Abstract
Domesticated Cucurbita has been remarked as one of the plant genera with the highest diversity in color, shape and fruit dimensions. Their economic and cultural values are related to the consumption of the mature or immature fruits, seeds, flowers, and to the use as decoration. The wild ancestor of C. maxima, the ssp. andreana has an actual scattered and disjointed distribution, associated with megafauna seed disperser syndrome. It was domesticated in South America around 9000–7000 years BP. The cultivar-group is a subspecific category for assembling cultivars on the basis of defined similarity. The work describes and pictures nine cultivar-groups for the species, Banana, Turban, Hubbard, Show, Buttercup, Zapallito, Plomo, Zipinka and Nugget. The molecular and a morphological join data analysis scatter biplot showed Turban and Buttercup in a central position, suggesting a first step in the domestication pathway associated with seed and immature fruit consumption; afterward, bigger bearing fruits groups were selected for their mature fruit flesh quality on one hand, and bush type, short day induction and temperate climate adaptation on the other hand. The striking domesticated Brazilian accession MAX24 intermediate between cultigens and ssp. andreana strengthens, in concordance with archeological remains, the possible domestication place of the species more easternward than previously believed.
1. Cucurbita
1.1. Extant Wild Species
Cucurbita (2n = 20) is a well-defined New World genus within the tribe Cucurbitae [1]. Wild species are herbaceous, multi-branched, procumbent or climbing vines. The stems are slender, and the leaves are arranged on them alternately [1]. Plants are monoecious and predominantly staminate, with large yellow-orange (rarely pale yellow) flowers pollinated in early morning by solitary bees of the genera Poponapis and Xenoglossa [2]. Fruits are small (5–10 cm long), round, indehiscent, with a lignified rind, thin fibrous, light-colored or pale usually bitter flesh and numerous, relatively small seeds [3]. Around twelve wild species are generally recognized; six belong to the perennial, xerophytic group, Cucurbita cordata Watson, C. digitata Gray, C. foetidissima Kunth, C. palmata Watson, C. pedatifolia Bailey and C. radicans Naudin. This group has a distribution range from southern-western United States and Baja California to northern-central and southern Mexico [4]. The annual, mesophytic group comprises Cucurbita argyrosperma Huber ssp. sororia Merrick & Bates, C. lundelliana Bailey, C. okeechobeensis Bailey ssp. martinezii Andres and Nabhan and ssp. okeechobeensis, C. pepo L. ssp. fraterna Lira et al., ssp. ovifera Decker var. ozarkana Decker and var. texana Filov, C. ecuadorensis Cutler and Whitaker and C. maxima Duchesne ssp. andreana Filov. For this group, the distribution is more scattered and disjointed, ranging from central United States, Florida, Texas, the pacific and gulf coast of Mexico, southern Mexico, northern Central America, Ecuador, Peru, Bolivia, northwestern and central Argentina and Uruguay [4,5]. As it is evident, the actual Mexico territory is the center of diversity of wild Cucurbita species.
1.2. Seed Dispersal
In the majority of the botanical and ecological descriptions of the wild Cucurbita species, the seed dispersal system is overlooked. One of the reasons is that until the Newson and Mihlbachler [6] finding of abundant wild Cucurbita seeds in mastodon dung at the Page-Ladson archeological site, no clear evidence for a zoochory mechanism was recorded. This confirmed the previous hypothesis of Barlow [7] that C. foetidissima was a clear example of the megafauna (>1000 kg) anachronisms syndrome, proposed by Jansen and Martin [8]. The mass megafauna extinction around 12,000 years ago left wild Cucurbita species stranded as anachronisms in a post-megafauna world, producing fruit that few or no animals eat, with seeds that go undispersed and orphans of mutualistic partners that shaped a mosaic-like landscapes, offering an abundance of disturbed habitat in a niche-diverse ecosystem suitable for climbing vines plants as Cucurbita [9,10]. This is crucial to understand the actual distribution of these species that relied, since the beginning of the Holocene, on alternative seed dispersals as buoyancy of fruits along streams, gravity, seed predators and eventually anthropogenic activity. This accounted for a shrinking and fragmentation in the range, and even that some species could have gone extinct. As an example, C. okeechobeensis has an allopatric distribution in Mexico and Florida, and in the latter site, the population was restricted to the endoreic basin of the Lake Okeechobee. Moreover, C. maxima ssp. andreana shows patchy distribution in small specific populations in Peru and Bolivia [11], and in Argentina is associated with disturbed roadsides, agriculture field fences, fallows and field drainages [12] (Figure 1).

Figure 1.
Fruits of C. maxima ssp. andreana in an agriculture field wire fence at Zavalla, Santa Fe province, Argentina. Lack of fruits and seeds’ dispersal is apparent; dry light brown ones are from the previous growing season, yellow-green ones from the current year.
1.3. Anthropogenic Seed Dispersal
It has been pointed out that bottle gourd (Lagenaria siceraria, Cucurbitaceae) was carried as a utilitarian already domesticated (mutualism) species through Beringia into the new world and was thereafter involved and reliant on humans [13]. In this sense, we can hypothesize that Paleoindians as gatherers recognized the smaller gourds of wild Cucurbita with a potential use and started a virtuous circle in which the gourds, that were maladaptive of seed dispersers, as they became extinct, found in humans a proxy, already familiarized with gourds, that also provided disturbed niches for their climbing growth. Paleoindians by their side were benefited by the use of gourds as buoys, rattles, small containers and by the consumption of their protein and oil-rich seeds. This anthropogenic seed dispersal mechanism was envisioned as a starting point of domestication in many plant taxa including Cucurbita [14].
1.4. Domestication
Over the last decade, the idea that domestication was a rapid process (Holocene revolution) began to be seriously questioned. New insights into the pace of domestication arose from archaeobotanical studies which show the time it takes for domestication phenotypes to arise and fix in evolving domesticated species is longer than previously thought [15]. One of the reasons of this protracted time could be that many of the crop domestication traits have a polygenic basis, with multiple genes of small effect that could take many generations to be fixed in a population, such as seed size enlargement and fruit size. Another reason is that much of domestication was not, as generally believed, carried out by deliberate, strong selection, but rather is the result of unconscious (natural) selection of incipient species as they were cultivated in the novel, managed environments of Neolithic agricultural fields [15]. This accounts for traits as seed non-dormancy and synchronous germination, but also seed size; larger seed size may be adaptive in several ways, improving competition in early growth patches and survival of the seedlings [16]. One peculiar character that was selected in parallel in three Cucurbita taxa whose fruits are consumed in the immature state is the short internode phenotype that rendered bushy plants, with precocious anthesis and an augmented number of fruits per plant [17,18,19]. Other traits such as fruit and pulp color and possibly taste clearly were driven by conscious human choice [20].
1.5. Cucurbita in South America
Around 3 million years ago, the Isthmus of Panama rose to the actual level connecting the previous isolated Americas and starting what is known as the Great American Biotic Interchange [21]. South America’s newcomer mammals as seed dispersers were involved in the distribution of new plant genera as Cucurbita. Gomphotheres (Proboscidea) is one of the families that colonized and radiated southerly along two corridors; one along the Andes (Cuvieronius hyodon and Stegomastodon waringi); the other route was along the eastern coastal area southward down to the pampas (Stegomastodon platensis) [22]. We can infer that wild Cucurbita spread and speciation in South America were related to these Proboscidae, and the actual distribution and species survival, as mentioned above, was affected by their extinction.
1.6. Natural Cucurbita Pollinators in South America
The bees of the Peponapis genus, specialized in Cucurbita species, have a Neotropical distribution with the center of diversity located in Mexico. Peponapis fervens is the only species of the genus known from southern South America [23]. Using ecological niche modeling, the potential geographical areas of this species were determined and found to extend north beyond the latitude of 20° S, including some areas of northeastern and central western Brazil, northern Argentina and Bolivia, which is much larger than the actual distribution range of ssp. andreana [23].
