Abstract
Climatic conditions represent one of the main constraints that influence avian calling behavior. Here, we monitored the daily calling activity of the Undulated Tinamou (Crypturellus undulatus) and the Chaco Chachalaca (Ortalis canicollis) during the dry and wet seasons in the Brazilian Pantanal. We aimed to assess the effects of climate predictors on the vocal activity of these focal species and evaluate whether these effects may vary among seasons. Air temperature was positively associated with the daily calling activity of both species during the dry season. However, the vocal activity of both species was unrelated to air temperature during the wet season, when higher temperatures occur. Daily rainfall was positively related to the daily calling activity of both species during the dry season, when rainfall events are scarce and seem to act as a trigger for breeding phenology of the focal species. Nonetheless, daily rainfall was negatively associated with the daily calling activity of the Undulated Tinamou during the wet season, when rainfall was abundant. This study improves our understanding of the vocal behavior of tropical birds and their relationships with climate, but further research is needed to elucidate the mechanisms behind the associations found in our study.
1. Introduction
Bird vocalizations have important roles in territory establishment and mate attraction, but they are also uttered to maintain group contact and to signal about food or danger, such as threats or fights [1,2,3]. Therefore, understanding the context of avian vocal behavior might be useful to elucidate the function of vocal activity in birds and to plan surveys and develop effective monitoring protocols [4]. Despite the large number of studies assessing bird vocalizations, the main causes of changes in bird vocal activity seem to be context dependent and to differ among species [3,5] and even within species according to the type of vocalization analyzed [6,7].
Bird behavior, and hence vocal activity, is affected by endogenous factors (e.g., breeding seasonality and mating status [8]) as well as by exogenous factors (e.g., photoperiod [9,10], climatic conditions [11], habitat structure [12], moon phase [13,14], background noise [15], and artificial light [16]). The influence of climatic conditions on the vocal activity of birds is not restricted to direct impacts on bird vocal behavior, as it is also related to changes in sound transmission (e.g., an increase in temperature results in an increase in the absorption of low-frequency sounds [17]). As a general rule, rainfall is the climatic predictor with the most influence on bird vocal activity [11,18]. Birds tend to reduce their display and vocal activity during rainfall [11,19,20] since they usually look for cover. The noise of the rain also overlaps with most bird vocalizations, and therefore, the decreases in vocal activity during rainfall might also be due to a reduction in the ability of birds to communicate with their conspecifics [21,22]. Similarly, several bird species adjust their vocal activity according to cloudy conditions [18,23] and air temperature [24,25,26].
Most studies assessing the vocal behavior of birds have been performed in northern temperate zones [3]. However, the study of vocal behavior in tropical birds is interesting since song function and communication roles typical of northern temperate areas may not be extrapolated to tropical regions [27]. For example, in tropical areas, singing activity is usually less restricted to the breeding period than in temperate areas [28,29,30]. Moreover, there is a large contribution of duets and female songs [27], and most birds are residents [31].
Due to the longer calling periods, the impact of climatic conditions on the vocal activity of birds may differ according to the season and temporal scale considered. For example, rainfall may have a negative impact on a daily scale due to the masking effect of bird vocalizations [21,22], but its effect might be positive on a longer scale. This positive impact might be particularly true for tropical areas with high seasonality, where rainfall is a strong determinant of food abundance, and thus acts as a trigger regulating the breeding phenology and vocal activity of several tropical birds [14,30,31]. This suggests that to understand the complex nature of the vocal activity of tropical birds, there is a need to monitor bird vocal behavior over large temporal scales.
The current knowledge regarding the effects of climatic conditions and seasonal changes on vocal activity in tropical birds comes mainly from studies that used passerines as study species [4,28,32], but see, e.g., Pérez-Granados and Schuchmann [14,33]. In this work, we aimed to contribute to the current knowledge about the impact of climatic conditions on daily vocal activity of tropical birds, making use of an extended database collected for two tropical nonpasserine birds. We evaluated whether the effects of daily air temperature and daily rainfall on daily bird vocal activity vary among seasons. We hypothesized that climatic conditions would affect bird vocal activity by altering vocal behavior and that their effects may differ among seasons. We predicted that daily rainfall would have a positive impact on bird vocal activity during the dry season, when rainfall is scarce and may improve food availability, but a negative effect during the wet season, when heavy rainfalls usually occur. We also predicted that the daily minimum temperature would have a negative impact on the species’ vocal activity according to previous studies that found greater vocal activity in non-passerines at cooler temperatures, including studies with other bird species in the study area [23,34,35].
