An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception
Abstract
1. Introduction
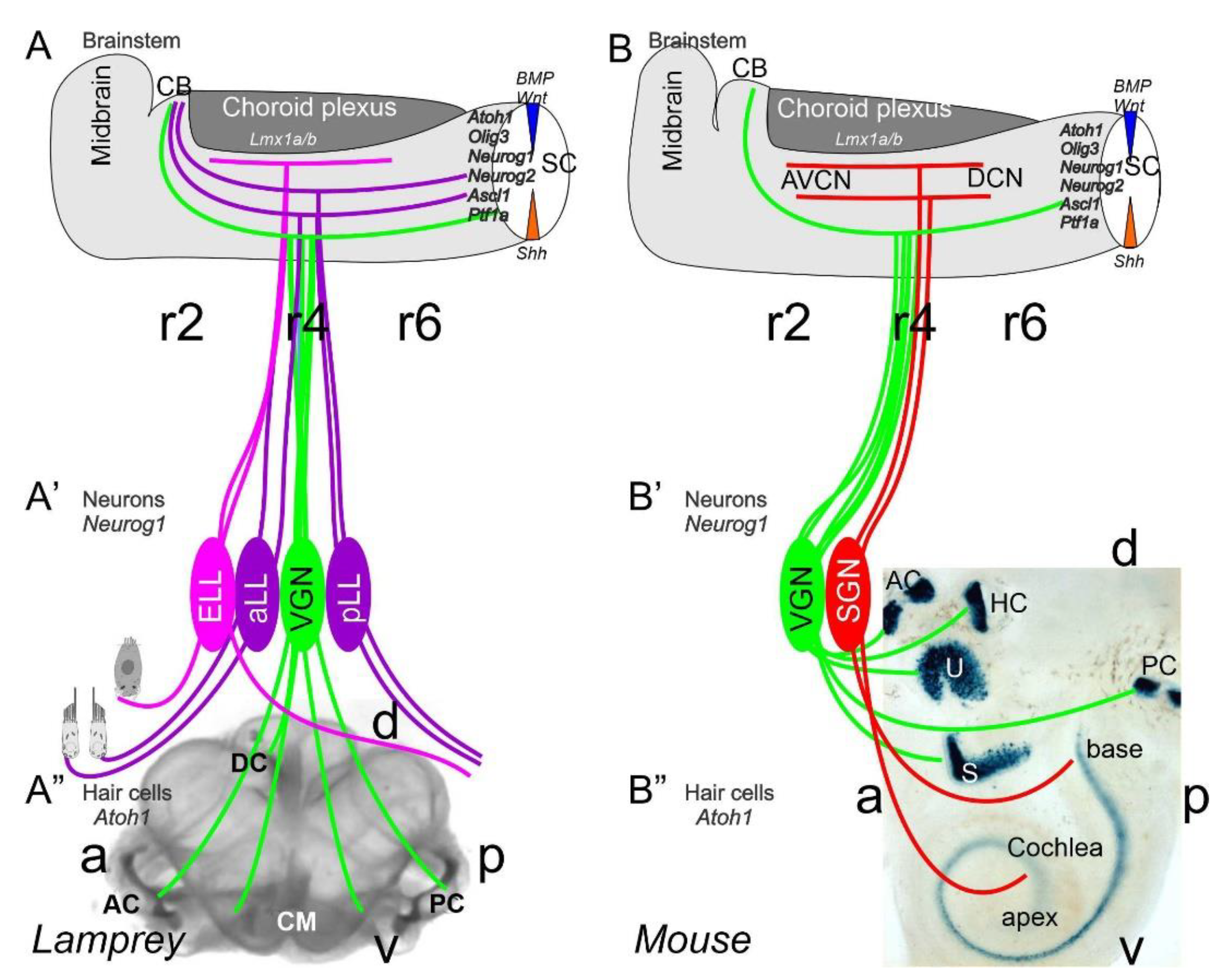
2. Neurons Depend upon Eya1, Sox2, Neurog1 and Neurod1

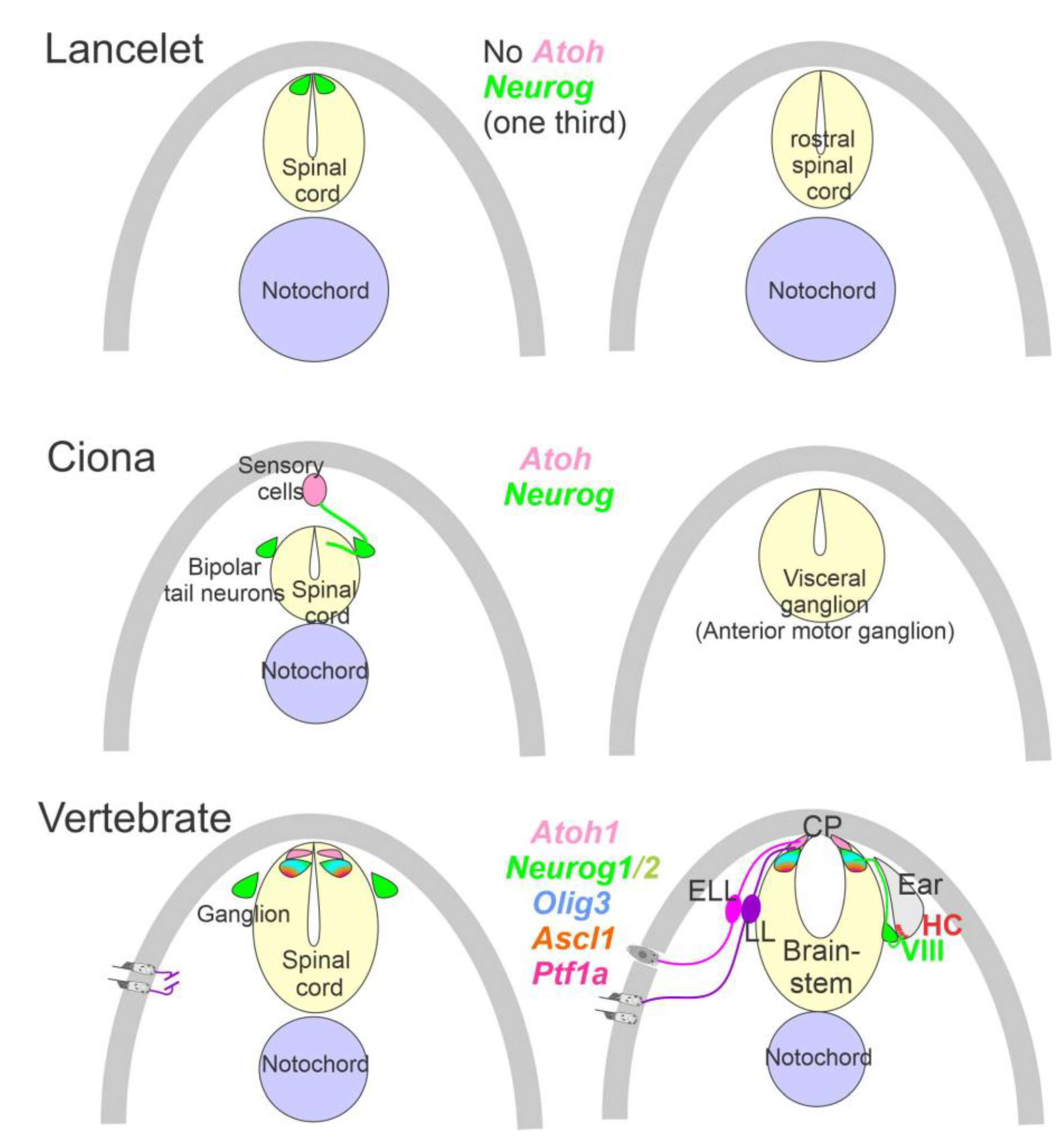
3. The Brainstem Is Transformed from the Spinal Cord

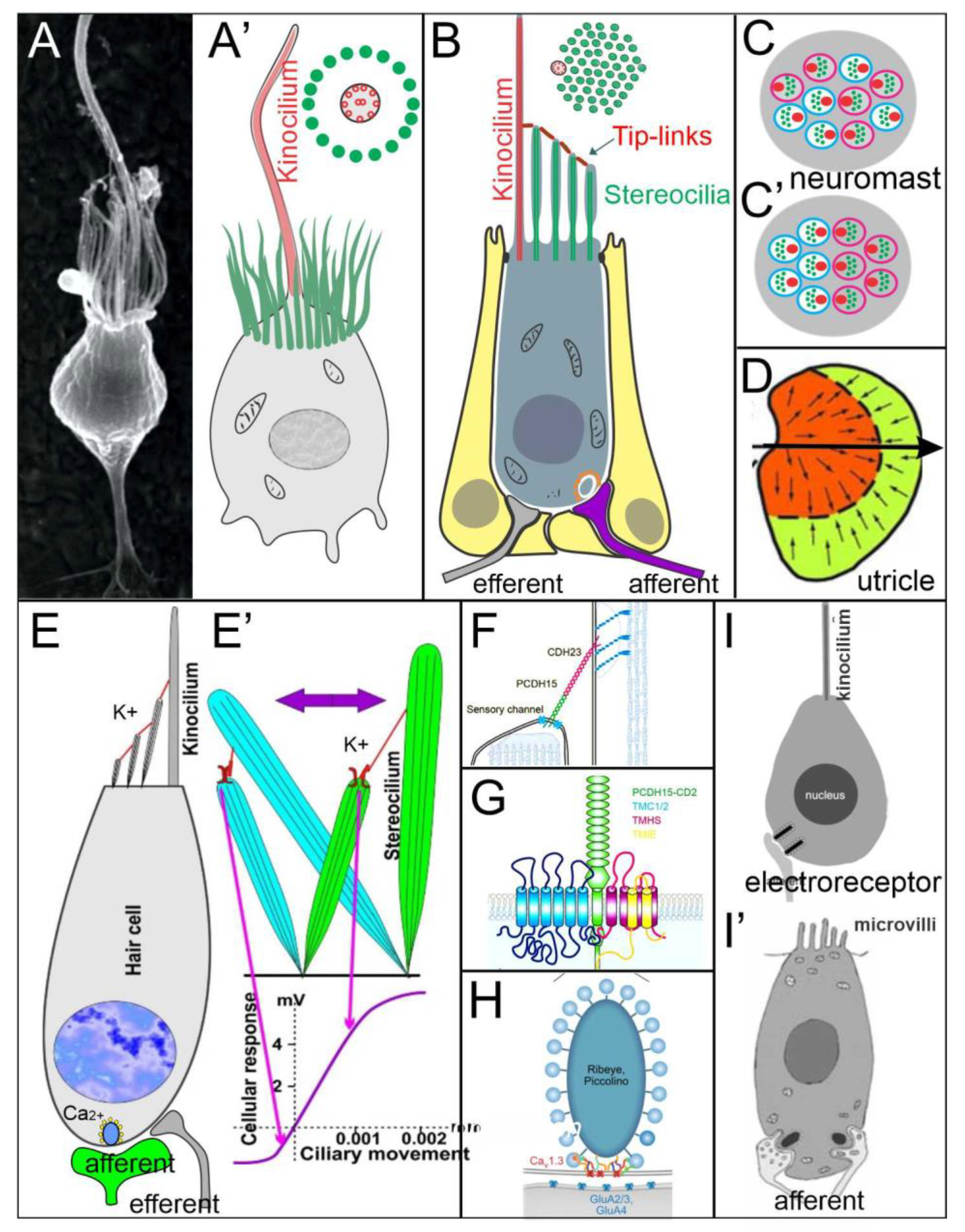
4. Hair Cells Depend on Eya1, Sox2 and Atoh1
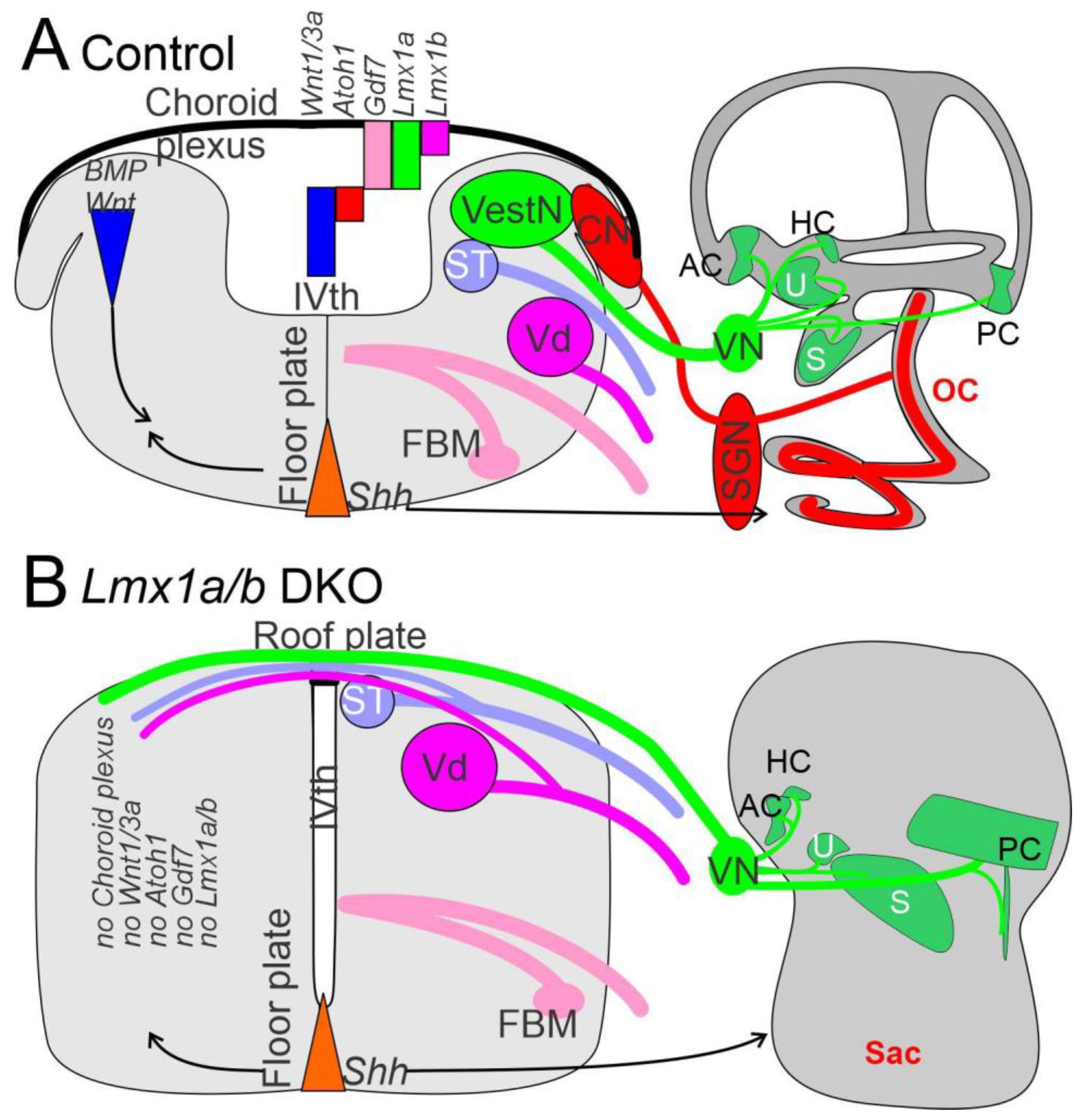
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heger, P.; Zheng, W.; Rottmann, A.; Panfilio, K.A.; Wiehe, T. The genetic factors of bilaterian evolution. eLife 2020, 9, e45530. [Google Scholar] [CrossRef] [PubMed]
- Holland, L. Invertebrate origins of vertebrate nervous systems. In Evolutionary Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–73. [Google Scholar]
- Chagnaud, B.P.; Engelmann, J.; Fritzsch, B.; Glover, J.C.; Straka, H. Sensing external and self-motion with hair cells: A comparison of the lateral line and vestibular systems from a developmental and evolutionary perspective. Brain Behav. Evol. 2017, 90, 98–116. [Google Scholar] [CrossRef]
- Fritzsch, B.; Elliott, K.L.; Glover, J.C. Gaskell revisited: New insights into spinal autonomics necessitate a revised motor neuron nomenclature. Cell Tissue Res. 2017, 370, 195–209. [Google Scholar] [CrossRef]
- Bermingham, N.A.; Hassan, B.A.; Wang, V.Y.; Fernandez, M.; Banfi, S.; Bellen, H.J.; Fritzsch, B.; Zoghbi, H.Y. Proprioceptor pathway development is dependent on Math1. Neuron 2001, 30, 411–422. [Google Scholar] [CrossRef]
- Kim, K.; Gibboney, S.; Razy-Krajka, F.; Lowe, E.K.; Wang, W.; Stolfi, A. Regulation of neurogenesis by FGF signaling and Neurogenin in the invertebrate chordate Ciona. Front. Cell Dev. Biol. 2020, 8, 477. [Google Scholar] [CrossRef]
- Stolfi, A.; Ryan, K.; Meinertzhagen, I.A.; Christiaen, L. Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature 2015, 527, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.Z.; Daza, D.O. A new look at an old question: When did the second whole genome duplication occur in vertebrate evolution? Genome Biol. 2018, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Jahan, I.; Pan, N.; Elliott, K.L. Evolving gene regulatory networks into cellular networks guiding adaptive behavior: An outline how single cells could have evolved into a centralized neurosensory system. Cell Tissue Res. 2015, 359, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Elliott, K.L. Gene, cell, and organ multiplication drives inner ear evolution. Dev. Biol. 2017, 431, 3–15. [Google Scholar] [CrossRef]
- Elliott, K.L.; Pavlínková, G.; Chizhikov, V.V.; Yamoah, E.N.; Fritzsch, B. Development in the Mammalian Auditory System Depends on Transcription Factors. Int. J. Mol. Sci. 2021, 22, 4189. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Chen, J.S.; Zeller, R.W. Transcriptional regulation of the peripheral nervous system in Ciona intestinalis. Dev. Biol. 2013, 378, 183–193. [Google Scholar] [CrossRef]
- Ryan, K.; Lu, Z.; Meinertzhagen, I.A. The peripheral nervous system of the ascidian tadpole larva: Types of neurons and their synaptic networks. J. Comp. Neurol. 2018, 526, 583–608. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Anselmi, C.; Burighel, P.; Martini, M.; Gasparini, F. Differentiation and induced sensorial alteration of the coronal organ in the asexual life of a tunicate. Integr. Comp. Biol. 2018, 58, 317–328. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Zhang, T.; Jiang, H.; Ramakrishnan, A.; Fritzsch, B.; Shen, L.; Xu, P.X. Chromatin remodelers and lineage-specific factors interact to target enhancers to establish proneurosensory fate within otic ectoderm. Proc. Natl. Acad. Sci. USA 2021, 118, 1–12. [Google Scholar] [CrossRef]
- Dvorakova, M.; Macova, I.; Bohuslavova, R.; Anderova, M.; Fritzsch, B.; Pavlinkova, G. Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev. Biol. 2020, 457, 43–56. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, Z.; del Barco Barrantes, I.; de la Pompa, J.L.; Anderson, D.J. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 1998, 20, 469–482. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Fritzsch, B.; Serls, A.; Bakel, L.A.; Huang, E.J.; Reichardt, L.F.; Barth, D.S.; Lee, J.E. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 2001, 128, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Anderson, D.J.; Fritzsch, B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J. Assoc. Res. Otolaryngol. 2000, 1, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Kersigo, J.; Pan, N.; Fritzsch, B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010, 341, 95–110. [Google Scholar] [CrossRef]
- Macova, I.; Pysanenko, K.; Chumak, T.; Dvorakova, M.; Bohuslavova, R.; Syka, J.; Fritzsch, B.; Pavlinkova, G. Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. J. Neurosci. 2019, 39, 984–1004. [Google Scholar] [CrossRef]
- Baker, C.V. The Development and Evolution of Lateral Line Electroreceptors: Insights from Comparative Molecular Approaches. In Electroreception: Fundamental Insights from Comparative Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–62. [Google Scholar]
- Elliott, K.L.; Fritzsch, B. Evolution and development of lateral line and electroreception: An integrated perception ofneurons, hair cells and brainstem nuclei. In The Senses; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 95–115. [Google Scholar]
- Lai, H.C.; Seal, R.P.; Johnson, J.E. Making sense out of spinal cord somatosensory development. Development 2016, 143, 3434–3448. [Google Scholar] [CrossRef]