1.7. Domesticated Cucurbita Species
Domesticated Cucurbita has been remarked as one of the plant genera with the highest diversity in color, shape and fruit dimensions. Five species in decreasing order of importance are now cultivated worldwide, i.e., C. pepo; C. moschata; C. maxima; C. argyrosperma and C. ficifolia; and, in production as a whole, they are positioned among the ten most important world vegetables crops [24]. Their economic and cultural values are related to the consumption of the mature or immature fruits, seeds, flowers, and their use as decoration. Nutritionally, they contribute with fibers, minerals, vitamins and carotenes, and have been, along with maize and beans, the base of many pre-Columbian cultures’ diets in the Americas [25]. As mentioned, it has been suggested that the genus was originally gathered for their gourds and consumption of their nutritious and palatable seeds [2,3]. The cultivated species are all annually herbaceous, with a great adaptation to diverse soils and climatic conditions, being grown from the tropic (C. moschata) to temperate-cold zones (C. maxima), and from sea level up to 2800 m.a.s.l. (C. ficifolia) [26]. The current genetic, biogeographical and archaeological data suggest that the cultivated forms are not derived from a common ancestor, whereas each species represents an independent domestication event [27] (Figure 2). Except for C. moschata and C. ficifolia, the wild species from which they were domesticated have been identified [5,27,28,29].
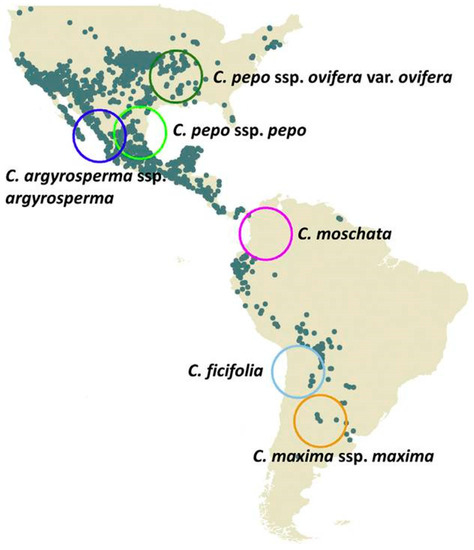
Figure 2.
Distribution map of wild species of Cucurbita with occurrences based on 7000 records from GBIF (www.gbif.org, accessed on 15 November 2020). Hypothesized origins of domestication for the five Cucurbita species are indicated by open circles. C pepo was split into the two cultivated spp. C. pepo ssp. ovifera: dark green; C. pepo ssp. pepo: light green; C. argyrosperma ssp. argyrosperma: blue; C. moschata: magenta; C. ficifolia: light blue; C. maxima ssp. maxima: orange [30]. Copyright 2021 Kates, Lopez-Anido, Sánchez-de la Vega, Eguiarte, Soltis and Soltis.
In C. pepo, independent domestication events were proposed for the two cultivated subspecies (texana and pepo). The first one, in the southwestern USA related to the wild ssp. texana var. ozarkana; the second, in Mexico, associated with actual cultivated ornamental gourds [28,31], or to wild populations of C. pepo ssp. fraterna [27,32]. In C. argyrosperma, the wild ssp. sororia is recognized as the ancestor of cultivated forms, presumably domesticated in the lowlands of Jalisco, Mexico [29]. In C. moschata, even when no wild progenitor was found, it is believed that it could have been domesticated either in northern South America, where domesticated forms of small fruits and thick fruit shells were described, or in Mexico, where the greatest diversity of cultivated forms occurs [33]. The only domesticated species of Cucurbita with an exclusively South American origin is C. maxima; there is no record that places this species north of the equator before the Spanish colonization of the Americas [3]. It had a great importance in the pre-Inca and Inca civilizations (Peru, Bolivia and northwestern Argentina) [34,35] where, due to its nutritional quality and adaptation to a temperate climate, it gradually replaced C. moschata and C. ficifolia [24]. C. maxima was also cultivated by Arawak, Guarani, Qom and Charrua ethnics in the southeastern Amazonas river basin (including northern Bolivia and southeastern Brazil), Paraguay, northeastern Argentina and the La Plata river basin (eastern Argentina and Uruguay) [17,36,37,38,39]. It was domesticated from the free living ssp. andreana supposedly in north-central Argentina and Bolivia [2,17,40].
2. Cucurbita Archeobotanics
2.1. South American Archeological Sites
In South America the longest lists of crops domesticated origins are from areas where good conditions exist for archaeological artifact preservation and where complex societies with high population densities and advanced agricultural technologies existed (Inca and pre-Inca civilizations in actual Peru, Bolivia, Northern Argentina) [41]. In Peru, on the western slopes of the northern Andes, the earliest macrofossil remains of the Cucurbita sp. were dated 8435 ± 40 B.P. These are small seeds (6 to 7 mm long, 2.5 to 4 mm wide) of uniform dark brown color, prominent raised margins, and an elliptical shape [42]. These authors attributed these remains to C. moschata since a similar brown color and size seeds were reported in Colombian landraces. However, C. maxima cannot be ruled out, since seed brown color cultivars are also common, as I will show below, in the Banana cultivar-group. In Amazonia, areas with higher population density in the pre-Columbian period also exhibited a rich crop genetic heritage, but the poor environments for archaeological preservation and lack of research effort have not yielded as much information [43]. The Amazon river basin has been highlighted as an important area of plant domestication and diversity associated with the swidden/fallow management, micro-environmental heterogeneity and small population sizes (genetic drift); moreover, the Beni savanna, which extends from northern Bolivia to Brazil and Peru, was proposed as a center of diversity for C. maxima and C. moschata [44]. In Cucurbita, phytoliths (microscopic structures made of silica persistent after plant decay) have been studied and evidence proven that domesticated forms were present on the northern South American lowlands’ landscape and tropical forest by at least 7000, and quite possibly by 9000 BP. The identification of these microremains was referenced to the genus level (not species), and it was suggested that they could correspond either to C. moschata or C. maxima [45]. More recently Watling [38] found phytoliths of cultivated Cucurbita and possible wild Cucurbita at the Teotoni site (southwestern Amazonia), on the Madeira river, which its headwater comes from the Bolivian central Andes. The authors suggested that they may correspond to C. maxima, since as Whitaker and Cutler [3] stated, “The cultural history of the cucurbits indicates a very strong tendency towards conservative crop husbandry among pre-Columbian and post-Columbian peoples in that they tended to grow essentially the same cultivars over long periods of time”, and nowadays, C. maxima is most cultivated in the Brazilian Rodonia State (where the site is located). In northeastern Argentina, well-preserved seeds, rinds, peduncles and even entire fruits remains of domesticated and intermediate (semidomesticated) C. maxima were found in many sites, dated up to 2700 BP [34,46]. In the La Plata river basin lowlands, small-scale horticultural practices including Cucurbita were recorded in mounds distributed either in south-eastern Argentina and Uruguay but in a more recently time frame (2000 BP) associated with Guarani and possible Arawak cultures, both descendants from populations that originated in the amazon basin [39].
