Abstract
Seagrass meadows are known to be rich in fauna, with complex food webs that provide trophic subsidy to species and habitats way beyond the extent of their distribution. Birds are an often-overlooked part of marine ecosystems; not only are they crucial to the health of marine ecosystems, but their populations are also supported by the productivity and biodiversity of marine ecosystems. The links of birds to specific habitat types such as seagrass meadows are largely not considered except in the context of direct herbivorous consumption. Here, we examine the linkages between seagrass and birds and propose a conceptual framework for how seagrasses may support bird populations beyond their distribution in both direct and indirect pathways. We present evidence that seagrass meadows are globally foraged for fish and invertebrates by coastal birds. They are also targeted by herbivorous wildfowl and potentially benefit birds further afield indirectly as a result of their support for offshore marine fish species at critical times in their life cycle (e.g., Atlantic Cod and King George Whiting). Evidence from the literature indicates that seagrass does provide support for birds, but reveals a field of research requiring much gap filling as studies are globally sparse, mechanistically limited, and small in spatial and temporal scales.
1. Introduction
Seagrass meadows are rich biodiverse ecosystems that occur all over the globe, in both tropical and temperate seas [1]. They contain complex food webs that provide trophic subsidy to species and habitats way beyond the extent of their distribution [2]. Given the wide variety of food sources provided by this productive habitat, it is no surprise that seagrass meadows support an equally wide array of grazers and predators. However, despite its importance for sustaining biodiversity and many other ecosystem services [3], the global distribution of seagrass is a fraction of what was historically present [4,5]. Recent estimates from where records exist indicate that at least 20% of the world’s seagrass has been lost [5]. Seagrasses also provide other services in the coastal zone such as preventing coastal erosion, storing and trapping carbon [6] and filtering the water column [7].
The true ecosystem-level consequences of such decline and the benefits that can be afforded through habitat restoration are poorly understood. Given the relatively high-per-unit area costs of marine habitat restoration [8], making the case for such work requires a thorough examination of the ecosystem service benefits of such new habitat creation.
Birds are an often-overlooked part of marine ecosystems, not only are they crucial to the health of marine ecosystems, but their populations are also supported by the productivity and biodiversity of marine and coastal ecosystems [9,10,11,12]. The links of birds to specific habitat types such as seagrass meadows are largely not considered except in the context of direct herbivorous consumption by wildfowl [13]. This is despite the fact that both bottom-up and top-down processes have been considered as pathways for the population maintenance of some coastal birds [14].