- Hernandez-Miranda, L.R.; Müller, T.; Birchmeier, C. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev. Biol. 2017, 432, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Grothe, B.; Carr, C.E.; Casseday, J.H.; Fritzsch, B.; Köppl, C. The evolution of central pathways and their neural processing patterns. In Evolution of the Vertebrate Auditory System; Springer: Berlin/Heidelberg, Germany, 2004; pp. 289–359. [Google Scholar]
- Wullimann, M.F.; Grothe, B. The central nervous organization of the lateral line system. In The Lateral Line System; Springer: Berlin/Heidelberg, Germany, 2013; pp. 195–251. [Google Scholar]
- Chizhikov, V.V.; Iskusnykh, I.Y.; Fattakhov, N.; Fritzsch, B. Lmx1a and Lmx1b are Redundantly Required for the Development of Multiple Components of the Mammalian Auditory System. Neuroscience 2021, 452, 247–264. [Google Scholar] [CrossRef]
- Lee, K.J.; Dietrich, P.; Jessell, T.M. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature 2000, 403, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.L.; Weston, M.D.; Fritzsch, B.; Gabel, H.W.; Ruvkun, G.; Soukup, G.A. MicroRNA—183 family conservation and ciliated neurosensory organ expression. Evol. Dev. 2008, 10, 106–113. [Google Scholar] [CrossRef]
- Nichols, D.H.; Pauley, S.; Jahan, I.; Beisel, K.W.; Millen, K.J.; Fritzsch, B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008, 334, 339–358. [Google Scholar] [CrossRef]
- Arendt, D.; Musser, J.M.; Baker, C.V.; Bergman, A.; Cepko, C.; Erwin, D.H.; Pavlicev, M.; Schlosser, G.; Widder, S.; Laubichler, M.D. The origin and evolution of cell types. Nat. Rev. Genet. 2016, 17, 744–757. [Google Scholar] [CrossRef]
- Schlosser, G. A short history of nearly every sense—The evolutionary history of vertebrate sensory cell types. Integr. Comp. Biol. 2018, 58, 301–316. [Google Scholar] [CrossRef]
- Tarchini, B.; Lu, X. New insights into regulation and function of planar polarity in the inner ear. Neurosci. Lett. 2019, 709, 134373. [Google Scholar] [CrossRef]
- Montcouquiol, M.; Rachel, R.A.; Lanford, P.J.; Copeland, N.G.; Jenkins, N.A.; Kelley, M.W. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003, 423, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.R.; Deans, M.R. Frizzled3 and Frizzled6 Cooperate with Vangl2 to Direct Cochlear Innervation by type II Spiral Ganglion Neurons. J. Neurosci. 2019, 39, 8013–8023. [Google Scholar] [CrossRef]
- Qiu, X.; Müller, U. Mechanically gated ion channels in mammalian hair cells. Front. Cell. Neurosci. 2018, 12, 100. [Google Scholar] [CrossRef]
- Shibata, S.B.; Ranum, P.T.; Moteki, H.; Pan, B.; Goodwin, A.T.; Goodman, S.S.; Abbas, P.J.; Holt, J.R.; Smith, R.J. RNA interference prevents autosomal-dominant hearing loss. Am. J. Hum. Genet. 2016, 98, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; López-Schier, H. Evolution of polarized hair cells in aquatic vertebrates and their connection to directionally sensitive neurons. In Flow Sensing in Air and Water; Springer: Berlin/Heidelberg, Germany, 2014; pp. 271–294. [Google Scholar]
- Kozak, E.L.; Palit, S.; Miranda-Rodriguez, J.R.; Janjic, A.; Bottcher, A.; Lickert, H.; Enard, W.; Theis, F.J.; Lopez-Schier, H. Epithelial Planar Bipolarity Emerges from Notch-Mediated Asymmetric Inhibition of Emx2. Curr. Biol. 2020, 30, 1142–1151.e6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Kindt, K.; Wu, D.K. Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Akyuz, N.; Liu, X.P.; Asai, Y.; Nist-Lund, C.; Kurima, K.; Derfler, B.H.; Gyorgy, B.; Limapichat, W.; Walujkar, S.; et al. TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron 2018, 99, 736–753.e6. [Google Scholar] [CrossRef] [PubMed]
- Erives, A.; Fritzsch, B. A Screen for Gene Paralogies Delineating Evolutionary Branching Order of Early Metazoa. G3 Genes Genom. Genet. 2020, 10, 811–826. [Google Scholar] [CrossRef]
- Schwander, M.; Kachar, B.; Müller, U. The cell biology of hearing. J. Cell Biol. 2010, 190, 9–20. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Brändle, K.; Fritzsch, B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev. Biol. 1995, 168, 358–373. [Google Scholar] [CrossRef][Green Version]
- Leitch, D.B.; Julius, D. Electrosensory Transduction: Comparisons Across Structure, Afferent Response Properties, and Cellular Physiology. In Electroreception: Fundamental Insights from Comparative Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 63–90. [Google Scholar]
- Fritzsch, B.; Straka, H. Evolution of vertebrate mechanosensory hair cells and inner ears: Toward identifying stimuli that select mutation driven altered morphologies. J. Comp. Physiol. A 2014, 200, 5–18. [Google Scholar] [CrossRef]
- Xu, P.X.; Adams, J.; Peters, H.; Brown, M.C.; Heaney, S.; Maas, R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 1999, 23, 113–117. [Google Scholar] [CrossRef]
- Zou, D.; Silvius, D.; Fritzsch, B.; Xu, P.-X. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development 2004, 131, 5561–5572. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D.; Somorjai, I.M. The sensory peripheral nervous system in the tail of a cephalochordate studied by serial blockface scanning electron microscopy. J. Comp. Neurol. 2020, 528, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Northcutt, G. Cranial and spinal nerve organization in amphioxus and lampreys: Evidence for an ancestral craniate pattern. Cells Tissues Organs 1993, 148, 96–109. [Google Scholar] [CrossRef]
- Negrón-Piñeiro, L.J.; Wu, Y.; Di Gregorio, A. Transcription Factors of the bHLH Family Delineate Vertebrate Landmarks in the Nervous System of a Simple Chordate. Genes 2020, 11, 1262. [Google Scholar] [CrossRef]
- Kageyama, R.; Shimojo, H.; Ohtsuka, T. Dynamic control of neural stem cells by bHLH factors. Neurosci. Res. 2019, 138, 12–18. [Google Scholar] [CrossRef]