2.2. Diagnostic Macroremains
The existence of a domestication relationship between ancient human societies and plant species can be inferred from morphological changes in the archaeological record of target plant populations [47]. In Cucurbita, the major archeological morphological diagnostic markers associated with domestication environments are an increase in seed, fruit and peduncle size and color changes in relation to the wild ancestor counterpart. In C. maxima, peduncles and seeds were proposed to be the best diagnostic archeological macrorest to identify Cucurbita spp., and the increase in their diameter and length, respectively, a good indicator of changes occurring under domestication [46]. C. maxima seeds are flat, oval and with no pronounced marginal bulge; ssp. maxima (domesticated) can be white or brown; ssp. andreana is always light brown. Seeds between 13 and 30 mm long and 7–17 mm wide correspond to cultivated ssp. maxima, while seeds between 6.5 and 10 mm long and 4–6.5 mm wide to ssp. andreana. The peduncles of ssp. maxima can be distinguished from those of ssp. andreana by their basal diameter. Diameters less than, or equal to, 8 mm belong to the wild ancestor; meanwhile, diameters greater than or equal to 17 mm, to the domestic ssp. [46]. Fruit rind remains of both bottle gourds (L. siceraria) and Cucurbita are very commons in archeological sites [34]. In the case of Lagenaria, a substantial increase in rind thickness is associated with domesticated forms (fruit durability as containers) in contrast to the brittle and rapidly disintegrating exocarp of wild African counterparts [13]. Interestingly in the case of C. maxima, no clear association between rind thickness and cultivated remains was found [48]; this could be explained by the fact that cultivated forms for immature fruit consumption (summer squash) generally kept the thick exocarp of the wild ssp. along domestication, and the same was observed in C. pepo [49].
3. Cultivar-Groups
The cultivar-group concept is a formal subspecific category for assembling cultivars on the basis of defined similarity [50]. In C. pepo, eight Cultivar-groups were proposed on the basis of fruit shape and length/width ratio; four belong to ssp. texana (Straightneck, Crookneck, Scallop and Acorn), and the remaining to ssp. pepo (Vegetable Marrow, Zucchini, Cocozelle and Pumpkin) [51]. In general, each cultivar-group is related to a distinct origin, i.e., Cocozelle appeared in Italy, where there is a preference for long immature fruits; Zucchini emerged at the USA market in the beginning of the last century, presumably derived from Cocozelle; Vegetable Marrow varieties, of a short tapered cylindrical fruit, were first developed in England in the 19th century; and the Acorn forms were raised by the Arikara Indians of North Dakota [52].
In C. moschata, Jeffrey [53] recognized that there is no wholly satisfactory subspecific classification, and, mainly based on geographical origin, proposed six cultivar-groups. Out of them, two assemblages of cultivars could be distinguished, the Caribbean-Colombian, related to short-day flowering requirements and in general brown seeds; and the Mexican, of long-day induction and light tan-colored seeds [54].
In C. maxima, a great variation of cultivated forms exists, and many attempts were conducted to classify them. Castetter [55], based in the cultivars of ample distribution among North American growers, proposed the first grouping of seven horticultural groups (Hubbard, Delicious, Orange Marrows, Turban, Banana, Warty or Pebbled, Show or Display); however, the Zapallito type cultivars, of recognized importance as summer squash in the southern Latin American countries, were not included. Later, Grebencikov [56] considered a subspecific classification of six convarieties (Turbaniformis, Bananina, Hubbardiana, Maxima, Parvifructina and Zapallitina), which included the Zapallito type, but is not comprehensive regarding the cultivars forms that emerged in most seed catalogues since the last 60 years. Among these are the Nugget and Buttercup types in North America, New Zealand and Japan, and the Plomo and Moranga varieties in Argentina and Brazil. Moreover, the Zipinca type, very popular in Northwestern Argentina local markets [36], was not considered. More recently, Goldman [57] presented a very nice pictured list of C. maxima cultivars, grouping them in Australian, Hubbard, Buttercup, Banana, Turban, Mammoth and Zapallito cultivar-groups. In the following, I will describe a comprehensive list, in which I merge previously separated groups and include new ones that deserve consideration.
3.1. Hubbard
The Hubbard group or convariety Hubbardiana is characterized by its elliptical-acorn or ovate fruit shape, tapering curved to one or both ends, of medium to large size (2–6 kg). Rind color varies from green, blue-gray, orange to cream-white, with or without longitudinal stripes (Figure 3). The rind surface is uneven to warted (generally not smooth). Flesh is orange-yellow with a good aptitude for mature fruits consumption. Plants show a viney growth habit; some new cultivars may be intermediate in internode length, as a semi-bush type and very few show mottled leaves. The Hubbard name was given in 1820 after a washerwomen of a sea captain who brought the seed from the West Indies [58]. I agree with [57] in including in this group the Delicious and Pebbled groups of [55].

Figure 3.
Mature fruits from different accessions of C. maxima Hubbard Cultivar-group. Public domain photos, Germplasm Resources Information Network (GRIN), USA.
3.2. Turban
The Turban group or convariety Turbaniformis is particular for its bizarre mature fruits where the pericarp does not enclose completely the fruit, leaving stylar tissue to come out as if one fruit was growing inside the other one (Figure 4). Fruits are generally medium to small in size, varying in rind color, with orange very frequent. Flesh may be light orange, yellow to cream, and some cultivars are not so good for mature consumption, rather sold for ornamental purpose. Plants have a viney growth habit with somehow smaller leaves than the rest of the cultivar-groups, and long staminate flowers’ pedicels that make male flowers protrude over the foliage at anthesis [59]. Seeds are white; however, some accessions are segregating for a brown seed coat, since its inheritance is complex, ruled by two loci with a dominant and recessive epistasis [60]. A distinguishing characteristic is that the ratio length to width of the seeds is the shortest of all cultivar-groups, ranging 1.4–1.5 [59]. The first known illustration of a Turban fruits was drew by Nicolas Duchesne in 1785 [61].

Figure 4.
Mature fruits from different accessions of Turban cultivar-group. Blue ruler length 20 cm. Sliced fruits, left light color soft flesh, right more intense color and firm flesh.
3.3. Banana
The Banana group or convariety Bananina is characterized by its elongated medium size mature fruits, with a length to width ratio from 2.3 to 3.1 [59] (Figure 5), similar to the ratio of the Vegetable Marrow cultivar-group of C. pepo [51]. Fruits have generally three stylar locules (mean 3.43). Mature fruit rind color varies as in the Hubbard group, but the pink is much more frequent. The flesh color is orange with a good aptitude for mature fruits consumption (winter squash). The presence of a protuberance in the stylar end (nipple) and thick brown seeds are distinctive of the group [59]. Plants are of the viney type and leaves not mottled.

Figure 5.
Mature fruits from different accessions of C. maxima Banana cultivar-group. Blue ruler 20 cm. Sliced fruit bearing three locules.
3.4. Show
The Show group also referenced as Display or Mammoth has been characterized by its big globular mature fruits, long been known to produce the largest fruit in the plant kingdom (Figure 6). Interest in large pumpkins derives from exhibits in agricultural fairs either in North American or European rural life since the last 150 years [62]. Rind color is generally orange, yellow or cream. Flesh color is light orange, yellow or even cream, with poor quality for consumption as winter squash, flesh dry matter between 4.3% and 5.1% [59]. Seeds’ length to width ratio of this group is above 1.8, being the highest of all C. maxima cultivar-groups; similar results were found for the globular Pumpkin cultivar-group of C. pepo [63]. Plants are viney; some cultivars bear yellow stems [64], and mottled leaves are absent.
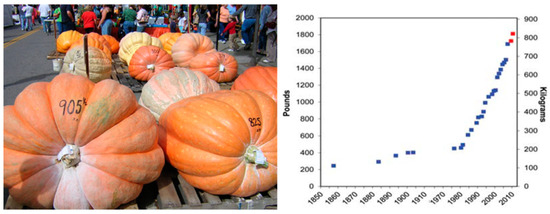
Figure 6.
Right, fruits of Show cultivar-group at Barnesville Pumpkin Festival in Barnesville, Ohio, USA, picture credit Lee Paxton (https://en.wikipedia.org/wiki/User:Leepaxton, accessed on 15 June 2021). Left, world records of pumpkin fruit weight [65].
3.5. Buttercup
The Buttercup cultivar-group is characterized by its medium-small size (1.6–1.7 kg), flattened transverse broad elliptic-rectangular shape. Rind color is dark green, with or without light green stripes. Pericarp may incompletely encompass the fruit showing a small crown in the stylar end (Figure 7). Flesh is orange with a good quality for mature consumption, sweetness and nutty flavor [66]. This group is very popular in New Zealand and Japan (known as Kabocha). Plants are semi-bush with an intermediate internode length, and mottled leaves absent.