Given the long-term decline in the population of many coastal and seabirds, the known response of many seabird populations to fluctuations in their prey, and the need for compensatory restorative actions to enhance their populations, it is imperative we improve our understanding of the role of key marine habitats such as seagrass in supporting coastal and seabirds.
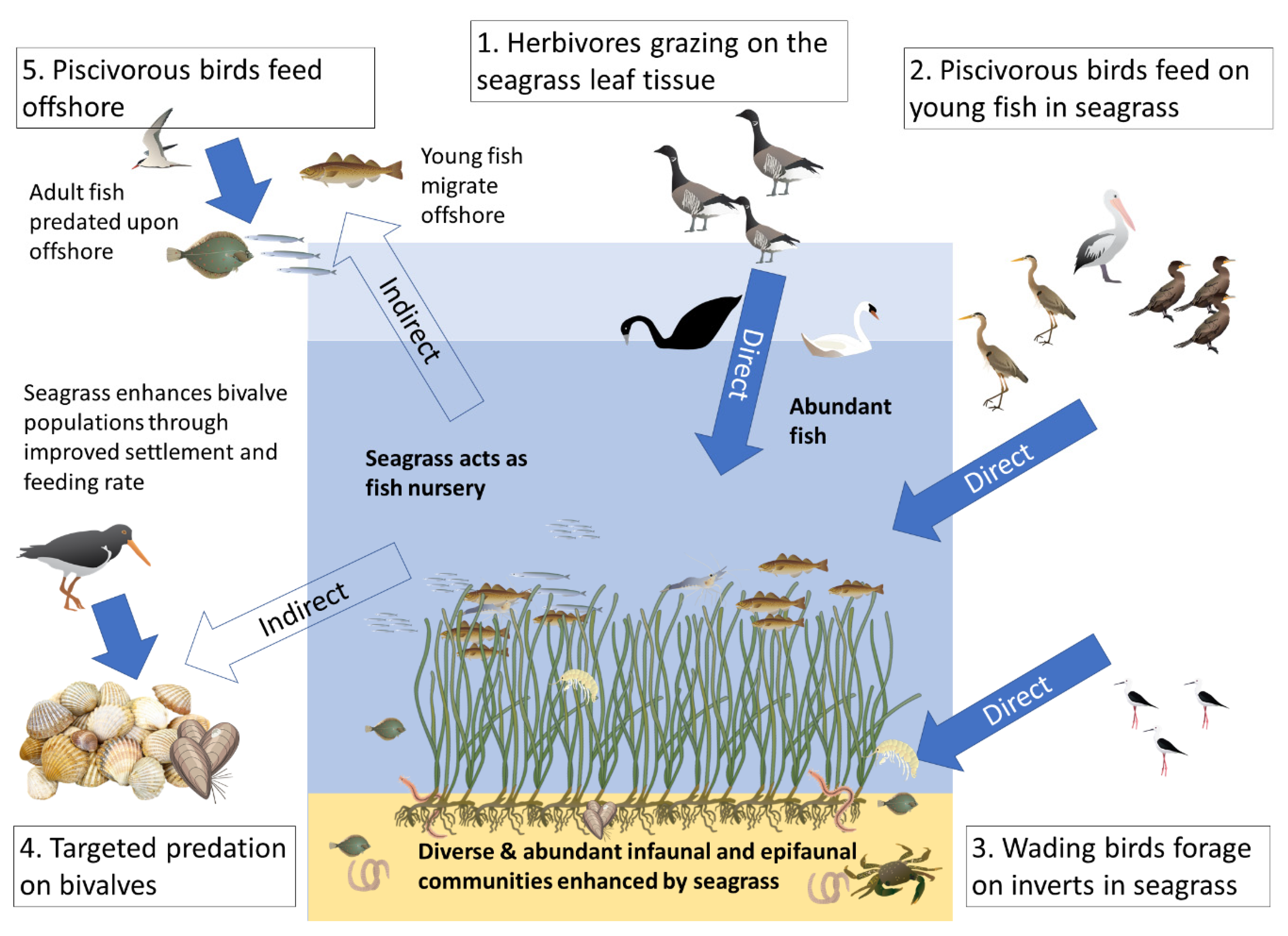
In the present mini review we examine the links between seagrass and seabirds and propose a conceptual framework for how seagrasses within and beyond their distribution may potentially support some bird populations in both direct and indirect pathways (see Figure 1). Such roles will be species and habitat specific and discussion of this is considered.
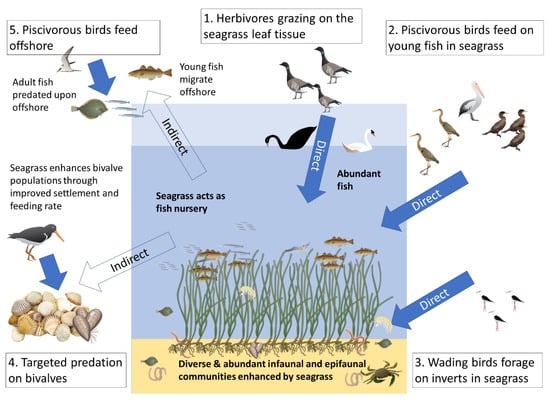
Figure 1.
Conceptual diagram examining the potential links between seagrass and seabirds in NE Atlantic coastal seas. These hypothesised links are split between direct consumption of seagrass (1) or seagrass-associated fauna (2,3) and those indirect links from consumption of populations benefitting from seagrass (4,5).
2. Direct Support: Grazing Birds
One of the most well-known links between seagrass and avian foraging is when birds graze on the seagrass vegetation. There are several waterfowl species that feed on seagrass worldwide, which have been summarised in literature reviews [10,15]. The paper by Kollars et al., 2017 [10] conducted an extensive and detailed review of this topic and found that 39 species or subspecies of birds had seagrass as a component of their diet, although it is a dominant or frequent part for just 26 of those species. Typical examples of such species that graze of seagrass are the Brant Geese (Branta bernicla), Widgeon (Anas Penelope), Black Swans (Cygnus atratus) and Teal (Anas crecca). Grazing on seagrass mostly occurs over the intertidal. However, some swans may also graze over shallow waters. The intense grazing activity of large flocks of Geese can in some localities lead to the complete harvesting on the seagrass shoot material [13].
3. Direct Support: Coastal Birds Foraging on Seagrass Invertebrates
The presence of seagrass increases both the abundance and diversity of the benthic invertebrates that inhabit the sediment it grows in [16,17,18]. This abundance is also present as epifauna living within the seagrass leaves [19]. In turn, it would be reasonable to hypothesise that seagrass would indirectly have an effect on the foraging behaviour of bird species that feed on those invertebrates. However, studies that test such an hypothesis are limited.
The Wadden Sea, one of the largest tidal flats in the world, is recognised as a World Heritage Site in part due to its importance for migratory birds [20]. Horn et al. [21] found that the seagrass meadows of the Wadden Sea show significantly higher biomass of the prey species of a number of shorebirds. Within the study, Dunlin (Calidris alpina), Red Knot (Calidris canutus), and Bar-Tailed Godwits (Limosa lapponica) all heavily used the areas of seagrass. Both the Red Knot and the Eurasian Oystercatcher (Haematopus ostralegus) showed a marked preference for seagrass meadows over unvegetated areas. Given that their prey was in greater abundance in the seagrass meadows, this is in support of the hypothesis that seagrass influences shorebird foraging behaviour.