- Reiprich, S.; Wegner, M. From CNS stem cells to neurons and glia: Sox for everyone. Cell Tissue Res. 2015, 359, 111–124. [Google Scholar] [CrossRef]
- Riddiford, N.; Schlosser, G. Dissecting the pre-placodal transcriptome to reveal presumptive direct targets of Six1 and Eya1 in cranial placodes. eLife 2016, 5, e17666. [Google Scholar] [CrossRef]
- Millimaki, B.B.; Sweet, E.M.; Riley, B.B. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev. Biol. 2010, 338, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Sugahara, F.; Pascual-Anaya, J.; Takagi, W.; Oisi, Y.; Kuratani, S. Inner ear development in cyclostomes and evolution of the vertebrate semicircular canals. Nature 2019, 565, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.; de Caprona, D.; Busslinger, M.; Xu, P.; Fritzsch, B. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev. Biol. 2010, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.S.; Fritzsch, B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS ONE 2013, 8, e62046. [Google Scholar] [CrossRef] [PubMed]
- Pirvola, U.; Spencer-Dene, B.; Xing-Qun, L.; Kettunen, P.; Thesleff, I.; Fritzsch, B.; Dickson, C.; Ylikoski, J. FGF/FGFR-2 (IIIb) signaling is essential for inner ear morphogenesis. J. Neurosci. 2000, 20, 6125–6134. [Google Scholar] [CrossRef] [PubMed]
- Riccomagno, M.M.; Martinu, L.; Mulheisen, M.; Wu, D.K.; Epstein, D.J. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002, 16, 2365–2378. [Google Scholar] [CrossRef]
- Kersigo, J.; D’Angelo, A.; Gray, B.D.; Soukup, G.A.; Fritzsch, B. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre-mediated microRNA loss. Genesis 2011, 49, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Pauley, S.; Wright, T.J.; Pirvola, U.; Ornitz, D.; Beisel, K.; Fritzsch, B. Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 227, 203–215. [Google Scholar] [CrossRef]
- Pauley, S.; Lai, E.; Fritzsch, B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 2470–2482. [Google Scholar] [CrossRef]
- Hwang, C.H.; Simeone, A.; Lai, E.; Wu, D.K. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 2725–2734. [Google Scholar] [CrossRef]
- Ma, Q.; Fode, C.; Guillemot, F.; Anderson, D.J. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999, 13, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Elliott, K.L.; Pavlinkova, G. Primary sensory map formations reflect unique needs and molecular cues specific to each sensory system. F1000Research 2019, 8, 345. [Google Scholar] [CrossRef]
- Dennis, D.J.; Han, S.; Schuurmans, C. bHLH transcription factors in neural development, disease, and reprogramming. Brain Res. 2019, 1705, 48–65. [Google Scholar] [CrossRef]
- Erzurumlu, R.S.; Murakami, Y.; Rijli, F.M. Mapping the face in the somatosensory brainstem. Nat. Rev. Neurosci. 2010, 11, 252–263. [Google Scholar] [CrossRef]
- Fode, C.; Gradwohl, G.; Morin, X.; Dierich, A.; Le Meur, M.; Goridis, C.; Guillemot, F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode–derived sensory neurons. Neuron 1998, 20, 483–494. [Google Scholar] [CrossRef]
- Alsina, B. Mechanisms of cell specification and differentiation in vertebrate cranial sensory systems. Curr. Opin. Cell Biol. 2020, 67, 79–85. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, P.; Mak, S.-S.; Fritzsch, B.; Ladher, R.K.; Baker, C.V. The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat. Commun. 2012, 3, 1041. [Google Scholar] [CrossRef]
- Sapede, D.; Dyballa, S.; Pujades, C. Cell lineage analysis reveals three different progenitor pools for neurosensory elements in the otic vesicle. J. Neurosci. 2012, 32, 16424–16434. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.A.; La Mantia, A.-S. Transcriptional regulation of cranial sensory placode development. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 111, pp. 301–350. [Google Scholar]
- Dykes, I.M.; Tempest, L.; Lee, S.-I.; Turner, E.E. Brn3a and Islet1 act epistatically to regulate the gene expression program of sensory differentiation. J. Neurosci. 2011, 31, 9789–9799. [Google Scholar] [CrossRef]
- Huang, E.J.; Liu, W.; Fritzsch, B.; Bianchi, L.M.; Reichardt, L.F.; Xiang, M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development 2001, 128, 2421–2432. [Google Scholar] [CrossRef]
- Mackowetzky, K.; Yoon, K.H.; Mackowetzky, E.J.; Waskiewicz, A.J. Development and evolution of the vestibular apparatuses of the inner ear. J. Anat. 2021. [Google Scholar] [CrossRef]
- Muthu, V.; Rohacek, A.M.; Yao, Y.; Rakowiecki, S.M.; Brown, A.S.; Zhao, Y.-T.; Meyers, J.; Won, K.-J.; Ramdas, S.; Brown, C.D.; et al. Genomic architecture of Shh-dependent cochlear morphogenesis. Development 2019, 146, dev181339. [Google Scholar] [CrossRef] [PubMed]
- Petitpré, C.; Wu, H.; Sharma, A.; Tokarska, A.; Fontanet, P.; Wang, Y.; Helmbacher, F.; Yackle, K.; Silberberg, G.; Hadjab, S. Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system. Nat. Commun. 2018, 9, 3691. [Google Scholar] [CrossRef]
- Shrestha, B.R.; Chia, C.; Wu, L.; Kujawa, S.G.; Liberman, M.C.; Goodrich, L.V. Sensory neuron diversity in the inner ear is shaped by activity. Cell 2018, 174, 1229–1246.e17. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Babola, T.; Pregernig, G.; So, K.S.; Nguyen, M.; Su, S.-S.M.; Palermo, A.T.; Bergles, D.E.; Burns, J.C.; Müller, U. Hair cell mechanotransduction regulates spontaneous activity and spiral ganglion subtype specification in the auditory system. Cell 2018, 174, 1247–1263.e15. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.L.; Kersigo, J.M.; Lee, J.H.; Jahan, I.; Pavlinkova, G.; Fritzsch, B.; Yamoah, E.N. Changes in Peripherin-eGFP expression in spiral ganglion neurons; from development to maturity. Front. Cell. Neurosci. 2021, 15, 181. [Google Scholar] [CrossRef]