2. Materials and Methods
2.1. Study Species
We selected the Undulated Tinamou (Crypturellus undulatus) and the Chaco Chachalaca (Ortalis canicollis) as focal species to evaluate seasonal changes in vocal activity and how such changes are related to climatic conditions. We chose these species because they are common and resident species with high vocal activity over the annual cycle [30] (authors’ unpublished data). The Undulated Tinamou is a resident species, and similar to other tinamou species, it is a polygynous (males take care of the nest and incubate the eggs, which are laid by different females), shy, and elusive bird typical of dense tropical habitats [36]. It is a ground-dwelling omnivore that is widespread in South America. Although no specific information is available, we expect that both sexes may call, according to the typical calling behavior described for the Tinamidae [37,38]. The breeding period of the species in the study area (Brazilian Pantanal, see below) has been proposed to occur between September and April, the months during which the vocal activity of the species is maximum (see the seasonal pattern of vocal activity throughout a whole year [30]). The diel pattern of vocal activity shows a bimodal scheme with a peak during the early morning and a second peak in late afternoon, although the species was vocally active throughout the day and night (see the diel pattern of vocal activity [30]). The only known call of the Undulated Tinamou is composed of three distinctive notes (Supplemental Figure S1).
The Chaco Chachalaca is the smallest of the Chachalacas (Ortalis genus) and is a common and resident bird of lowland forests and dry and semideciduous forests of central South America [39]. The breeding period of the species in the Brazilian Pantanal, based on seasonal changes in vocal activity, has been proposed to occur between August and December, when 66.2% of the total calls were detected during a year (n = 112,650 calls, authors’ unpublished data). In that species the female is the only sex incubating. The Chaco Chachalaca vocalizes during day and night but shows its maximum vocal activity during the early morning (77.5% of the calls identified over a year were detected between 5 a.m. and 9 a.m., n = 112,650 calls, authors’ unpublished data). The call of the species is loud and transcribed as “Chata-ra-ta” [39] and is a short series of harsh, raucous syllables (Supplemental Figure S1) that are uttered (indistinguishable) by both sexes.
2.2. Study Area
We conducted a field study from 8 June 2015 to 31 May 2016 in the northeastern part of the Brazilian Pantanal (Pantanal Matogrossense), the largest seasonal floodplain in the world. The study area is located near the SESC Pantanal (Poconé municipality, Mato Grosso, Brazil; 16°30′ S, 56°25′ W, see Online Resource 1) and close to the Cuiabá River, one of the main tributaries of the Paraguay River within the Pantanal. This area is seasonally inundated from October to April due to the flooding of the Paraguay River, while during May–September, it exhibits a pronounced dry season [40]. More specifically, the annual cycle in the study area covers four seasons: (1) the dry season (July–September), with a strong hydrological deficit; (2) the rising water season (October–December), when rain starts and the water level begins to increase; (3) the wet season (January–March), with the highest level of inundation; and (4) the receding season (April–June), when the water level starts to decline [41]. More detailed information on the impact of flood pulses on the local avian and vegetation communities in the study area can be found in de Deus et al. [42].
The study area comprised three acoustic monitoring stations separated by 1530 and 2017 m (Supplemental Figure S2) within a mosaic of forested and savanna areas. The regional climate is tropical and humid; the average annual rainfall is between 1000 and 1500 mm, and the mean annual temperature is approximately 24 °C. During the monitored period, the total annual rainfall in the study area was 1130 mm, and the mean annual temperature was 25.5 °C (authors’ own data).
2.3. Acoustic Monitoring
We placed one Song Meter SM2 recorder (Wildlife Acoustics, Maynard, MA, USA, www.wildlifeacoustics.com, accessed on 12 July 2021) on each of the three acoustic monitoring stations. Acoustic monitoring was performed daily during the study period and therefore covered one annual cycle at each site. The Song Meter recorder was programmed to record (in stereo and .wav format) the first 15 min of each hour in 24/7 mode according to the winter local time (GMT -4) and was configured with a sampling rate of 48 kHz and a resolution of 16 bits per sample. We have no data how far calls of the focal species can be detected. Previous studies using SM2 recorder and birds as models have found that the effective detection radius of the recorder was around 150–160 m [43,44]. Although different bird species were used for such assessments, the published effective radius may provide some approximate radius at which vocalizations of the Undulated Tinamou and Chaco Chachalaca might be recorded. Recordings were stored on SD memory cards capable of storing ~250 h of recordings. The recorders were powered by four 1.5 V alkaline batteries (Duracell MN1,3000) (~160 h autonomy) and checked weekly to download data and change batteries.
