. pearsonii | Pearson’s horseshoe bat | Thong 2011 [7]; Abramov & Kruskop 2012 [9]; Kruskop 2013 [14]; Thong, 2014a [16]; Thong et al., 2016 [18]; Furey 2002 [20]; This study | Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] ; Thong et al., 2021 [43] |
| R. pusillus | Least horseshoe bat | Thong 2011 [7]; Abramov & Kruskop 2012 [9] (= R. pusillus and R. cf. pusillus); Kruskop 2013 [14]; Thong, 2014a [16]; Thong et al., 2016 [18]; Furey 2002 [20]; This study | Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27]; Thong et al., 2021 [43] |
| Hipposideridae | Roundleaf bats | ||
| Aselliscus cf. stoliczkanus | Dong Bac trident bat | Thong 2011 [7] (=A. stoliczkanus); Abramov & Kruskop 2012 [9] =A. stoliczkanus; Kruskop 2013 [14] =A. stoliczkanus; Thong et al., 2016 [18]; Furey 2002 [20] (=A. stoliczkanus); This study | Thong 2008 [23] =A. stoliczkanus; Thong & Furey 2008 [24] =A. stoliczkanus; Can et al., 2008 [25] =A. stoliczkanus; Thong et al., 2020 [27] |
| Coelops frithii | Tailless leaf-nosed bat | Furey 2002 [20] | Abramov & Kruskop 2012 [9]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| Hipposideros alongensis | Ha Long leaf-nosed bat | Bourret 1942a [2] (=H. larvatus alongensis); Bourret 1942b [3] (=H. larvatus alongensis); Topal 1993 [4] (=H. turpis); Thong 2011 [7]; Abramov & Kruskop 2012 [9] (=H. turpis alongensis); Thong et al., 2012a [11]; Kruskop 2013 [14]; Thong 2013 [15]; Thong et al., 2016 [18]; Furey 2002 [20] (=H. turpis); This study | Thong 2008 [23] (=H. cf. turpis alongensis); Thong & Furey 2008 [24] (=H. turpis); Can et al., 2008 [25] (=H. turpis); Thong et al., 2020 [27] |
| H. armiger | Great Himalayan leaf-nosed bat | Thong 2011 [7]; Abramov & Kruskop 2012 [9]; Thong et al., 2016 [18]; Furey 2002 [20]; This study | Kruskop 2013 [14]; Canh et al., 1997 [21]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| H. gentilis | Andersen’s roundleaf bat | Thong 2011 [7] (=H. pomona); Abramov & Kruskop 2012 [9] (=H. pomona); Thong et al., 2012c [13]; Kruskop 2013 [14] (=H. pomona); Thong et al., 2016 [18] (=H. pomona); Yuzefovich et al., 2021 [19]; Furey 2002 [20] (=H. pomona); This study | Thong 2008 [23] (=H. pomona); Thong & Furey 2008 [24] (=H. pomona); Can et al., 2008 [25] (=H. pomona); Thong et al., 2020 [27] (=H. pomona). |
| H. larvatus | Intermediate leaf-nosed bat | Bourret 1942b [3]; Thong 2011 [7] (=H. grandis); Abramov & Kruskop 2012 [9]; Kruskop 2013 [14]; Thong et al., 2016 [18] (=H. grandis); Furey 2002 [20]; This study | Canh et al., 1997 [21]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] (=H. grandis). |
| H. griffini | Griffin’s leaf-nosed bat | Thong 2011 [7]; Thong et al., 2012b [12] | Abramov & Kruskop 2012 [9]; Thong 2008 [23] (=Hipposideros sp.nov.); Kruskop 2013 [14]; Thong et al., 2020 [27] |
| H. khaokhouayensis | Phou Khao Khouay leaf-nosed bat | Thong et al., 2008 [6]; Thong 2011 [7]; Abramov & Kruskop 2012 [9]; Kruskop 2013 [14]; Thong et al., 2016 [18]; This study | Thong 2008 [23]; Thong & Furey 2008 [24]; Thong et al., 2020 [27] |
| Vespertilionidae | Common bats | ||
| Harpiocephalus harpia | Lesser hairy-winged bat | Furey 2002 [20]; | Abramov & Kruskop 2012 [9]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| Hypsugo pulveratus | Chinese pipistrelle | Thong et al., 2016 [18]; This study | Abramov & Kruskop 2012 [9]; Thong 2008 [23]; Thong & Furey 2008 [24]; Thong et al., 2020 [27] |