The attraction to vegetated habitat may not always hold true, as studies on the Western Sandpiper (Calidrus mauri) indicate a preference for unvegetated areas on exposed tidal flats in British Columbia. However, such congregations align with known areas of high abundance of invertebrates [22]. This indicates that where seagrass habitat does contain an abundance of invertebrates, it may become a more targeted habitat. The Western Sandpiper feeds primarily by pecking prey from the surface of the sediment, rather than probing into it for infauna [23]. This may provide an explanation for the preference of unvegetated areas, as seagrass leaf cover would give prey species far more protection and a place to hide from the sandpiper. A similar result was found by [24] in Tasmania, where shorebirds, such as Pied Oystercatchers (Haematopus longirostris), Red-Necked Stints (Calidris ruficollis), and Double-banded Plovers (Charadrius bicinctus) were studied. On a small scale, the shorebirds showed preference for feeding in areas with less seagrass leaf biomass, as seen with the Western Sandpipers (Calidris mauri) in British Columbia. However, when using a larger scale analysis, the presence of seagrass and associated increased invertebrate biomass correlates with a higher feeding density of shorebirds. When analysed, the larger scale relationship accounted for far more of the variability than on the small scale. Therefore, it can be inferred that while these birds tend to feed in areas with less leaf cover, seagrass meadows are still preferred.
In addition to shorebirds, waterfowl predating on invertebrates may also be influenced by seagrass when selecting feeding sites. A 50% reduction in the number of Common Goldeneyes (Bucephala clangula) sighted in the Antigonish Harbour, Canada was observed after the seagrass biomass declined by 95% [25]. This species feeds on invertebrates associated with seagrass beds, such as small crustaceans and molluscs [26,27].
There also exists a number of indirect benefits of seagrass on invertebrate populations that can potentially benefit avifauna. Seagrasses are known to provide population level benefits to some species of abundant bivalves that are later predated upon by avifauna. For example, the filter feeding rate of the common cockle Cerastoderma edule has been found to double in the presence of the seagrass Zostera noltii with potential population level benefits [28]. These cockles are a target food for species of birds such as oystercatchers (Haematopus ostralegus) and Knot (Calidris canutus), and populations dependent upon the availability of this resource [29]. Some bivalves such as the Blue Mussel (Mytulis edulis) are known to have enhanced settlement in seagrass prior to secondary settlement in harder substrates leading to population level benefits [30]. Mussels are a commonly utilised resource for many bird species and studies in the UK found enhanced mussel populations to result in increased abundance of the Curlew (Numenius arquata) and the Redshank (Tringa tetanus) [31].
Understanding of the links between seagrass and the foraging of shore birds for invertebrate prey is far from complete, with studies limited to only a handful. There is some conflicting evidence available, with some studies finding that the presence of seagrass increases bird abundance [21,25] and others finding that shorebirds prefer areas with less seagrass cover, but this is again a scale dependent relationship [22,32]. While it appears clear that seagrass abundance does influence these birds, it is unlikely to have the same effect across the broad range of species that utilise seagrass meadows worldwide. This area of study reflects a major knowledge gap in our understanding of the direct support that seagrass provides to Avifauna.
4. Direct Support: Piscivorous Bird Foraging within Seagrass
Many piscivorous birds feed on species of fish that are known to associate with seagrass meadows (e.g., Atlantic Herring Clupea harengus). Many of these species of fish do not just frequent seagrass meadows, but their populations are known to benefit overall from using seagrass at key times in their lifecycle (e.g., Atlantic Cod Gadus morhua) [33,34].
Given that numerous academic papers exist in the literature documenting the positive relationship between aspects of predatory seabird species condition relative to the availability of forage fish [35], it is reasonable to hypothesise that habitats such as seagrass that support fisheries production provide a means of support for foraging fish in both direct and indirect means. Seagrass habitats show greater diversity and abundance of fish, particularly juveniles, than unvegetated areas [33,36,37] with the large, healthy, well-connected meadows showing the greatest diversity [38].
Although we hypothesise that the elevated abundance of fish in seagrass would result in piscivorous birds selecting to hunt within it, literature examining such an hypothesis is limited. Significant anecdotal evidence indicates an abundance of potentially piscivorous birds targeting seagrass, with searches of images online commonly showing evidence of bird species globally foraging within seagrass (e.g., herons and pelicans) (Figure 2 and Figure 3).

Figure 2.
(a) Dunlin (Calidris alpina) and Knot (Calidris canutus) are observed in abundance at intertidal seagrass sites in the UK (author: Emma Butterworth). (b) Widgeon and Brant Geese grazing on seagrass at Lindisfarne.

Figure 3.
Significant photographic evidence of the links between bird foraging and seagrass can be found through searching through imagery available on the website flikr.com (photo source: Flikr.com (accessed on 1 July 2021)).
Great Blue Herons (Ardea herodias fannini) feed on a variety of fish, wading in relatively shallow water to catch their prey [39,40]. Huang et al. [41] found that herons hunted within seagrass meadows enough to have a significant impact on the abundance of fish. While their paper does not directly link the presence of seagrass to the herons’ choice of foraging location, it can be inferred that the species has heavy usage of the meadows. Another factor to be considered, however, is that herons (Ardeidae) are limited in their choice of location by the height of the water [42]. This means that herons are limited to the use of seagrass meadows that are shallower and more exposed, potentially imposing the time constraints of low tide. It appears that seagrass habitats are selected if the water is low enough for that species. Ref. [39] found that shallow lagoons with kelp beds or seagrass meadows were a preferred choice for the Great Blue Heron when feeding at the coast.