2.4. Acoustic Data Analyses
The channel recordings were scanned with Kaleidoscope Pro 5.1.8, an automated signal-recognition software program provided by Wildlife Acoustics (Wildlife Acoustics, Maynard, MA, USA, www.wildlifeacoustics.com, accessed on 12 July 2021). Kaleidoscope scans the recordings, searching for candidate sounds according to the signal parameters introduced in the software. To introduce the most adequate signal parameters, we characterized 52 calls of the Undulated Tinamou and 47 of the Chaco Chachalaca in the study area (Supplemental Table S1). These calls were measured from spectrograms using Raven Pro 1.5 (Cornell Lab of Ornithology, Ithaca, NY, USA) [45]. According to call parameters, we introduced the following signal parameters in Kaleidoscope and scanned the recordings once for each species:
- Undulated Tinamou: minimum and maximum frequency range: 1150 and 1350 Hz, respectively; minimum and maximum detection length: 1.3 and 3 s, respectively; maximum intersyllable gap: 0.2 s; distance from the cluster center: 2.0.
- Chaco Chachalaca: minimum and maximum frequency range: 300 and 2500 Hz, respectively; minimum and maximum detection length: 0.5 and 20 s, respectively; maximum intersyllable gap: 0.1 s; distance from the cluster center: 2.0.
The “distance from the cluster center” parameter ranges from 0 to 2 (1.0–1.4 are the values recommended by Wildlife Acoustics) and has an impact on the number of detected signals. Large values result in a relatively large number of detected signals, therefore, increasing the number of target signals detected (true positives) as well as the number of false positives (misclassified signals). We are aware that this selection will increase the number of false positives, but we aimed to detect as many Tinamou calls as possible. An analysis of the impact of using different values of the distance from the cluster center parameter for detecting the calls of the Undulated Tinamou was provided by Pérez-Granados et al. [30]. Finally, each candidate sound that was identified by Kaleidoscope Pro as a potential call of the Undulated Tinamou or the Chaco Chachalaca was visually and/or acoustically checked, always by the same observer (CPG), to remove incorrect detections (false positives). Therefore, the final database used in posterior analyses only contained calls of the focal species.
Finally, we evaluated the performance of the recognizers by measuring their recall rate [46]. The recall rate is defined as the proportion of target sounds (Undulated Tinamou or Chaco Chachalaca calls in our case) automatically detected by the recognizer. The recall rate of the Undulated Tinamou recognizer was 0.74 (1545 calls detected of the 2097 calls annotated in the 240 15-min recordings of the validation dataset; see Pérez-Granados and Schuchmann [47] for full details of the assessment of the recognizer employed in this study). We followed the same approach to estimate the recall rate of the Chaco Chachalaca, so we divided the total number of calls of the Chaco Chachalaca detected by Kaleidoscope by the total number of calls within sound recordings [45]. The total number of calls of the species per 15-min recording was always counted by the same experienced observer (CPG) by visually and acoustically checking 200 selected recordings. We reviewed a total of 100 recordings with known presence of the species (according to Kaleidoscope results, one-third of the recordings per site) and 100 randomly selected recordings from the period between 7 a.m. and 8 a.m., which is when the vocal activity of the species was maximum (authors’ unpublished data) and is therefore the period with a higher probability of finding recordings with the presence of the species. The recordings were reviewed blind with respect to station identification, date of recording and whether the species had been detected by Kaleidoscope.
2.5. Environmental Variables
During the study period, climate data was registered by a weather station in the study area (Supplemental Figure S2). We collected the following daily information: minimum air temperature (°C), mean air temperature (°C), maximum air temperature (°C), rainfall (mm), and relative air humidity (%). Air temperature and relative air humidity were measured with the Thermohyrgrometer (HMP155A, Vaisala, Helsinki, Finland) installed at 2 m, while daily rainfall was measured with the weighing rain gauge TR-525M (Texas Electronics, Dallas, TX, USA) installed at 20 m. All sensors were purchased with calibration from the manufacturer.
2.6. Statistical Analyses
We focused our comparison during the two most different periods: the dry season (July–September) and the wet season (January–March). We reduced the potential collinearity among climatic variables following a two-step process. First, we conducted a principal component analysis (PCA) for the three air temperature variables (minimum, mean, and maximum air temperature). The scores of the first principal component were then used as a surrogate of daily air temperature in posterior analyses (air temperature hereafter). Lastly, we checked the correlation between air temperature, daily relative air humidity, and daily rainfall and removed those variables with Spearman rank correlations higher than 0.7 [48]. The daily rainfall and daily relative air humidity were highly correlated (Spearman rank correlation of 0.73), with higher relative air humidity on those days with higher daily rainfall. We decided to remove relative air humidity from further analyses because we expected a higher impact of daily rainfall on the daily vocal activity of the focal species.