| Murina cyclotis | Round-eared tube-nosed bat | Furey 2002 [20]; This study | Abramov & Kruskop 2012 [9]; Kruskop 2013 [14]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| M. harrisoni | Harrison’s murine bat | Thong et al., 2011 [8] | Abramov & Kruskop 2012 [9] (=M.tiensa); Kruskop 2013 [14]; Thong 2008 [23] (=M. tiensa); Thong et al., 2020 [27] |
| Myotis alticraniatus | Small-toothed myotis | Abramov & Kruskop 2012 [9] (=M. siligorensis); Kruskop 2013 [14] (=M. siligorensis); Thong et al., 2016 [18] (=M. siligorensis); This study | Thong 2008 [23] (=M. siligorensis); Thong & Furey 2008 [24] (=M. siligorensis); Thong et al., 2020 [27] (=M. siligorensis) |
| M. hasseltii | Lesser large-footed myotis | This study | |
| M. muricola | Nepalese whiskered bat | Furey 2002 [20]; | Abramov & Kruskop 2012 [9]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| M. pilosus | Rickett’s big-footed myotis | Thong et al., 2016 (=Myotis sp.) [18]; This study | Thong et al., 2020 [27] (=M. cf. pilosus) |
| Phoniscus jagorii | Peters’ trumpet-eared bat | This study | |
| Pipistrellus abramus | Japanese Pipistrelle | This study | Abramov & Kruskop 2012 [9]; Kruskop 2013 [14]; Borrisenko&Kruskop 2003 [22]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| P. javanicus | Javan pipistrelle | Furey 2002 [20] | Abramov & Kruskop 2012 [9]; Kruskop 2013 [14]; Canh et al., 1997 [21]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| P. tenuis | Least pipistrelle | Thong et al., 2016 [18]; Furey 2002 [20] | Abramov & Kruskop 2012 [9]; Thong 2008 [23]; Thong & Furey 2008 [24]; Can et al., 2008 [25]; Thong et al., 2020 [27] |
| Tylonycteris fulvida | Small bamboo bat | This study | |
| Miniopteridae | Bent-wing bats | ||
| Miniopterus fuliginosus | Eastern bent-winged bat | Thong et al., 2016 [18] (=Miniopterus cf. magnater); This study | Abramov & Kruskop 2012 [9] (=Miniopterus cf. fuliginosus); Kruskop 2013 [14]; Thong 2008 [23] =Miniopterus cf. fuliginosus); Thong & Furey 2008 [24] (=Miniopterus sp.); Nga & Tung 2018 [26]; Thong et al., 2020 [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thong, V.D.; Denzinger, A.; Sang, N.V.; Huyen, N.T.T.; Thanh, H.T.; Loi, D.N.; Nha, P.V.; Viet, N.V.; Tien, P.D.; Tuanmu, M.-N.; et al. Bat Diversity in Cat Ba Biosphere Reserve, Northeastern Vietnam: A Review with New Records from Mangrove Ecosystem. Diversity 2021, 13, 376. https://doi.org/10.3390/d13080376
Thong VD, Denzinger A, Sang NV, Huyen NTT, Thanh HT, Loi DN, Nha PV, Viet NV, Tien PD, Tuanmu M-N, et al. Bat Diversity in Cat Ba Biosphere Reserve, Northeastern Vietnam: A Review with New Records from Mangrove Ecosystem. Diversity. 2021; 13(8):376. https://doi.org/10.3390/d13080376
Chicago/Turabian StyleThong, Vu Dinh, Annette Denzinger, Nguyen Van Sang, Nguyen Thi Thu Huyen, Hoang Trung Thanh, Dao Nhan Loi, Pham Van Nha, Nguyen Van Viet, Pham Duc Tien, Mao-Ning Tuanmu, and et al. 2021. "Bat Diversity in Cat Ba Biosphere Reserve, Northeastern Vietnam: A Review with New Records from Mangrove Ecosystem" Diversity 13, no. 8: 376. https://doi.org/10.3390/d13080376
APA StyleThong, V. D., Denzinger, A., Sang, N. V., Huyen, N. T. T., Thanh, H. T., Loi, D. N., Nha, P. V., Viet, N. V., Tien, P. D., Tuanmu, M.-N., Huang, J. C.-C., Thongphachanh, L., Luong, N. T., & Schnitzler, H.-U. (2021). Bat Diversity in Cat Ba Biosphere Reserve, Northeastern Vietnam: A Review with New Records from Mangrove Ecosystem. Diversity, 13(8), 376. https://doi.org/10.3390/d13080376