Figure 7.
Fruits of Buttercup cultivar-group, left unripe fruit in plant, right, mature fruits, one upside-down showing the crown. Blue ruler 20 cm.
3.6. Nugget
The Nugget is a relatively new group, commonly sold nowadays in United States vegetable seeds companies. The precocious bicolor fruit attribute is characteristic of the group, which gives a bright yellow color when immature, turning to orange when ripe (Figure 8). Fruits are flat-globular in shape and intermediate to small in size, same as Buttercup. Flesh is orange, suitable for mature consumption as winter squash. The presence of a nipple as in the Banana cultivar-group is very frequent. Plants are semi-bush, a characteristic also pursued in modern C. pepo and C. moschata cultivars for its amenability to high-density planting [24]. Mottled leaves in the foliage are absent. Goldman [57] included the nugget type in the zapallito group, but as I show below they are quite different.

Figure 8.
Upper row, mature entire and sliced fruits of Nugget cultivar-group. Blue ruler 20 cm. Lower row, typical semi-bush plant bearing immature fruits.
3.7. Zapallito
Zapallo is the Quechua language word referring to pumpkin. Zapallito is the Spanish diminutive form of Zapallo (small pumpkin). The Zapallito cultivar-group or convariety Zapallitina is only grown for the consumption of the immature fruits as summer squash (Figure 9). The market bright light green color size fruit is reached, depending on air temperature, between six up to ten days after anthesis (daa); it takes longer than C. pepo summer squash that generally are harvested between 2 and 3 daa. This could be due to the smaller ovary of pistillate flowers at anthesis of C. maxima in comparison to C. pepo. Mature fruits are flattened, with a length to width ratio ranging 0.6–0.7; rinds are dark green turning brown, and may show a small crown in the stylar end, same as in the Buttercup cultivar-group. Mature fruit flesh is light orange, yellow or cream, with very poor quality for consumption as winter squash (flesh dry matter 4.9–5.5%). In fact, mature fruits have a very short shelf-life. Plants are bush type, with the shortest internode length of all cultivar-groups (11–20 mm), and mottled leaves very rare. Seeds are white. I consider that varieties included as Zapallito by Goldman [57] are not accurately assigned; one is a Nugget type and the other (SilverBell) is a small Hubbard type.

Figure 9.
Upper row, Zapallito cultivar-group bush plant at anthesis and bucket at harvest. Lower row, entire and sliced mature fruits bearing typical thin spongy light colored flesh. Blue ruler length 20 cm.
3.8. Plomo
This cultivar-group is characterized by medium-large globular or flatted grey-blue lobed fruits (Figure 10). Plomo in Spanish means lead, for the particular grey color. In Brazil, they are known as “Abóbora gaúcha” [67]. In Spain, they are called Valencia’s squash. I consider that it belongs to the Plomo cultivar-group, the Australian-blue group of [57]. In Italy, the popular variety Marina di Chioggia is also representative of this cultivar-group and was depicted in eighteenth century drawings [61]. Fruits are excellent as winter squash owing to their meaty and deep orange flesh. In Australia and South Africa, many improved cultivars have been developed, some of them with virus resistance transferred from C. ecuadorensis along backcrosses and selfings [68]. Plants are viney, and mottled leaves can be found in some cultivars. Seeds could be either brown or white.

Figure 10.
Fruits of Plomo cultivar-group. Lower row, left globular, right flattened type. Blue ruler length 20 cm.
3.9. Moranga
C. maxima cultivars grown in Brazil have rarely been included in descriptions. Moranga means squash in Portuguese, and the characteristic of this cultivar-group deserves, in my opinion, its inclusion. Fruits are orange with light colored stripes, medium to large size (3.7–5.3 kg), flattened, with marked lobes and between 4–5 locules (Figure 11). Flesh is light orange, with an aptitude for mature consumption. Plants are viney, and leaves non-mottled. Seeds can be brown or white [59].

Figure 11.
Fruits of Moranga cultivar-group, lower row, sliced fruit showing five locules. Blue ruler length 20 cm.
3.10. Zipinka
The word Zipinka comes from the Quechua language Sip’u that means warts. Zipinka cultivar-group fruits are acorn shaped, small (0.7–1.3 kg), with abundant warts when ripe; color varies from brown, dark green to grey (Figure 12). It is distinguishable by their hard thick rind (2.6–3.5 mm), by far the thickest rinds of all cultivar-groups including the wild ssp. andreana [59]. The orange flesh is meaty but thin, with a pretty high dry matter (9.6–9.8%). Plants are semi-bush with an internode length between 33 and 67 mm, and mottled leaves are quite frequent. Another particular characteristic of this cultivar-group is the amount of seeds produced, between 8200 and 9900 seeds per m2. Whitaker and Cutler [3] argued that in Cucurbita, this attribute was related to primitive varieties, originally selected for the consumption of both flesh and seeds. Millán [17] included Zipinka in the Zapallito group, but even when the fruits are small, fruits and plant habit are quite different.

Figure 12.
Fruits of Zipinka cultivar-group. Lower row, left, sliced mature fruit; right, seeds and flesh were removed to show thick rind. Blue ruler length 20 cm.
4. Diversity and Domestication Pathways
In C. maxima, a couple studies have been conducted in order to elucidate genetic relationships among cultivars or accessions, but neither was comprehensive to include as many cultivar-groups as described above nor was the ssp. andreana considered. Ferriol [69] studied fifty landraces mostly collected in Spain, and nine from South American origin. They used SRAP (sequenced related amplified polymorphism) and AFLP (amplified fragment length polymorphism) markers, obtaining 50 and 145 polymorphic fragments, respectively. Principal coordinate analysis (PCoA) were advanced, where SRAP markers differentiated accessions in relation to fruit attributes (flesh color and shape), those for cattle feeding (globular with light colored flesh), human consumption (flattened to globular with orange flesh) and turban type. Meanwhile AFLP separated mainly in relation to provenances (America vs. Spain), and then, within the Spanish, human consumption from cattle feeding (Figure 13).
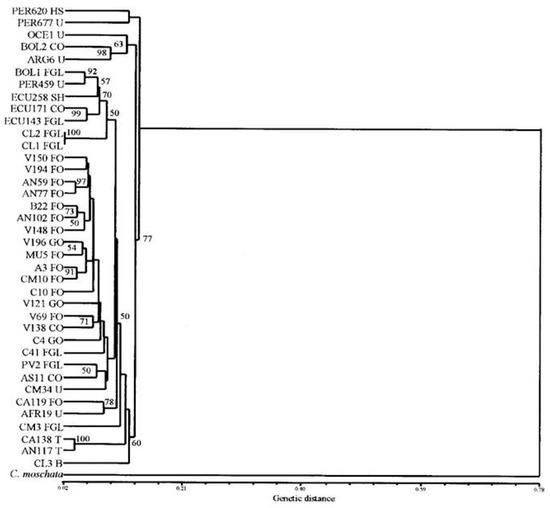
Figure 13.
Relationships among 38 accessions of C. maxima using AFLP markers based on Dice distance. Bootstrap values are indicated. Entry codes beginning with PER, ARG, BOL, ECU, OCE and AFR are from Peru, Argentina, Bolivia, Ecuador, New Zealand and Morocco, respectively. The rest of the entries are from Spain. Adapted from [69].