- Mao, Y.; Reiprich, S.; Wegner, M.; Fritzsch, B. Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS ONE 2014, 9, e94580. [Google Scholar] [CrossRef]
- Morris, J.K.; Maklad, A.; Hansen, L.A.; Feng, F.; Sorensen, C.; Lee, K.-F.; Macklin, W.B.; Fritzsch, B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006, 1091, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Freyer, L.; Aggarwal, V.; Morrow, B.E. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development 2011, 138, 5403–5414. [Google Scholar] [CrossRef] [PubMed]
- Billings, S.E.; Myers, N.M.; Quiruz, L.; Cheng, A.G. Opposing effects of Wnt/β-catenin signaling on epithelial and mesenchymal cell fate in the developing cochlea. Development 2021, 148, dev199091. [Google Scholar] [CrossRef]
- Goodrich, L.V. Early development of the spiral ganglion. In The Primary Auditory Neurons of the Mammalian Cochlea; Springer: Berlin/Heidelberg, Germany, 2016; pp. 11–48. [Google Scholar]
- Fritzsch, B.; Tessarollo, L.; Coppola, E.; Reichardt, L.F. Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. Prog. Brain Res. 2004, 146, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.M.; Conover, J.C.; Fritzsch, B.; De Chiara, T.; Lindsay, R.M.; Yancopoulos, G.D. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development 1996, 122, 1965–1973. [Google Scholar] [CrossRef]
- Fritzsch, B.; Kersigo, J.; Yang, T.; Jahan, I.; Pan, N. Neurotrophic factor function during ear development: Expression changes define critical phases for neuronal viability. In The Primary Auditoryneurons of the Mammaliancochlea; Dabdoub, A., Fritzsch, B., Popper, A., Fay, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 52, pp. 49–84. [Google Scholar]
- Fariñas, I.; Jones, K.R.; Tessarollo, L.; Vigers, A.J.; Huang, E.; Kirstein, M.; De Caprona, D.C.; Coppola, V.; Backus, C.; Reichardt, L.F. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J. Neurosci. 2001, 21, 6170–6180. [Google Scholar] [CrossRef] [PubMed]
- Kersigo, J.; Fritzsch, B. Inner ear hair cells deteriorate in mice engineered to have no or diminished innervation. Front. Aging Neurosci. 2015, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Sarai, P.; Barbacid, M.; Silos-Santiago, I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int. J. Dev. Neurosci. 1997, 15, 563–576. [Google Scholar] [CrossRef]
- Silos-Santiago, I.; Fagan, A.M.; Garber, M.; Fritzsch, B.; Barbacid, M. Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur. J. Neurosci. 1997, 9, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Hallböök, F.; Wilson, K.; Thorndyke, M.; Olinski, R.P. Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav. Evol. 2006, 68, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. Ngf, bdnf, nt3, and nt4. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–15. [Google Scholar]
- Kopecky, B.; Santi, P.; Johnson, S.; Schmitz, H.; Fritzsch, B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev. Dyn. 2011, 240, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Schimmang, T.; Pirvola, U. Coupling the cell cycle to development and regeneration of the inner ear. Semin. Cell Dev. Biol. 2013, 24, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Tateya, T.; Omori, K.; Kageyama, R. Id genes are required for morphogenesis and cellular patterning in the developing mammalian cochlea. Dev. Biol. 2020, 460, 164–175. [Google Scholar] [CrossRef]
- Chen, S.-D.; Yang, J.-L.; Lin, Y.-C.; Chao, A.; Yang, D.-I. Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer’s Disease: Cell Cycle Reentry and Beyond. Cells 2020, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.E.; Brown, N.L. All in the family: Proneural bHLH genes and neuronal diversity. Development 2018, 145, dev159426. [Google Scholar] [CrossRef]
- Pierce, E.T. Histogenesis of the dorsal and ventral cochlear nuclei in the mouse. An autoradiographic study. J. Comp. Neurol. 1967, 131, 27–53. [Google Scholar] [CrossRef]
- Chou, S.-W.; Chen, Z.; Zhu, S.; Davis, R.W.; Hu, J.; Liu, L.; Fernando, C.A.; Kindig, K.; Brown, W.C.; Stepanyan, R. A molecular basis for water motion detection by the mechanosensory lateral line of zebrafish. Nat. Commun. 2017, 8, 2234. [Google Scholar] [CrossRef]
- Altman, J.; Bayer, S.A. Atlas Of Prenatal Rat Brain Development; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Kopecky, B.; Fritzsch, B. The Myc road to hearing restoration. Cells 2012, 1, 667–698. [Google Scholar] [CrossRef]
- Alonso, M.B.D.; Hernandez, I.L.; de la Fuente, M.A.; Garcia-Sancho, J.; Giraldez, F.; Schimmang, T. Transcription factor induced conversion of human fibroblasts towards the hair cell lineage. PLoS ONE 2018, 13, e0200210. [Google Scholar]
- Mantela, J.; Jiang, Z.; Ylikoski, J.; Fritzsch, B.; Zacksenhaus, E.; Pirvola, U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 2005, 132, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, E.N.; Li, M.; Shah, A.; Elliott, K.L.; Cheah, K.; Xu, P.X.; Phillips, S.; Young, S.M., Jr.; Eberl, D.F.; Fritzsch, B. Using Sox2 to alleviate the hallmarks of age-related hearing loss. Ageing Res. Rev. 2020, 59, 101042. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Gregory, D.; Rosa-Molinar, E. The development of the hindbrain afferent projections in the axolotl: Evidence for timing as a specific mechanism of afferent fiber sorting. Zoology 2005, 108, 297–306. [Google Scholar] [CrossRef][Green Version]
- Yang, T.; Kersigo, J.; Jahan, I.; Pan, N.; Fritzsch, B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear. Res. 2011, 278, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, J.F.; Dubuc, R. Relationship between vestibular primary afferents and vestibulospinal neurons in lampreys. J. Comp. Neurol. 2000, 427, 255–273. [Google Scholar] [CrossRef]