Phalacrocorax, commonly known as Cormorants, are a genus of birds that mainly hunt fish by diving [43,44,45]. In Western Australia, Pied Cormorants (Phalacrocorax varius) show strong preference towards seagrass meadows over bare sand to hunt in [45]. It was likely not the seagrass itself that caused the birds to choose those locations, rather that cormorants are able to track prey abundance which is highest within the seagrass. Though the habitat use was also impacted by predators, seagrass is certainly not the only factor. Dorfman and Kingsford [43] found a similar preference for seagrass meadows in four cormorant species, Great (Phalacrocorax carbo), Little Black (Phalacrocorax sulcirostris), Pied and Little Pied (Microcarbo melanoleucos). The cormorants appeared to choose to both hunt and roost near seagrass meadows. Unlike herons, whose usage of seagrass beds is limited by water level and diel changes, cormorants showed no trends when it comes to these factors. The implication of this is that the distribution of the birds was determined by characteristics of the locations, for example seagrass abundance, rather than any temporal changes.
As with those species that feed on invertebrates, potentially near seagrass, there was limited literature exploring whether seagrass has an influence on the foraging of piscivorous fish. Many seabird species (e.g., Kittiwakes, Guillemots) are known to forage in coastal shallow water areas when nesting [46,47] and consume young fish known to be abundant in seagrass such as the Atlantic cod [33,47].
With so few species studied with regard to their links to seagrass, it would be hard to come to a significant conclusion as to whether seagrass generates direct impacts on any specific birds themselves. However, available evidence does indicate the need for an expanded evidence base on this broader topic. We propose that this area is a particular knowledge gap within the scientific literature.
5. Indirect Support: Seabirds Benefitting from Seagrass Fish Nursery Function
When looking at the effect seagrass has on birds, it would be an oversight to ignore the link between pelagic birds, their prey species, and seagrass meadows. While these birds often hunt miles away from any seagrass, the species that they prey on, such as Gadoids and Clupeids, often utilise seagrass as nursery habitats [36,37,48,49]. At ocean basin scales, seagrass is incredibly important in supporting fish stocks far from land, with 20% of the world’s biggest fisheries supported by seagrass meadows through the provision of a nursery function to juvenile fish [34]. Where seagrass meadows decline, there is evidence that this has negative effects on the pelagic fish stocks [50,51]. This in turn, may impact the success of the species such as birds that feed on them. Given that many fish stocks are overfished already [52], a reduction in the number of juvenile fish surviving to maturity could severely affect their predators.
For example, a high abundance of juvenile Atlantic Herring (Clupea harengus) was found in seagrass in studies that took place in the United Kingdom, Denmark and the Baltic Sea [36,53,54]. Clupeids form a part of the diet of numerous seabirds, notable in the diet of Common Guillemot (Uria aalge) chicks and Razorbills (Alca torda) [12,55,56,57]. For adult Guillemots, capable of catching and eating larger prey, gadoids were a significant prey item [12,55].
While some preferences are observed, generally studies have found that many seabirds are relatively flexible with their diet. Larger birds such as Northern Gannets (Morus bassanus) and Cory’s Shearwaters (Calonectris diomedea) show particularly great flexibility in prey species [58,59,60] and are therefore likely to utilise fish species found to be in abundance, so the generality of seagrass support for many fish species may have wide support to these prey flexible birds.
Overall, seagrass may have an indirect effect on many species of pelagic birds by acting as a nursery to their prey items. However, there does not appear to be any literature that connects the birds with seagrass. Connections must be made through improved understanding of the diets of pelagic bird species linked to knowledge of how individual fish species utilise seagrass. Approaches such as the use of isotope food web analysis and examination of otolith microchemistry from bird stomach contents provide tools to answer these questions.
6. Seagrass Loss and Shifting Baselines
Conceptions of environmental degradation tend to shift depending on our temporal reference point. In a seagrass context, this “shifting baseline syndrome” (SBS) [61] is commonly the earliest known data of areal extent of seagrass which is then assumed as an unaffected baseline condition [62]. This is further exacerbated by data being supported by qualitative and quantitative accounts that refer to healthier conditions within a scientist’s lifetime. With each generation, the concept of a healthy ecosystem shifts, depending on their perceived baseline. In many parts of the world there is extensive evidence that vast amounts of seagrass have disappeared and with it the support it provided for fish stocks and biodiversity [5,62]. Factors driving this loss range from poor water quality, to coastal development, land reclamation and boating and fishing impacts [4]. Globally, where data are available, this loss has been extensive) often over 50% of a nations seagrass) [62,63], leaving areas of lower diversity with limited structural complexity supporting less associated species. The challenge remains as to how to halt this continued loss and provide a supporting environment for recovery and restoration to occur [64].
Whilst we cannot for sure understand the pristine state of our coastal seas, we can assume that the contribution from pristine habitats in their support for a more complex and productive food web must have been orders of magnitude higher. Our very understanding as to what is bird diet in many parts of the world only reflects what they have been recorded to consume in an anthropogenic modified environment, for example studies on Kittiwake diet in the Firth of Forth [65], an environment fundamentally altered through human modification [66]. In the context of the links between seagrass and seabirds, we can only assume that the availability of abundant food sources for seabirds linked to seagrass would have been historically orders of magnitude higher.