Kazminska [70] surveyed 85 C. maxima cultivated accessions from Europe, North America, Asia, Australia and New Zealand, which include representatives of six cultivar-groups. They used 23 simple sequence repeat markers (SSR) that generated 99 alleles. Cluster analysis was advanced in which one major cluster comprised accessions from Banana, Buttercup and Hubbard cultivars-groups. The second major cluster was divided in two sub-clusters, one of which included mostly cultivars from the USA and western Europe of the Show cultivar-group, and the second comprised old cultivars from central and eastern Europe not assigned with any cultivar-group. A third minor cluster was distinguished, more outlying, that involved two accessions of the Australian Blue cultivar-group (Plomo), one representative of the Turban cultivar-group and a Ukrainian provenance. The mean distance among the 85 accessions of C. maxima was 0.56. PCoA was advanced only for the modern cultivars, and divided the accessions into three groups [70] (Figure 14). One included accessions from the Banana, Hubbard and Buttercup cultivar-groups. Another comprised five entries from the Show cultivar-group, and the third consisted of three cultivars from the Australian Blue cultivar-group (Plomo) [70].
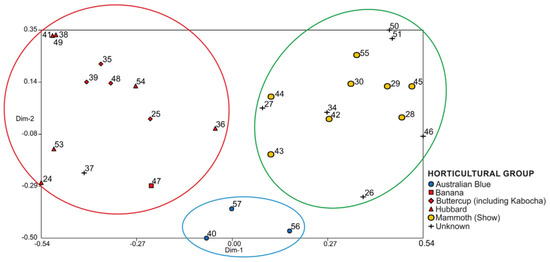
Figure 14.
PcoA grouping results obtained with the distance matrix for C. maxima cultivars; color circles indicate three clusters of cultivars [70]. Cultivar-groups after [57]. Copyright 2017 Elsevier.
I conducted a study including 159 entries of C. maxima covering entries from all cultivar-groups above described with wild ssp. andreana provenances and out-groups from C. ecuadorensis, C. moschata, C. ficifolia and cultivated C. argyrosperma [59]. The evaluation was conducted along two seasons for morphological descriptors of leaf, stem, flower, fruit, seed [71,72]. In a subset of 78 accessions, a molecular analysis was conducted along SRAP markers, which yielded 208 polymorphic bands. In Figure 15 is presented the hierarchical cluster analysis dendrogram.
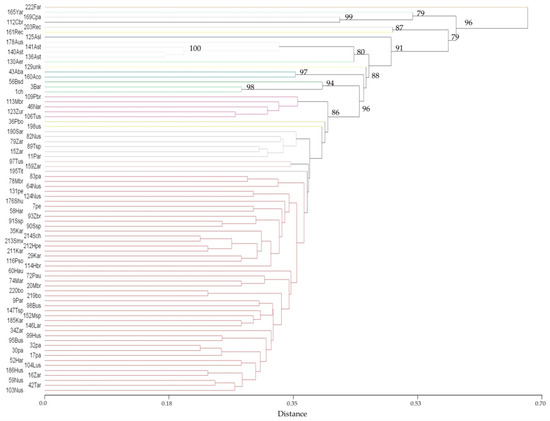
Figure 15.
Hierarchical cluster dendrogram of 78 Cucurbita accessions for SRAP markers polymorphisms. Unbiased p-values for multiscale bootstrap resampling are showed in some branching. For entries, a tripartite abbreviation code was used, in which the number is a household accession code (see supplementary Table S1 for details); uppercase letter refers to species other than C. maxima or cultivar-group in C. maxima, F (C. ficifolia), Y (C. argyrosperma), C (C. moschata), R (C. ecuadorensis), A (ssp. andreana), B (Buttercup), P (Plomo), M (Moranga), Z (Zapallito), T (Turban), S (Show), H (Hubbard), K (Zipinka), L (Banana), N (Nugget). When C. maxima accessions could not be assigned to any cultivar-group, uppercase letter is missing. The rest of the lower case letters in the code refers to country of origin us (USA), ar (Argentina), pe (Peru), br (Brazil), ch (Chile), bo (Bolivia), pa (Paraguay), ur (Uruguay), ec (Ecuador), mx (Mexico), sp (Spain), it (Italy), hu (Hungary), au (Australia), so (South Africa) and unk (not specified in passport data). For Argentinian entries of ssp. Andreana, lowercase letters indicate Province of origin, st (Santiago del Estero), co (Córdoba), er (Entre Ríos), sl (San Luis) and ba (Buenos Aires).
In the cluster analysis of Figure 15, the out-group Cucurbita species were clearly separated. Subspecies andreana entries were also disjointed, but interestingly accession 129unk (MAX 24 from IPK Genebank, Gatersleben, Germany) clustered with the ssp. andreana entries. The accession name is “Ovo de Ganso” (meaning goose´s egg in Portuguese), and even when in the IPK Genebank passport data are referred as unknown origin, it was donated by the Agronomic Institute of Campinas, Brazil. This entry was included in the parvifructina convariety along with wild ssp. andreana and pictured by [56]. Remarkably 129unk produce very small pink-grey but not bitter fruits (Figure 16). Flesh is orange and thin, and fruit weight and peduncle width are not significant different from those of ssp. andreana [59]; seeds are white, intermediate in length between the cultivar-group with the smallest seeds (Zipinka) and ssp. andreana; and do not have dormancy as reported for ssp. andreana [73]. Unusually, dry matter content is very high (13.25%). The plant habit is viney, and it took to onset pistillate anthesis 25 more days than ssp. andreana as a whole when grown at latitude 33°1′ S. It is clear that it has short-day floral induction, as Cucurbita provenances from low latitudes. Hammer and Gladis [74] mentioned within the convariety parvifructina the presence of a small-fruited, non-bitter, trailing form with hard shells wide-spread in Brazil. In [67], fruits resembling those of 129unk are pictured as ornamental gourds with edible flesh cultivated in Brazil.

Figure 16.
Mature entire and sliced fruits of Ovo de Ganso (MAX 24, IPK Genebank, Gatersleben, Germany). Blue ruler length 20 cm.
Moreover, within ssp. andreana, provenances from the Santiago del Estero province were differentiated from those of Cordoba, Entre Rios and Buenos Aires provinces (Figure 15). Within the domesticated C. maxima, a sub-cluster with a 96 bootstraps was composed by Buttercup accessions 56Bsd (PI 234608 Queensland Blue from GRIN Genebank, USA) and 3Bar, and the Chilean 1ch. Interestingly, in the study of [70], the same Queensland Blue along with other Buttercup accessions conformed the most distant cluster of the domesticates. For the rest of the accessions, there was not a clear clustering in relation to cultivar-group belonging; meanwhile, some entries with a common origin clustered together. One particular point that was resolved is referred to accession 198us (PI 165558 from India) which has been long included in the Gene List of Cucurbita [49] as a donor of the Bicolor precocious yellow fruit pigmentation (Bmax), which is true, but is referred as ssp. Andrena; however, it clustered within the domesticated, has a medium size fruit and has a lack of seed dormancy, so it is clearly not ssp. andreana (Figure 14).
Kates [40], using ~15,000 single nucleotide polymorphisms (SNPs), evaluated 32 improved and landraces entries of C. maxima, plus 15 ssp. andreana provenances. In the phylogenetic tree, the wild and domesticated samples each formed well-defined clades (Figure 17). Domesticated entries conformed two sub-clusters; the first one comprised mostly Hubbard, Banana and Buttercup accessions, which is in concordance with [70]; meanwhile, the second encompassed Zapallito, Nugget and Moranga counterparts. Plomo entries were scattered along the two sub-clusters suggesting a less specific genetic makeup. In the ssp. andreana clade, a sub-clustering was in concordance with provinces (within Argentina) and also to fruit characteristics; entries from Buenos Aires, Santa Fe, Córdoba and San Luis showed globular green fruits with light yellow stripes. Those from Entre Ríos and Santiago del Estero, in general, presented from flattened, elliptical to pear shape, mottled light green and cream fruits (Figure 17).