- Duncan, J.S.; Cox, B.C. Anatomy and Development of the Inner Ear. In The Senses; Fritzsch, B., Grothe, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 253–276. [Google Scholar]
- Bell, C.C.; Han, V.; Sawtell, N.B. Cerebellum-like structures and their implications for cerebellar function. Annu. Rev. Neurosci. 2008, 31, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, R.G. The phylogenetic distribution and innervation of craniate mechanoreceptive lateral lines. In The Mechanosensory Lateral Line; Springer: Berlin/Heidelberg, Germany, 1989; pp. 17–78. [Google Scholar]
- Webb, J.F. Morphology of the mechanosensory lateral lien system of fishes. In The Senses; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 29–46. [Google Scholar]
- Chitnis, A.B. Development of the zebrafish posterior lateral line system. In The Senses; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 66–84. [Google Scholar]
- Fritzsch, B.; Beisel, K.; Jones, K.; Farinas, I.; Maklad, A.; Lee, J.; Reichardt, L. Development and evolution of inner ear sensory epithelia and their innervation. J. Neurobiol. 2002, 53, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Pombal, M.A.; Megías, M. Development and functional organization of the cranial nerves in lampreys. Anat. Rec. 2019, 302, 512–539. [Google Scholar] [CrossRef]
- Fritzsch, B. Evolution of the vestibulo-ocular system. Otolaryngol. Head Neck Surg. 1998, 119, 182–192. [Google Scholar] [CrossRef]
- Fritzsch, B.; Signore, M.; Simeone, A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev. Genes Evol. 2001, 211, 388–396. [Google Scholar] [CrossRef]
- Fritzsch, B.; Wake, M. The inner ear of gymnophione amphibians and its nerve supply: A comparative study of regressive events in a complex sensory system (Amphibia, Gymnophiona). Zoomorphology 1988, 108, 201–217. [Google Scholar] [CrossRef]
- Fritzsch, B.; Pan, N.; Jahan, I.; Duncan, J.S.; Kopecky, B.J.; Elliott, K.L.; Kersigo, J.; Yang, T. Evolution and development of the tetrapod auditory system: An organ of Corti-centric perspective. Evol. Dev. 2013, 15, 63–79. [Google Scholar] [CrossRef]
- Maklad, A.; Fritzsch, B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res. Bull. 2003, 60, 497–510. [Google Scholar] [CrossRef]
- Zecca, A.; Dyballa, S.; Voltes, A.; Bradley, R.; Pujades, C. The order and place of neuronal differentiation establish the topography of sensory projections and the entry points within the hindbrain. J. Neurosci. 2015, 35, 7475–7486. [Google Scholar] [CrossRef] [PubMed]
- Matei, V.; Pauley, S.; Kaing, S.; Rowitch, D.; Beisel, K.; Morris, K.; Feng, F.; Jones, K.; Lee, J.; Fritzsch, B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 2005, 234, 633–650. [Google Scholar] [CrossRef]
- Rubel, E.W.; Fritzsch, B. Auditory system development: Primary auditory neurons and their targets. Annu. Rev. Neurosci. 2002, 25, 51–101. [Google Scholar] [CrossRef] [PubMed]
- Ruben, R.J. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol. 1967, 220, 1–44. [Google Scholar]
- De No, R.L. The Primary Acoustic Nuclei; Raven Press: New York, NY, USA, 1981. [Google Scholar]
- Schmidt, H.; Fritzsch, B. Npr2 null mutants show initial overshooting followed by reduction of spiral ganglion axon projections combined with near-normal cochleotopic projection. Cell Tissue Res. 2019, 378, 15–32. [Google Scholar] [CrossRef]
- Elliott, K.L.; Kersigo, J.; Pan, N.; Jahan, I.; Fritzsch, B. Spiral Ganglion Neuron Projection Development to the Hindbrain in Mice Lacking Peripheral and/or Central Target Differentiation. Front. Neural Circuits 2017, 11, 25. [Google Scholar] [CrossRef][Green Version]
- Fritzsch, B.; Matei, V.; Nichols, D.; Bermingham, N.; Jones, K.; Beisel, K.; Wang, V. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Milinkeviciute, G.; Cramer, K.S. Development of the ascending auditory pathway. In The Senses; Fritzsch, B., Grothe, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 337–353. [Google Scholar]
- Duncan, J.S.; Fritzsch, B.; Houston, D.W.; Ketchum, E.M.; Kersigo, J.; Deans, M.R.; Elliott, K.L. Topologically correct central projections of tetrapod inner ear afferents require Fzd3. Sci. Rep. 2019, 9, 10298. [Google Scholar] [CrossRef] [PubMed]
- Maklad, A.; Kamel, S.; Wong, E.; Fritzsch, B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010, 340, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C.; Maler, L. Central neuroanatomy of electrosensory systems in fish. In Electroreception; Springer: Berlin/Heidelberg, Germany, 2005; pp. 68–111. [Google Scholar]
- Millimaki, B.B.; Sweet, E.M.; Dhason, M.S.; Riley, B.B. Zebrafish atoh1 genes: Classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 2007, 134, 295–305. [Google Scholar] [CrossRef]
- Northcutt, R.G. Distribution and innervation of lateral line organs in the axolotl. J. Comp. Neurol. 1992, 325, 95–123. [Google Scholar] [CrossRef]
- Pan, N.; Jahan, I.; Kersigo, J.; Duncan, J.S.; Kopecky, B.; Fritzsch, B. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS ONE 2012, 7, e30358. [Google Scholar] [CrossRef]
- Xiang, M.; Maklad, A.; Pirvola, U.; Fritzsch, B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003, 4, 2. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Puelles, L. Towards a New Neuromorphology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Watson, C.; Shimogori, T.; Puelles, L. Mouse Fgf8-Cre-LacZ lineage analysis defines the territory of the postnatal mammalian isthmus. J. Comp. Neurol. 2017, 525, 2782–2799. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Steshina, E.Y.; Chizhikov, V.V. Loss of Ptf1a leads to a widespread cell-fate misspecification in the brainstem, affecting the development of somatosensory and viscerosensory nuclei. J. Neurosci. 2016, 36, 2691–2710. [Google Scholar] [CrossRef]