7. Seabirds Support Seagrass
The focus of this review has been on the role that seagrass plays in supporting a range of species of birds. However, these benefits can potentially also flow back to seagrass to help improve their status. In calcareous sediments, seagrasses are commonly phosphorus limited [67,68]. Where seagrass restoration practitioners have recognised such a limitation, they have been able to utilise birds to alter this by placing perching poles in shallow waters encouraging birds to fertilise sediments with faeces, releasing seagrass from phosphorus limitation [69]. Birds may also be of benefit to seagrasses through the spread of seed in faeces, helping to genetically mix populations through space and time [70].
8. Conclusions
The present review hypothesises that seagrass meadows support avifauna in a range of direct and indirect means. Whilst the available evidence is insufficient to answer all those hypotheses in any detail, there is sufficient evidence to indicate that mechanisms behind these links do exist and that the intricacies of these links deserve more thorough examination.
Seagrass meadows have been found globally to be targeted for invertebrates and fish by a range of bird species, many bird species do target fish species known to have critical links to seagrass and numerous bivalve species that are highly exploited by birds do gain population level benefits from seagrass. Given the increasing trend of the global decline in birds (as well as of seagrass), with some of these related to declining resource availability, we believe that greater focus needs to be placed on how marine habitats, namely seagrass as well as others such as Kelp and Oyster, help support bird populations and therefore how other aspects of marine conservation can in turn potentially enhance bird populations.
Author Contributions
Both authors contributed equally to all aspects of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McKenzie, L.; Nordlund, L.M.; Jones, B.L.; Cullen-Unsworth, L.C.; Roelfsema, C.M.; Unsworth, R.K.F. The global distribution of seagrass meadows. Environ. Res. Lett. 2020, 15, 074041. [Google Scholar] [CrossRef]
- Heck, K.L.; Carruthers, T.J.B.; Duarte, C.M.; Hughes, A.R.; Kendrick, G.; Orth, R.; Williams, S.W. Trophic Transfers from Seagrass Meadows Subsidize Diverse Marine and Terrestrial Consumers. Ecosystems 2008, 11, 1198–1210. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Koch, E.W.; Barbier, E.B.; Creed, J. Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed]
- Dunic, J.C.; Brown, C.J.; Connolly, R.M.; Turschwell, M.P.; Côté, I.M. Long-term declines and recovery of meadow area across the world’s seagrass bioregions. Glob. Chang. Biol. 2021, 27, 4096–4109. [Google Scholar] [CrossRef] [PubMed]
- Röhr, M.E.; Holmer, M.; Baum, J.K.; Björk, M.; Boyer, K.; Chin, D.; Chalifour, L.; Cimon, S.; Cusson, M.; Dahl, M.; et al. Blue Carbon Storage Capacity of Temperate Eelgrass (Zostera marina) Meadows. Glob. Biogeochem. Cycles 2018, 32, 1457–1475. [Google Scholar] [CrossRef]
- Maxwell, P.S.; Eklöf, J.; van Katwijk, M.; O’Brien, K.; De La Torre-Castro, M.; Boström, C.; Bouma, T.J.; Krause-Jensen, D.; Unsworth, R.; van Tussenbroek, B.I.; et al. The fundamental role of ecological feedback mechanisms for the adaptive management of seagrass ecosystems—A review. Biol. Rev. 2016, 92, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Bayraktarov, E.; Saunders, M.I.; Abdullah, S.; Mills, M.; Beher, J.; Possingham, H.; Mumby, P.J.; Lovelock, C. The cost and feasibility of marine coastal restoration. Ecol. Appl. 2016, 26, 1055–1074. [Google Scholar] [CrossRef]
- Green, A.J.; Elmberg, J. Ecosystem services provided by waterbirds. Biol. Rev. 2013, 89, 105–122. [Google Scholar] [CrossRef]
- Kollars, N.M.; Henry, A.K.; Whalen, M.A.; Boyer, K.; Cusson, M.; Eklöf, J.S.; Hereu, C.M.; Jorgensen, P.; Kiriakopolos, S.L.; Reynolds, P.L.; et al. Meta-Analysis of Reciprocal Linkages between Temperate Seagrasses and Waterfowl with Implications for Conservation. Front. Plant Sci. 2017, 8, 2119. [Google Scholar] [CrossRef]
- Cury, P.M.; Boyd, I.L.; Bonhommeau, S.; Anker-Nilssen, T.; Crawford, R.J.M.; Furness, R.W.; Mills, J.A.; Murphy, E.J.; Österblom, H.; Paleczny, M.; et al. Global Seabird Response to Forage Fish Depletion—One-Third for the Birds. Science 2011, 334, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, J.; Leopold, M.F.; Camphuysen, K. A comparative study of the diet of Guillemots Uria aalge and Razorbills Alca torda killed during the Tricolor oil incident in the south-eastern North Sea in January 2003. Atl. Seab. 2004, 6, 147–164. [Google Scholar]