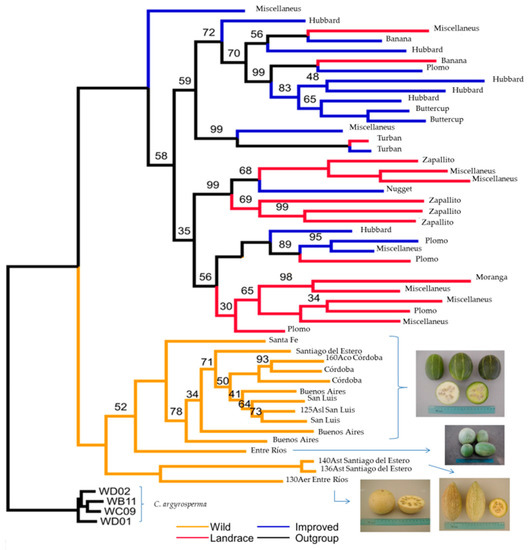
Figure 17.
Phylogenetic tree of C. maxima adapted from [40]. In the domesticated clade, entries are assigned to cultivar-groups according to external fruits characteristics, otherwise indicated as miscellaneous. In the wild ssp. andreana clade, Argentinian provinces of provenances are given, and for those included in [59], the original tripartite code is also indicated.
The joint analysis of morphological and molecular data of the 159 accessions along the Procrustes approach [75] is presented in Figure 18.
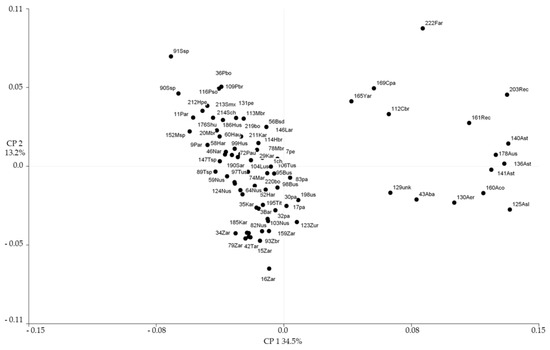
Figure 18.
Procrustes analysis biplot for morphological and molecular data of 159 accessions of C. maxima and out-group species. Codes are the same as in Figure 15.
The first axis that accounted for 34.5% of the variation clearly separates ssp. andreana entries from the rest of the cultivated forms, with the exception of 129unk (Ovo de Ganso). The second axis segregates the C. maxima domesticated entries where Zapallito, Zipinka and Nugget cultivar-groups were placed to the lower quadrant; Turban and Buttercup cultivar-groups have a more central placement; meanwhile, Hubbard, Plomo, Moranga and Banana cultivar-groups are positioned to the upper part, irradiating uppermost the Show cultivar-group (Figure 18).
In C. pepo, clear separations in relation to cultivar-groups were observed for ssp. texana but not for ssp. pepo domesticates [31]. In C. pepo ssp. texana, the cultivar-group Scallop was centrally positioned in relation to the remaining cultivar-groups, and assigned a putative first domestication associated with the consumption of seeds and immature fruits [31]. In this sense, in C. maxima, the central core tendency of Turban and Buttercup cultivar-groups may reflect a first step in the domestication pathway, irradiating subsequently to more specialized selection for fruit flesh consumption, where fruit size and flesh quality were more desirable attributes, leading to Moranga, Hubbard, Banana, Plomo and Show cultivar-groups on one hand, and for immature fruit consumption related to long-day induction, precocity (bush habit) and adaptation to temperate climate, yielding Zapallito, Nugget and Zipinka cultivar-groups on the other hand.
The striking placement of 129unk (Ovo de Ganso, MAX 24), more related to wild ssp. andreana than any of the assessed cultigens so far, can be reflecting either a relic of an old intermediate domesticated form that scarcely survived as weedy related to anthropogenic disturbance, or a completely specialized small size domesticate for use as buoy in fishing nets, small containers, rattles or decorative purposes. This finding strengthens the possible domestication place of the species more easternward than previously believed in concordance with the potential presence of the natural Peponapis pollinator and archeological remains, confirming the intuition of Whitaker and Carter [76] of the Brazilian origin of the Turban cultivar-group.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13080354/s1, Table S1: Details of accessions included in Lopez-Anido 2017 study.
Funding
This research was funded by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) grant number PUE0043.
Acknowledgments
Germplasm Resources Information Network (GRIN), USA, for providing data and pictures of mature squash accessions. IPK Genebank, Germany, for passport data on some accessions.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Paris, H.S. Genetic Resources of Pumpkins and Squash, Cucurbita spp. In Genetics and Genomics of Cucurbitaceae. Plant Genetics and Genomics: Crops and Models; Grumet, R., Katzir, N., Garcia-Mas, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 20, pp. 11–154. [Google Scholar] [CrossRef]
- Nee, M. The domestication of Cucurbita (Cucurbitaceae). Econ. Bot. 1990, 44, 56–68. [Google Scholar] [CrossRef]
- Whitaker, T.W.; Cutler, H.C. Cucurbits and cultures in the Americas. Econ. Bot. 1965, 19, 344–349. [Google Scholar] [CrossRef]
- Khoury, C.K.; Carver, D.; Kates, H.R.; Achicanoy, H.A.; van Zonneveld, M.; Thomas, E.; Heinitz, C.; Jarret, R.; Labate, J.; Reitsma, K.; et al. Distributions, conservation status, and abiotic stress tolerance potential of wild cucurbits (Cucurbita L.). Plants People Planet. 2020, 2, 269–283. [Google Scholar] [CrossRef]
- Millán, R. Variaciones el zapallito amargo Cucurbita andreana y el origen de Cucurbita maxima. Rev. Arg. Agron. 1945, 12, 86–93. [Google Scholar]
- Newsom, L.A.; Mihlbachler, M.C. Mastodons (Mammut americanum) Diet Foraging Patterns Based on Analysis of Dung Deposits. In First Floridians and Last Mastodons: The Page-Ladson Site in The Aucilla River; Webb, S.D., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 263–332. [Google Scholar]
- Barlow, C. The Ghost of Evolution, Nonsensical Fruit, Missing Partners, and Other Ecological Anachronisms; Basic Books: New York, NY, USA, 2000; pp. 51–69. [Google Scholar]
- Janzen, D.H.; Martin, P.S. Neotropical Anachronisms: The Fruits the Gomphotheres Ate. Science 1982, 215, 19–27. [Google Scholar] [CrossRef]
- Johnson, C.N. Ecological consequences of Late Quaternary extinctions of megafauna. Proc. R. Soc. B 2009, 276, 2509–2519. [Google Scholar] [CrossRef]
- Kistler, L.; Newsom, L.A.; Ryan, T.M.; Clark, A.C.; Smith, B.D.; Perry, G.H. Gourds and squashes (Cucurbita spp.) adapted to megafaunal extinction and ecological anachronism through domestication. Proc. Natl. Acad. Sci. USA 2015, 112, 15107–15112. [Google Scholar] [CrossRef]
- Tropicos Connecting the World to Botanical Data Since 1982. Available online: http://legacy.tropicos.org/Name/50172465?projectid=13 (accessed on 8 July 2021).