- Yamada, M.; Seto, Y.; Taya, S.; Owa, T.; Inoue, Y.U.; Inoue, T.; Kawaguchi, Y.; Nabeshima, Y.-i.; Hoshino, M. Specification of spatial identities of cerebellar neuron progenitors by ptf1a and atoh1 for proper production of GABAergic and glutamatergic neurons. J. Neurosci. 2014, 34, 4786–4800. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, T.; Yamada, M.; Terao, M.; Terashima, T.; Hioki, H.; Inoue, Y.U.; Inoue, T.; Masuyama, N.; Obata, K.; Yanagawa, Y. Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development 2009, 136, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, E.D.; Rusanova, A.; Stelzer, J.; Hernaiz-Llorens, M.; Schroer, A.E.; Epifanova, E.; Bladt, F.; Isik, E.G.; Buchert, S.; Jia, S.; et al. Olig3 regulates early cerebellar development. eLife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Yellajoshyula, D.; Zhang, B.; Teets, B.; Rockweiler, N.; Kroll, K.L. Gene regulatory networks in neural cell fate acquisition from genome-wide chromatin association of Geminin and Zic1. Sci. Rep. 2016, 6, 37412. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, H.-S.; Moody, S.A. Neural transcription factors: From embryos to neural stem cells. Mol. Cells 2014, 37, 705. [Google Scholar] [CrossRef]
- Aruga, J.; Hatayama, M. Comparative genomics of the Zic family genes. In Zic Family; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–26. [Google Scholar]
- Merzdorf, C.S.; Sive, H.L. The zic1 gene is an activator of Wnt signaling. Int. J. Dev. Biol. 2004, 50, 611–617. [Google Scholar] [CrossRef]
- Merzdorf, C.; Forecki, J. Amphibian Zic Genes. In Zic Family; Springer: Berlin/Heidelberg, Germany, 2018; pp. 107–140. [Google Scholar]
- Litingtung, Y.; Dahn, R.D.; Li, Y.; Fallon, J.F.; Chiang, C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 2002, 418, 979–983. [Google Scholar] [CrossRef]
- Jahan, I.; Kersigo, J.; Elliott, K.L.; Fritzsch, B. Smoothened overexpression causes trochlear motoneurons to reroute and innervate ipsilateral eyes. Cell Tissue Res. 2021, 384, 59–72. [Google Scholar] [CrossRef]
- Glover, J.C.; Elliott, K.L.; Erives, A.; Chizhikov, V.V.; Fritzsch, B. Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Dev. Biol. 2018, 444, S14–S24. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Lindgren, A.G.; Chizhikov, V.V.; Johnson, R.L.; Millen, K.J. Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. J. Neurosci. 2009, 29, 11377–11384. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Xu, P.X. Eya2 expression during mouse embryonic development revealed by Eya2(lacZ) knockin reporter and homozygous mice show mild hearing loss. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2021. [Google Scholar] [CrossRef]
- Fritzsch, B.; Pauley, S.; Feng, F.; Matei, V.; Nichols, D. The molecular and developmental basis of the evolution of the vertebrate auditory system. Int. J. Comp. Psychol. 2006, 19, 1–25. [Google Scholar]
- Pan, N.; Jahan, I.; Lee, J.E.; Fritzsch, B. Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg (Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009, 337, 407–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.Y.; Rose, M.F.; Zoghbi, H.Y. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 2005, 48, 31–43. [Google Scholar] [CrossRef]
- Storm, R.; Cholewa-Waclaw, J.; Reuter, K.; Bröhl, D.; Sieber, M.; Treier, M.; Müller, T.; Birchmeier, C. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development 2009, 136, 295–305. [Google Scholar] [CrossRef]
- Imayoshi, I.; Kageyama, R. bHLH factors in self-renewal, multipotency, and fate choice of neural progenitor cells. Neuron 2014, 82, 9–23. [Google Scholar] [CrossRef]
- Jahan, I.; Pan, N.; Elliott, K.L.; Fritzsch, B. The quest for restoring hearing: Understanding ear development more completely. Bioessays 2015, 37, 1016–1027. [Google Scholar] [CrossRef]
- Kersigo, J.; Gu, L.; Xu, L.; Pan, N.; Vijayakuma, S.; Jones, T.; Shibata, S.B.; Fritzsch, B.; Hansen, M.R. Effects of Neurod1 Expression on Mouse and Human Schwannoma Cells. Laryngoscope 2021, 131, E259–E270. [Google Scholar] [CrossRef]
- Fritzsch, B.; Sonntag, R.; Dubuc, R.; Ohta, Y.; Grillner, S. Organization of the six motor nuclei innervating the ocular muscles in lamprey. J. Comp. Neurol. 1990, 294, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Murakami, Y.; Renaud, J.-S.; Pasqualetti, M.; Charnay, P.; Ren, S.-Y.; Rijli, F.M. Hoxa2-and rhombomere-dependent development of the mouse facial somatosensory map. Science 2006, 313, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef]
- Bermingham, N.A.; Hassan, B.A.; Price, S.D.; Vollrath, M.A.; Ben-Arie, N.; Eatock, R.A.; Bellen, H.J.; Lysakowski, A.; Zoghbi, H.Y. Math1: An essential gene for the generation of inner ear hair cells. Science 1999, 284, 1837–1841. [Google Scholar] [CrossRef]
- Maricich, S.M.; Xia, A.; Mathes, E.L.; Wang, V.Y.; Oghalai, J.S.; Fritzsch, B.; Zoghbi, H.Y. Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J. Neurosci. 2009, 29, 11123–11133. [Google Scholar] [CrossRef]
- Birol, O.; Ohyama, T.; Edlund, R.K.; Drakou, K.; Georgiades, P.; Groves, A.K. The mouse Foxi3 transcription factor is necessary for the development of posterior placodes. Dev. Biol. 2016, 409, 139–151. [Google Scholar] [CrossRef]
- Wright, T.J.; Mansour, S.L. Fgf3 and Fgf10 are required for mouse otic placode induction. Development 2003, 130, 3379–3390. [Google Scholar] [CrossRef]
- Urness, L.D.; Paxton, C.N.; Wang, X.; Schoenwolf, G.C.; Mansour, S.L. FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev. Biol. 2010, 340, 595–604. [Google Scholar] [CrossRef]