- Percival, S.; Sutherland, W.; Evans, P. Intertidal habitat loss and wildfowl numbers: Applications of a spatial depletion model. J. Appl. Ecol. 1998, 35, 57–63. [Google Scholar] [CrossRef]
- Frederiksen, M.; Edwards, M.; Richardson, A.; Halliday, N.C.; Wanless, S. From plankton to top predators: Bottom-up control of a marine food web across four trophic levels. J. Anim. Ecol. 2006, 75, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.; Heck, K. Seagrass herbivory:evidence for the continued grazing of marine grasses. Mar. Ecol. Prog. Ser. 1999, 176, 291–302. [Google Scholar] [CrossRef]
- Attrill, M.J.; Strong, J.A.; Rowden, A.A. Are macroinvertebrate communities influenced by seagrass structural complexity? Ecography 2000, 23, 114–121. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fong, C.; Wu, R. The effects of seagrass (Zostera japonica) canopy structure on associated fauna: A study using artificial seagrass units and sampling of natural beds. J. Exp. Mar. Biol. Ecol. 2001, 259, 23–50. [Google Scholar] [CrossRef]
- Barnes, R. Patterns of benthic invertebrate biodiversity in intertidal seagrass in Moreton Bay, Queensland. Reg. Stud. Mar. Sci. 2017, 15, 17–25. [Google Scholar] [CrossRef]
- Reynolds, P.L.; Stachowicz, J.J.; Hovel, K.; Boström, C.; Boyer, K.; Cusson, M.; Eklöf, J.S.; Engel, F.G.; Engelen, A.H.; Eriksson, B.K.; et al. Latitude, temperature, and habitat complexity predict predation pressure in eelgrass beds across the Northern Hemisphere. Ecology 2018, 99, 29–35. [Google Scholar] [CrossRef]
- Boere, G.C.; Piersma, T. Flyway protection and the predicament of our migrant birds: A critical look at international conservation policies and the Dutch Wadden Sea. Ocean Coast. Manag. 2012, 68, 157–168. [Google Scholar] [CrossRef]
- Horn, S.; Schwemmer, P.; Mercker, M.; Enners, L.; Asmus, R.; Garthe, S.; Asmus, H. Species composition of foraging birds in association with benthic fauna in four intertidal habitats of the Wadden Sea. Estuar. Coast. Shelf Sci. 2020, 233, 106537. [Google Scholar] [CrossRef]
- Harrison, P.G.; Dunn, M. Fraser River delta seagrass ecosystems, their distributions and importance to migratory birds. In Fraser River Delta, British Columbia: Issues of an Urban Estuary; Groulx, R.I., Mosher, D.C., Luternauer, I., Bilderback, D.E., Eds.; Geological Survey of Canada, Bulletin 567: Ottawa, ON, Canada, 2004; pp. 173–188. [Google Scholar]
- Sutherland, T.F.; Shepherd, P.C.F.; Elner, R.W. Predation on meiofaunal and macrofaunal invertebrates by western sandpipers (Calidris mauri): Evidence for dual foraging modes. Mar. Biol. 2000, 137, 983–993. [Google Scholar] [CrossRef]
- Spruzen, F.L.; Richardson, A.M.; Woehler, E.J. Influence of environmental and prey variables on low tide shorebird habitat use within the Robbins Passage wetlands, Northwest Tasmania. Estuar. Coast. Shelf Sci. 2008, 78, 122–134. [Google Scholar] [CrossRef]
- Seymour, N.R.; Miller, A.G.; Garbary, D.J. Decline of Canada geese (Branta canadensis) and common goldeneye (Bucephala clangula) associated with a collapse of eelgrass (Zostera marina) in a Nova Scotia estuary. Helgol. Mar. Res. 2002, 56, 198–202. [Google Scholar] [CrossRef]
- Eadie, J.M.; Mallory, M.L.; Lumsden, H.G. Common Goldeneye (Bucephala clangula), version 2.0. In The Birds of North America; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 1995. [Google Scholar]
- Pehrsson, O. Food and Feeding Grounds of the Goldeneye Bucephala clangula (L.) on the Swedish West Coast. Ornis Scand. 1976, 7, 91. [Google Scholar] [CrossRef]
- Brun, F.G.; Van Zetten, E.; Cacabelos, E.; Bouma, T.J. Role of two contrasting ecosystem engineers (Zostera noltii and Cymodocea nodosa) on the food intake rate of Cerastoderma edule. Helgol. Mar. Res. 2008, 63, 19–25. [Google Scholar] [CrossRef]
- Norris, K.; Bannister, R.; Walker, P.; Walker, W.D. Changes in the number of oystercatchers Haematopus ostralegus wintering in the Burry Inlet in relation to the biomass of cockles Cerastoderma edule and its commercial exploitation. J. Appl. Ecol. 1998, 35, 75–85. [Google Scholar] [CrossRef]