- Ashwoth, L.; Galetto, L. Pollinators and Reproductive Success of the Wild Cucurbit Cucurbita maxima ssp. andreana (Cucurbitaceae). Plant. Biol. 2001, 3, 398–404. [Google Scholar] [CrossRef]
- Erickson, D.L.; Smith, B.D.; Clarke, A.C.; Sandweiss, D.H.; Tuross, N. An Asian origin for a 10,000-year-old domesticated plant in the Americas. Proc. Natl. Acad. Sci. USA 2005, 102, 18315–18320. [Google Scholar] [CrossRef] [PubMed]
- Spengler, R.N. Anthropogenic Seed Dispersal: Rethinking the Origins of Plant Domestication. Trends Plant. Sci. 2020, 25, 340–348. [Google Scholar] [CrossRef]
- Perugganan, M.D. Evolutionary Insights into the Nature of Plant Domestication. Curr. Biol. 2019, 29, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.Q.; Allaby, R.G.; Stevens, C. Domestication as innovation: The entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeol. 2010, 42, 13–28. [Google Scholar] [CrossRef]
- Millán, R. Los zapallitos de tronco de Sudamérica extratropical. Darwiniana 1947, 7, 333–345. [Google Scholar]
- Wu, T.; Zhou, J.; Zhang, Y.; Cao, J. Characterization and inheritance of a bush-type in tropical pumpkin (Cucurbita moschata Duchesne). Sci. Hortic. 2007, 114, 1–4. [Google Scholar] [CrossRef]
- Paris, H.S.; Lebeda, A.; Křistkova, E.; Andres, T.C.; Nee, M.H. Parallel Evolution Under Domestication and Phenotypic Differentiation of the Cultivated Subspecies of Cucurbita pepo (Cucurbitaceae). Econ. Bot. 2012, 66, 71–90. [Google Scholar] [CrossRef]
- Heiser, C.B. Aspects of unconscious selection and the evolution of domesticated plants. Euphytica 1988, 37, 77–81. [Google Scholar] [CrossRef]
- Marshall, L.G. Land Mammals and the Great American Interchange. Am. Sci. 1988, 76, 381–388. [Google Scholar]
- Sánchez, B.; Prado, J.L.; Alberdi, M.T. Feeding ecology, dispersal, and extinction of South American Pleistocene gomphotheres (Gomphotheriidae, Proboscidea). Paleobiology 2004, 30, 146–161. [Google Scholar] [CrossRef]
- Giannini, T.C.; Saraiva, A.M.; Alves-dos-Santos, I. Ecological niche modeling and geographical distribution of pollinator and plants: A case study of Peponapis fervens (Smith, 1879) (Eucerini: Apidae) and Cucurbita species (Cucurbitaceae). Ecol. Inform. 2010, 5, 59–66. [Google Scholar] [CrossRef]
- Ferriol, M.; Picó, B. Pumpkin and Winter Squash. In Handbook of Plant Breeding Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer Science Business Media: New York, NY, USA, 2008; pp. 317–349. [Google Scholar]
- Smith, B.D. The initial domestication of Cucurbita pepo in the Americas 10,000 years ago. Science 1997, 276, 932–934. [Google Scholar] [CrossRef]
- Andres, T.C. Biosystematics, theories on the origin, and breeding potential of Cucurbita ficifolia. In Biology and Utilization of the Cucurbitaceae; Bates, D.V., Robinson, R.W., Jeffrey, C., Eds.; Cornell University Press: Ithaca, NY, USA, 1990; pp. 102–119. [Google Scholar]
- Sanjur, O.I.; Piperno, D.R.; Andres, T.C.; Wessel-Beaver, L. Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitocondrial gene: Implications for crop plant evolution and areas of origin. Proc. Natl. Acad. Sci. USA 2002, 99, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Decker, D.S. Origin(s), Evolution and Systematics of Cucurbita pepo (Cucurbitaceae). Econ. Bot. 1988, 42, 4–15. [Google Scholar] [CrossRef]
- Barrera-Redondo, J.; Sánchez-de la Vega, J.; Aguirre-Liguori, J.A.; Castellanos-Morales, G.; Gutiérrez-Guerrero, Y.T.; Aguirre-Dugua, X.; Aguirre-Planter, E.; Tenaillon, M.I.; Rafael Lira-Saade, R.; Eguiarte, L.E. The domestication of Cucurbita argyrosperma as revealed by the genome of its wild relative. Hortic. Res. 2021, 8, 109–123. [Google Scholar] [CrossRef]
- Kates, H.R.; Soltis, P.S.; Soltis, D.E. Evolutionary and domestication history of Cucurbita (pumpkin and squash) species inferred from 44 nuclear loci. Mol. Phylogenetics Evol. 2017, 111, 98–109. [Google Scholar] [CrossRef]
- Gong, L.; Paris, H.S.; Nee, M.H.; Stift, G.; Pachner, N.; Vollmann, J.; Lelley, T. Genetic relationships and evolution in Cucurbita pepo (pumpkin, squash, gourd) as revealed by simple sequence repeat polymorphisms. Appl. Genet. 2012, 124, 875–891. [Google Scholar] [CrossRef]
- Castellanos-Morales, G.; Ruiz-Mondragón, K.Y.; Hernández-Rosales, H.S.; Guillermo Sánchez-de la Vega, G.; Gámez, N.; Aguirre-Planter, E.; Montes-Hernández, S.; Lira-Saade, R.; Eguiarte, L.E. Tracing back the origin of pumpkins (Cucurbita pepo ssp. pepo L.) in Mexico. Proc. R. Soc. B 2019, 286, 20191440. [Google Scholar] [CrossRef] [PubMed]
- Andres, T.C. Diversity in tropical pumpkin (Cucurbita moschata): Cultivar origin and history. In Progress in Cucurbit Genetics and Breeding Research, Proceeding of the Cucurbitaceae 2004, Olomouc, Czech Republic, 12–17 July 2004; Lebeda, A., Paris, H.S., Eds.; Palacky University: Olomouc, Czech Republic, 2004; pp. 113–119. [Google Scholar]
- Whitaker, T.W. Cucurbits in Andean prehistory. Am. Antiq. 1983, 48, 576–585. [Google Scholar] [CrossRef]
- Lema, V. Domesticación Vegetal y Grado de Dependencia ser Humano-Planta en el Desarrollo Cultural Prehispánico del Noroeste Argentino. Ph.D. Thesis, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata, Argentina, 2009. [Google Scholar]
- Parodi, L.R. La Agricultura Aborigen Argentina; Universitaria de Buenos Aires: Buenos Aires, Argentina, 1966; p. 45. [Google Scholar]
- Paucke, F. Hacia Allá y para Acá, 1st ed.; Ministerio de Innovación y Cultura: Santa Fe, Argentina, 2010; p. 168.
- Watling, J.; Shock, M.P.; Mongelo, G.Z.; Almeida, F.O.; Kater, T.; De Oliveira, P.E.; Neves, E.G. Direct archaeological evidence for Southwestern Amazonia as an early plant domestication and food production centre. PLoS ONE 2018, 13, 1–28. [Google Scholar] [CrossRef]
- Bonomo, M.; Politis, G.G. Mound Building, Social Complexity and Horticulture in the Lower Paraná River. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Kates, H.R.; López-Anido, F.; Sánchez-De La Vega, G.; Eguiarte, L.E.; Soltis, P.; Soltis, D.E. Targeted sequencing suggests wild-crop gene flow is central to different genetic consequences of two independent pumpkin domestications. Front. Ecol. Evol. 2021, 9, 405, in press. [Google Scholar] [CrossRef]
- Pearsall, D.M. Plant Domestication and the Shift to Agriculture in the Andes. In Handbook of South American Archaeology; Silverman, H., Isbell, W.H., Eds.; Springer: New York, NY, USA, 2008; pp. 105–120. [Google Scholar] [CrossRef]
- Dillehay, T.D.; Rossen, J.; Andres, T.C.; Williams, D.E. Preceramic Adoption of Peanut, Squash, and Cotton in Northern Peru. Science 2007, 316, 1890–1893. [Google Scholar] [CrossRef]
- Clement, C.R. 1492 and the Loss of Amazonian Crop Genetic Resources. I. The relation between Domestication and Human Population Decline. Econ. Bot. 1999, 53, 188–202. [Google Scholar] [CrossRef]
- Clement, C.R. 1492 and the Loss of Amazonian Crop Genetic Resources. II. Crop Biogeography at Contact. Econ. Bot. 1999, 53, 203–216. [Google Scholar] [CrossRef]
- Piperno, D.R.; Andres, T.C.; Stothert, K.E. Phytoliths in Cucurbita and other Neotropical Cucurbitaceae and their Occurrence in Early Archaeological Sites from the Lowland American Tropics. J. Archaeol. Sci. 2000, 27, 193–208. [Google Scholar] [CrossRef]
- Lema, V.S. Non-domesticated cultivation in the Andes: Plant management and nurturing in the Argentine northwest. Veg. Hist. Archaeobot. 2015, 24, 143–150. [Google Scholar] [CrossRef]
- Zeder, M.A.; Emshwille, E.; Smith, B.D.; Bradley, D.G. Documenting domestication: The intersection of genetics and archaeology. TRENDS Genet. 2006, 22, 139–156. [Google Scholar] [CrossRef]
- Martínez, A.; Lema, V.; Capparelli, A.; Bartoli, C.; López-Anido, F.; Perez, S. Multidisciplinary studies in Cucurbita maxima (squash) domestication. Veg. Hist. Archaeobot. 2018, 27, 207–217. [Google Scholar] [CrossRef]
- The Cucurbit Genetics Cooperative (CGC) Gen List of Cucurbita Species. Available online: https://cucurbit.info/wp-content/uploads/2018/10/gene14squash.pdf (accessed on 9 July 2021).