- Ahmed, M.; Xu, J.; Xu, P.-X. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 2012, 139, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Burton, Q.; Cole, L.K.; Mulheisen, M.; Chang, W.; Wu, D.K. The role of Pax2 in mouse inner ear development. Dev. Biol. 2004, 272, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Riccomagno, M.M.; Takada, S.; Epstein, D.J. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005, 19, 1612–1623. [Google Scholar] [CrossRef]
- Ohyama, T.; Basch, M.L.; Mishina, Y.; Lyons, K.M.; Segil, N.; Groves, A.K. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 15044–15051. [Google Scholar] [CrossRef]
- Chang, W.; Lin, Z.; Kulessa, H.; Hebert, J.; Hogan, B.L.; Wu, D.K. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008, 4, e1000050. [Google Scholar] [CrossRef]
- Munnamalai, V.; Fekete, D.M. Wnt signaling during cochlear development. Semin. Cell Dev. Biol. 2013, 24, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.D.; Rogers, A.A.M.; Zhang, J.; Shim, K. Cooperative and independent functions of FGF and Wnt signaling during early inner ear development. BMC Dev. Biol. 2015, 15, 1–15. [Google Scholar] [CrossRef]
- Sienknecht, U.J.; Fekete, D.M. Mapping of Wnt, frizzled, and Wnt inhibitor gene expression domains in the avian otic primordium. J. Comp. Neurol. 2009, 517, 751–764. [Google Scholar] [CrossRef]
- Tambalo, M.; Anwar, M.; Ahmed, M.; Streit, A. Enhancer activation by FGF signalling during otic induction. Dev. Biol. 2020, 457, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Fekete, D.M.; Wu, D.K. Revisiting cell fate specification in the inner ear. Curr. Opin. Neurobiol. 2002, 12, 35–42. [Google Scholar] [CrossRef]
- Groves, A.K.; Fekete, D.M. Shaping sound in space: The regulation of inner ear patterning. Development 2012, 139, 245–257. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Pelling, A.L.; Leung, K.K.; Tang, A.S.; Bell, D.M.; Tease, C.; Lovell-Badge, R.; Steel, K.P.; Cheah, K.S. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005, 434, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Filova, I.; Dvorakova, M.; Bohuslavova, R.; Pavlinek, A.; Elliott, K.L.; Vochyanova, S.; Fritzsch, B.; Pavlinkova, G. Combined Atoh1 and Neurod1 Deletion Reveals Autonomous Growth of Auditory Nerve Fibers. Mol. Neurobiol. 2020, 57, 5307–5323. [Google Scholar] [CrossRef]
- Hertzano, R.; Montcouquiol, M.; Rashi-Elkeles, S.; Elkon, R.; Yucel, R.; Frankel, W.N.; Rechavi, G.; Moroy, T.; Friedman, T.B.; Kelley, M.W.; et al. Transcription profiling of inner ears from Pou4f3(ddl/ddl) identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum. Mol. Genet. 2004, 13, 2143–2153. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Ramakrishnan, A.; Fritzsch, B.; Xu, J.; Wong, E.Y.M.; Loh, Y.E.; Ding, J.; Shen, L.; Xu, P.X. Dynamic changes in cis-regulatory occupancy by Six1 and its cooperative interactions with distinct cofactors drive lineage-specific gene expression programs during progressive differentiation of the auditory sensory epithelium. Nucleic Acids Res. 2020, 48, 2880–2896. [Google Scholar] [CrossRef]
- Wallis, D.; Hamblen, M.; Zhou, Y.; Venken, K.J.; Schumacher, A.; Grimes, H.L.; Zoghbi, H.Y.; Orkin, S.H.; Bellen, H.J. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development 2003, 130, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Jahan, I.; Bonde, G.; Sun, X.; Hildebrand, M.S.; Engelhardt, J.F.; Smith, R.J.; Cornell, R.A.; Fritzsch, B.; Bánfi, B. A mutation in the Srrm4 gene causes alternative splicing defects and deafness in the Bronx waltzer mouse. PLoS Genet. 2012, 8, e1002966. [Google Scholar] [CrossRef]
- Nakano, Y.; Wiechert, S.; Fritzsch, B.; Bánfi, B. Inhibition of a transcriptional repressor rescues hearing in a splicing factor–deficient mouse. Life Sci. Alliance 2020, 3, e202000841. [Google Scholar] [CrossRef] [PubMed]
- Bulfone, A.; Menguzzato, E.; Broccoli, V.; Marchitiello, A.; Gattuso, C.; Mariani, M.; Consalez, G.G.; Martinez, S.; Ballabio, A.; Banfi, S. Barhl1, a gene belonging to a new subfamily of mammalian homeobox genes, is expressed in migrating neurons of the CNS. Hum. Mol. Genet. 2000, 9, 1443–1452. [Google Scholar] [CrossRef]
- Chellappa, R.; Li, S.; Pauley, S.; Jahan, I.; Jin, K.; Xiang, M. Barhl1 regulatory sequences required for cell-specific gene expression and autoregulation in the inner ear and central nervous system. Mol. Cell. Biol. 2008, 28, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.R.; Leverenz, E.L.; Bialek, W.S. The Vertebrate Inner Ear; CRC Press: Boca Raton, FL, USA, 1985; p. 248. [Google Scholar]
- Lysakowski, A. Anatomy and microstrucctural organization of vestibular hair cells. In The Senses; Fritzsch, B., Straka, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 6, pp. 173–184. [Google Scholar]
- Fritzsch, B.; Elliott, K.L. Evolution and Development of the Inner Ear Efferent System: Transforming a Motor Neuron Population to Connect to the Most Unusual Motor Protein via Ancient Nicotinic Receptors. Front. Cell Neurosci. 2017, 11, 114. [Google Scholar] [CrossRef]
- Eatock, R.A.; Rusch, A.; Lysakowski, A.; Saeki, M. Hair cells in mammalian utricles. Otolaryngol. Head Neck Surg. 1998, 119, 172–181. [Google Scholar] [CrossRef]
- Platt, C.; Straka, H. Vestibular endorgans in vertebrates and adeqate sensory stimuli. In The Senses; Fritzsch, B., Straka, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 6, pp. 108–128. [Google Scholar]
- Curthoys, I.S. The anatomical and physiological basis of clinical tests of otolith function. A tribute to Yoshio Uchino. Front. Neurol. 2020, 11, 566895. [Google Scholar] [CrossRef]