- Bologna, P.A.; Fetzer, M.L.; McDonnell, S.; Moody, E.M. Assessing the potential benthic–pelagic coupling in episodic blue mussel (Mytilus edulis) settlement events within eelgrass (Zostera marina) communities. J. Exp. Mar. Biol. Ecol. 2005, 316, 117–131. [Google Scholar] [CrossRef]
- Caldow, R.W.G.; Beadman, H.A.; McGrorty, M.J.K.S.; Goss-Custard, J.D.; Mould, K.; Wilson, A. Effects of intertidal mussel cultivation on bird assemblages. Mar. Ecol. Prog. Ser. 2003, 259, 173–183. [Google Scholar] [CrossRef]
- Spruzen, F.L.; Richardson, A.M.M.; Woehler, E.J. Spatial variation of intertidal macroinvertebrates and environmental variables in Robbins Passage wetlands, NW Tasmania. Hydrobiologia 2007, 598, 325–342. [Google Scholar] [CrossRef]
- Lilley, R.J.; Unsworth, R.K. Atlantic Cod (Gadus morhua) benefits from the availability of seagrass (Zostera marina) nursery habitat. Glob. Ecol. Conserv. 2014, 2, 367–377. [Google Scholar] [CrossRef]
- Unsworth, R.K.; Nordlund, L.M.; Cullen-Unsworth, L.C. Seagrass meadows support global fisheries production. Conserv. Lett. 2018, 12, e12566. [Google Scholar] [CrossRef]
- Campbell, K.J.; Steinfurth, A.; Underhill, L.G.; Coetzee, J.; Dyer, B.M.; Ludynia, K.; Makhado, A.B.; Merkle, D.; Rademan, J.; Upfold, L.; et al. Local forage fish abundance influences foraging effort and offspring condition in an endangered marine predator. J. Appl. Ecol. 2019, 56, 1751–1760. [Google Scholar] [CrossRef]
- Bertelli, C.M.; Unsworth, R.K. Protecting the hand that feeds us: Seagrass (Zostera marina) serves as commercial juvenile fish habitat. Mar. Pollut. Bull. 2014, 83, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.; Orth, R. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]
- Henderson, C.J.; Gilby, B.L.; Lee, S.Y.; Stevens, T. Contrasting effects of habitat complexity and connectivity on biodiversity in seagrass meadows. Mar. Biol. 2017, 164, 117. [Google Scholar] [CrossRef]
- Butler, R.W. Habitat Selection and Time of Breeding in the Great Blue Heron, (Ardea herodias). University of British Columbia. Available online: https://open.library.ubc.ca/collections/ubctheses/831/items/1.01003921991 (accessed on 1 July 2021).
- Hom, C.W. Foraging Ecology of Herons in a Southern San Francisco Bay Salt Marsh. Colon. Waterbirds 1983, 6, 37. [Google Scholar] [CrossRef]
- Huang, A.C.; Essak, M.; O’Connor, M.I. Top-down control by great blue herons Ardea herodias regulates seagrass-associated epifauna. Oikos 2015, 124, 1492–1501. [Google Scholar] [CrossRef]
- Custer, T.W. The Use of Feeding Habitat by a Colony of Herons, Egrets, and Ibises near Beaufort, North Carolina (Abstract Only). Proc. Colon. Waterbird Group 1978, 1, 154. [Google Scholar] [CrossRef]
- Dorfman, E.J.; Kingsford, M. Environmental determinants of distribution and foraging behaviour of cormorants (Phalacrocorax spp.) in temperate estuarine habitats. Mar. Biol. 2001, 138, 1–10. [Google Scholar] [CrossRef]
- Grémillet, D.; Argentin, G.; Schulte, B.; Culik, B.M. Flexible foraging techniques in breeding Cormorants Phalacrocorax carbo and Shags Phalacrocorax aristotelis: Benthic or pelagic feeding? Ibis 2008, 140, 113–119. [Google Scholar] [CrossRef]
- Heithaus, M.R. Habitat use and group size of pied cormorants (Phalacrocorax varius) in a seagrass ecosystem: Possible effects of food abundance and predation risk. Mar. Biol. 2005, 147, 27–35. [Google Scholar] [CrossRef]
- Redfern, C.P.; Bevan, R.M.; Redfern, C. A comparison of foraging behaviour in the North Sea by Black-legged Kittiwakes Rissa tridactyla from an inland and a maritime colony. Bird Study 2014, 61, 17–28. [Google Scholar] [CrossRef]
- Bugge, J.; Barrett, R.T.; Pedersen, T. Optimal foraging in chick-raising Common Guillemots (Uria aalge). J. Ornithol. 2011, 152, 253–259. [Google Scholar] [CrossRef][Green Version]
- McDevitt-Irwin, J.; Iacarella, J.; Baum, J. Reassessing the nursery role of seagrass habitats from temperate to tropical regions: A meta-analysis. Mar. Ecol. Prog. Ser. 2016, 557, 133–143. [Google Scholar] [CrossRef]
- Furness, E.; Unsworth, R.K. Demersal Fish Assemblages in NE Atlantic Seagrass and Kelp. Diversity 2020, 12, 366. [Google Scholar] [CrossRef]
- Kritzer, J.P.; DeLucia, M.-B.; Greene, E.; Shumway, C.; Topolski, M.F.; Thomas-Blate, J.; Chiarella, L.A.; Davy, K.B.; Smith, K. The Importance of Benthic Habitats for Coastal Fisheries. BioScience 2016, 66, 274–284. [Google Scholar] [CrossRef]