- Brickell, C.D.; Baum, B.R.; Hetterscheid, W.L.A.; Leslie, A.C.; McNeill, J.; Trehane, P.; Vrugtman, F.; Wiersema, J.H. International Code of Nomenclature for Cultivated Plants. Acta Hortic. 2004, 647, 17–19. [Google Scholar] [CrossRef]
- Paris, H.S. A proposed subspecific classification of Cucurbita pepo. Phytologia 1986, 61, 133–138. [Google Scholar]
- Paris, H.S. Historical records, origin, and development of the edible cultivar groups of Cucurbita pepo (Cucurbitaceae). Econ. Bot. 1989, 43, 423–443. [Google Scholar] [CrossRef]
- Jeffrey, C. Cucurbita. In Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops (Except Ornamentals); Hanelt, P., Institute of Plant Genetics and Crop Plant Research, Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1541–1552. [Google Scholar]
- Andres, T.C. Diversity in tropical pumpkin (Cucurbita moschata): A review of infraspecific classifications. In Progress in Cucurbit Genetics and Breeding Research, Proceeding of the Cucurbitaceae 2004, Olomouc, Czech Republic, 12–17 July 2004; Lebeda, A., Paris, H.S., Eds.; Palacky University: Olomouc, Czech Republic, 2004; pp. 107–112. [Google Scholar]
- Castetter, E.F. Horticultural groups of Cucurbita. Proc. Am. Soc. Hortic. Sci. 1925, 22, 338–340. [Google Scholar]
- Goldman, A. The Compleat Squash: A Passionate Grower’s Guide to Pumpkins, Squash, and Gourds; Artisan: New York, NY, USA, 2004; p. 214. [Google Scholar]
- Grebenscikov, I. Notulae Cucurbitologicae III. Die Kult. 1958, 6, 38–60. [Google Scholar] [CrossRef]
- Cummings, M.B.; Jenkins, E.W. Pure Lines Studies with Ten Generations of Hubbard Squash; Free Press Printing Co: Burlington, VT, USA, 1928; p. 29. [Google Scholar]
- López-Anido, F.S. Diversidad Morfológica y Molecular en Cucurbita maxima Duchesne ex Lam. Ph.D. Thesis, Facultad de Ciencias Agrarias, Universidad Nacional de Rosario, Zavalla, Argentina, 2017. [Google Scholar]
- López-Anido, F. Genetics of seed coat colour in winter squash. In Proceedings of the 7th International Horticulture Research Conference, College of Horticulture and State Key Laboratory of Crop Stress Biology for Arid Areas of Northwest A&F University, Nanjing, China, 1–30 July 2020. [Google Scholar]
- Formiga, A.K.; Myers, J.R. Images and Descriptions of Cucurbita maxima in Western Europe in the Sixteenth and Seventeenth Centuries. Plant. Breed. Rev. 2020, 43, 317–356. [Google Scholar] [CrossRef]
- Janick, J. Giant Pumpkins: Genetic and Cultural Breakthroughs. Chron. Hortic. 2008, 48, 16–17. [Google Scholar]
- Paris, H.S.; Nerson, H. Seed dimension in the subspecies and cultivar-groups of Cucurbita pepo. Genet. Res. Crop Evol. 2003, 50, 615–625. [Google Scholar] [CrossRef]
- Paris, H.S.; Brown, R.N. The genes of pumpkin and squash. HortScience 2005, 40, 1620–1630. [Google Scholar] [CrossRef]
- Langevin, D.; Janick, J. New World Record of Giant Pumpkin, 2010. Chron. Hortic. 2011, 51, 24. [Google Scholar]
- Cumarasamy, R.; Corrigan, V.; Hurst, P.; Bendall, M. Cultivar differences in New Zealand “Kabocha” (buttercup squash, Cucurbita maxima). N. Z. J. Crop Hortic. Sci. 2002, 30, 197–208. [Google Scholar] [CrossRef][Green Version]
- Heiden, G.; Barbieri, R.L.; Neitzke, R.S. Chave para a Identificação das Espécies de Abóboras (Cucurbita, Cucurbitaceae) Cultivadas no Brasil; Embrapa Clima Temperado: Pelotas, Brazil, 2007; p. 32. [Google Scholar]
- Herrington, M.E.; Prytz, S.; Wright, R.M.; Walker, I.O.; Brown, P.; Persley, D.M.; Greber, R.S. ‘Dulong QHI’ and ‘Redlands Trailblazer’, PRSV-W-, ZYMV-, and WMV-resistant Winter Squash Cultivars. HortSience 2001, 36, 811–812. [Google Scholar] [CrossRef]
- Ferriol, M.; Picó, B.; Nuez, F. Morphological and molecular diversity of a collection of Cucurbita maxima landraces. J. Am. Soc. Hortic. Sci. 2004, 129, 60–69. [Google Scholar] [CrossRef]
- Kazminska, K.; Sobieszek, K.; Targonska-Karasek, M.; Korzeniewska, A.; Niemirowicz-Szczytt, K.; Bartoszewski, G. Genetic diversity assessment of a winter squash and pumpkin(Cucurbita maxima Duchesne) germplasm collection based ongenomic Cucurbita-conserved SSR markers. Sci. Hortic. 2017, 219, 37–44. [Google Scholar] [CrossRef]
- Esquinas Alcazar, J.T.; Gulick, P.J. Genetic Resources of Cucurbitaceae; IBPGR: Rome, Italy, 1983; p. 100. [Google Scholar]
- UPOV. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability, Pumpkin (Cucurbita maxima Duch.), TG/155/4; UPOV: Geneva, Switzerland, 2007; p. 29. [Google Scholar]
- Martínez, A.B.; Lema, V.S.; Capparelli, A.; López-Anido, F.; Benech-Arnold, R.L.; Bartoli, C.G. Differences in seed dormancy associated with the domestication of Cucurbita maxima: Elucidation of some mechanisms behind this response. Seed Sci. Res. 2018, 28, 1–7. [Google Scholar] [CrossRef]
- Hammer, K.; Gladis, T. Notes on infraspecific nomenclature and classifications of cultivated plants in Compositae, Cruciferae, Cucurbitaceae, Gramineae (with a remark on Triticum dicoccon Schrank) and Leguminosae. Genet. Resour. Crop Evol. 2014, 61, 1455–1467. [Google Scholar] [CrossRef]
- Gower, J.C. Generalized procrustes analysis. Psychometrika 1975, 40, 33–51. [Google Scholar] [CrossRef]
- Whitaker, T.W.; Carter, G.F. Critical Notes on the origin and Domestication of the Cultivated Species of Cucurbita. Am. J. Bot. 1946, 33, 10–15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).