- Elliott, K.L.; Fritzsch, B.; Duncan, J.S. Evolutionary and Developmental Biology Provide Insights Into the Regeneration of Organ of Corti Hair Cells. Front. Cell. Neurosci. 2018, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J. How the ear’s works work. Nature 1989, 341, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Géléoc, G.S.; Asai, Y.; Horwitz, G.C.; Kurima, K.; Ishikawa, K.; Kawashima, Y.; Griffith, A.J.; Holt, J.R. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 2013, 79, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.S.; Stoller, M.L.; Francl, A.F.; Tissir, F.; Devenport, D.; Deans, M.R. Celsr1 coordinates the planar polarity of vestibular hair cells during inner ear development. Dev. Biol 2017, 423, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ratzan, E.M.; Moon, A.M.; Deans, M.R. Fgf8 genetic labeling reveals the early specification of vestibular hair cell type in mouse utricle. Development 2020, 147. [Google Scholar] [CrossRef]
- Ketchum, E.M.; Sheltz-Kempf, S.N.; Duncan, J.S. Molecular basis of vestibular organ formation during ontogeny. In The Senses; Fritzsch, B., Straka, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 6, pp. 129–144. [Google Scholar]
- Deans, M.R. A balance of form and function: Planar polarity and development of the vestibular maculae. Semin. Cell Dev. Biol. 2013, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Keller, J.; Ramírez, O.L.; Garrido, A.G.; Zobeiri, O.A.; Chang, H.H.V.; Vijayakumar, S.; Ayiotis, A.; Duester, G.; Della Santina, C.C. Retinoic acid degradation shapes zonal development of vestibular organs and sensitivity to transient linear accelerations. Nat. Commun. 2020, 11, 63. [Google Scholar] [CrossRef]
- Balmer, T.S.; Trussell, L.O. Selective targeting of unipolar brush cell subtypes by cerebellar mossy fibers. eLife 2019, 8, e44964. [Google Scholar] [CrossRef]
- Bleckmann, H. Central lateral line pathways and central integration of lateral line information. In The Senses; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 163–184. [Google Scholar]
- Nicolson, T. The genetics of hair-cell function in zebrafish. J. Neurogenet. 2017, 31, 102–112. [Google Scholar] [CrossRef]
- Krey, J.F.; Chatterjee, P.; Dumont, R.A.; O’Sullivan, M.; Choi, D.; Bird, J.E.; Barr-Gillespie, P.G. Mechanotransduction-dependent control of stereocilia dimensions and row identity in inner hair cells. Curr. Biol. 2020, 30, 442–454.e7. [Google Scholar] [CrossRef]
- Vischer, H.A. The development of lateral-line receptors in Eigenmannia (Teleostei, Gymnotiformes). I. The mechanoreceptive lateral-line system (Part 1 of 2). Brain Behav. Evol. 1989, 33, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Martí, J.; Zecca, A.; Baudoin, J.P.; Faucherre, A.; Asakawa, K.; Kawakami, K.; López-Schier, H. Neuronal birth order identifies a dimorphic sensorineural map. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 2976–2987. [Google Scholar] [CrossRef]
- Fattal, D.; Hansen, M.; Fritzsch, B. Aging-related balance impairment and hearing loss. In The Wiley Handbook on the Aging Mind and Brain; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 315–336. [Google Scholar]
- Engelmann, J.; Fritzsch, B. Lateral line input to ‘almost’ all vertebrates shares a common organization with different distinct connections. In The Evolution Of Sensory System Revealed: Gene Regulation, Cellular Networks And Processes; Fritzsch, B., Elliott, K.L., Hall, B., Moody, S., Eds.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Jacobo, A.; Dasgupta, A.; Erzberger, A.; Siletti, K.; Hudspeth, A.J. Notch-Mediated Determination of Hair-Bundle Polarity in Mechanosensory Hair Cells of the Zebrafish Lateral Line. Curr. Biol. 2019, 29, 3579–3587.e7. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.R.; Warrier, S.; Jiang, T.; Wu, D.K.; Kindt, K.S. Directional selectivity of afferent neurons in zebrafish neuromasts is regulated by Emx2 in presynaptic hair cells. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Ji, Y.R.; Martin, D.; Wu, D.K. Emx2 regulates hair cell rearrangement but not positional identity within neuromasts. eLife 2020, 9, e60432. [Google Scholar] [CrossRef]
- Tarchini, B.; Jolicoeur, C.; Cayouette, M. A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Dev. Cell 2013, 27, 88–102. [Google Scholar] [CrossRef]
- Kimura, J.; Suda, Y.; Kurokawa, D.; Hossain, Z.M.; Nakamura, M.; Takahashi, M.; Hara, A.; Aizawa, S. Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5097–5108. [Google Scholar] [CrossRef]
- Holley, M.; Rhodes, C.; Kneebone, A.; Herde, M.K.; Fleming, M.; Steel, K.P. Emx2 and early hair cell development in the mouse inner ear. Dev. Biol. 2010, 340, 547–556. [Google Scholar] [CrossRef]
- Bellono, N.W.; Leitch, D.B.; Julius, D. Molecular tuning of electroreception in sharks and skates. Nature 2018, 558, 122–126. [Google Scholar] [CrossRef]
- Simmons, D.; Duncan, J.; de Caprona, D.C.; Fritzsch, B. Development of the inner ear efferent system. In Auditory and Vestibular Efferents; Springer: Berlin/Heidelberg, Germany, 2011; pp. 187–216. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritzsch, B. An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception. Diversity 2021, 13, 364. https://doi.org/10.3390/d13080364
Fritzsch B. An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception. Diversity. 2021; 13(8):364. https://doi.org/10.3390/d13080364
Chicago/Turabian StyleFritzsch, Bernd. 2021. "An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception" Diversity 13, no. 8: 364. https://doi.org/10.3390/d13080364
APA StyleFritzsch, B. (2021). An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception. Diversity, 13(8), 364. https://doi.org/10.3390/d13080364