- Seitz, R.D.; Wennhage, H.; Bergström, U.; Lipcius, R.N.; Ysebaert, T. Ecological value of coastal habitats for commercially and ecologically important species. ICES J. Mar. Sci. J. Cons. 2013, 71, 648–665. [Google Scholar] [CrossRef]
- Einoder, L. A review of the use of seabirds as indicators in fisheries and ecosystem management. Fish. Res. 2009, 95, 6–13. [Google Scholar] [CrossRef]
- Polte, P.; Asmus, H. Influence of seagrass beds (Zostera noltii) on the species composition of juvenile fishes temporarily visiting the intertidal zone of the Wadden Sea. J. Sea Res. 2006, 55, 244–252. [Google Scholar] [CrossRef]
- Rönnbäck, P.; Kautsky, N.; Pihl, L.; Troell, M.; Söderqvist, T.; Wennhage, H. Ecosystem Goods and Services from Swedish Coastal Habitats: Identification, Valuation, and Implications of Ecosystem Shifts. Ambio 2007, 36, 534–544. [Google Scholar] [CrossRef]
- Anderson, H.B.; Evans, P.G.H.; Potts, J.; Harris, M.P.; Wanless, S. The diet of Common Guillemot Uria aalge chicks provides evidence of changing prey communities in the North Sea. Ibis 2013, 156, 23–34. [Google Scholar] [CrossRef][Green Version]
- Barrett, R.T. The diet, growth and survival of Razorbill Alca torda chicks in the southern Barents Sea. Ornis Nor. 2015, 38, 25. [Google Scholar] [CrossRef]
- Riordan, J.; Birkhead, T. Changes in the diet composition of Common Guillemot Uria aalge chicks on Skomer Island, Wales, between 1973 and 2017. Ibis 2018, 160, 470–474. [Google Scholar] [CrossRef]
- Hamer, K.C.; Humphreys, E.M.; Garthe, S.; Hennicke, J.; Peters, G.; Grémillet, D.; Phillips, R.A.; Harris, M.P.; Wanless, S. Annual variation in diets, feeding locations and foraging behaviour of gannets in the North Sea: Flexibility, consistency and constraint. Mar. Ecol. Prog. Ser. 2007, 338, 295–305. [Google Scholar] [CrossRef]
- Paiva, V.; Geraldes, P.; Ramirez, I.; Meirinho, A.; Garthe, S.; Ramos, J. Foraging plasticity in a pelagic seabird species along a marine productivity gradient. Mar. Ecol. Prog. Ser. 2010, 398, 259–274. [Google Scholar] [CrossRef]
- Pettex, E.; Lorentsen, S.-H.; Grémillet, D.; Gimenez, O.; Barrett, R.T.; Pons, J.-B.; Le Bohec, C.; Bonadonna, F. Multi-scale foraging variability in Northern gannet (Morus bassanus) fuels potential foraging plasticity. Mar. Biol. 2012, 159, 2743–2756. [Google Scholar] [CrossRef]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef]
- Green, A.E.; Unsworth, R.K.F.; Chadwick, M.A.; Jones, P.J.S. Historical Analysis Exposes Catastrophic Seagrass Loss for the United Kingdom. Front. Plant Sci. 2021, 12, 261. [Google Scholar] [CrossRef]
- Baden, S.; Gullstrom, M.; Lunden, B.; Pihl, L.; Rosenberg, R. Vanishing seagrass (Zostera marina L.) in Swedish coastal waters. Ambio 2003, 32, 374–377. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; McKenzie, L.J.; Collier, C.J.; Cullen-Unsworth, L.C.; Duarte, C.M.; Eklöf, J.S.; Jarvis, J.C.; Jones, B.L.; Nordlund, L.M. Global challenges for seagrass conservation. Ambio 2019, 48, 801–815. [Google Scholar] [CrossRef]
- Lewis, S.; Wanless, S.; Wright, P.J.; Harris, M.P.; Bull, J.; Elston, D.A. Diet and breeding performance of black-legged kittiwakes Rissa tridactyla at a North Sea colony. Mar. Ecol. Prog. Ser. 2001, 221, 277–284. [Google Scholar] [CrossRef]
- Thurstan, R.H.; Hawkins, J.P.; Raby, L.; Roberts, C.M. Oyster (Ostrea edulis) extirpation and ecosystem transformation in the Firth of Forth, Scotland. J. Nat. Conserv. 2013, 21, 253–261. [Google Scholar] [CrossRef]
- Short, F.; Dennison, W.; Capone, D. Phosphorus-limited growth of the tropical seagrass Syringodium filiforme in carbonate sediments. Mar. Ecol. Prog. Ser. 1990, 62, 169–174. [Google Scholar] [CrossRef]
- Atkinson, M.J.; Smith, S.V. C:N:P ratios of benthic marine plants1. Limnol. Oceanogr. 1983, 28, 568–574. [Google Scholar] [CrossRef]
- Kenworthy, W.J.; Hall, M.O.; Hammerstrom, K.K.; Merello, M.; Schwartzschild, A. Restoration of tropical seagrass beds using wild bird fertilization and sediment regrading. Ecol. Eng. 2018, 112, 72–81. [Google Scholar] [CrossRef]
- McMahon, K.M.; Van Dijk, K.-J.; Ruiz-Montoya, L.; Kendrick, G.; Krauss, S.; Waycott, M.; Verduin, J.; Lowe, R.; Statton, J.; Brown, E.; et al. The movement ecology of seagrasses. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140878. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).