To analyze the variations in the effects of daily air temperature and daily rainfall on the daily vocal activities of the species, we fitted independent generalized linear models (GLMs) for each season and species (four models in total). All variables were z-standardized (for each season and species). The number of Undulated Tinamou or Chaco Chachalaca calls detected per day was used as the response variable, while climatic predictors (PCA scores, and daily rainfall) were included in the models as predictors. Likewise, the station was included as a covariate to control for variation owing to the site. The station was not included as a random effect due to the low number of levels within the factor, since a minimum number of five levels are recommended to consider a factor as random [49]. All statistical analyses were performed in R 3.4.1 [50]. The level of significance was α < 0.05, and the results are expressed as the mean ± SE.
3. Results
Kaleidoscope detected a total of 257,704 candidate sounds when looking for the call of the Undulated Tinamou during the two selected seasons. A total of 105,455 candidate sounds were identified as Undulated Tinamou calls and were used in subsequent analyses (57,252 calls during the dry season and 48,203 during the wet season). The number of calls detected per station ranged between 19,386 and 43,768. Undulated Tinamous were detected on 99.6% of the monitored days. A total of 341,589 candidate sounds were detected by Kaleidoscope when scanning the recordings looking for the Chaco Chachalaca, of which 51,569 were identified as calls of the species (38,825 calls during the dry season and 12,744 calls during the wet season). The species was detected on 100% of the monitored days, and the number of calls per station varied from 11,418 to 27,240. The recall rate of the Chaco Chachalaca recognizer was 0.78 (3575 calls detected by Kaleidoscope of the 4604 calls annotated in the 200 recordings of the validation dataset). The calling activity of both species (Figure 1A,B) and climatic conditions (Figure 1C,D) differed largely between seasons. For example, daily rainfall per day ranged from 0.7 mm during the dry season to 7.3 mm during the wet season (Figure 1D).
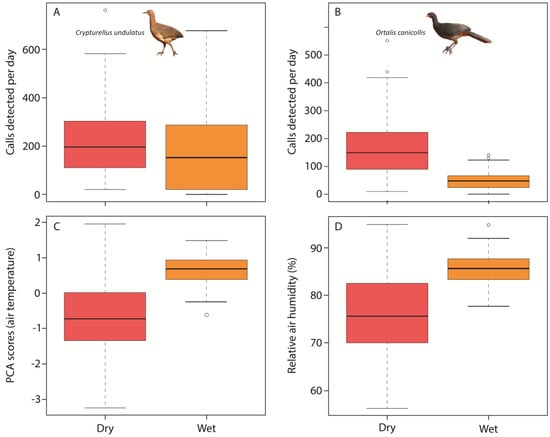
Figure 1.
Boxplots showing the seasonal variations in the daily calling activities of the Undulated Tinamou (A) and the Chaco Chachalaca (B) in the Pantanal Matogrossense (Brazil) during the dry and wet seasons. Seasonal variations in climatic conditions are also shown. Climatic conditions are indicated by air temperature (C), and daily rainfall (D). Air temperature is represented as the scores of the first Axis of a PCA performed to summarize daily maximum, minimum and mean air temperature.
The first Axis of the PCA (Axis I hereafter) explained 62.1% of the variance of our air temperature data. Axis I was positively associated with the three considered air temperature variables in both seasons (see Supplemental Table S2) and, therefore, Axis I is interpreted as a gradient of air temperature where lower values of Axis I correspond to days with lower air temperature while higher values of Axis I indicate those days with higher air temperature. According to the GLMs, the effects of climatic conditions on the daily calling activity of the Undulated Tinamou and the Chaco Chachalaca differed largely between seasons, and there were also variations among some of the acoustic monitoring stations (Table 1). During the dry season, the daily calling activities of both species were positively associated with air temperature and daily rainfall (Figure 2 and Figure 3). During the wet season, the relationship between climatic conditions and daily calling activity varied between the two monitored species (Table 1). The daily calling activity of the Undulated Tinamou was negatively associated with daily rainfall but was not related to air temperature (Table 1). In contrast, the daily calling activity of the Chaco Chachalaca was neither related to air temperature nor to daily rainfall during the wet season (Figure 2 and Figure 3).

Table 1.
Summary of generalized linear model analysis results regarding the effects of climatic conditions on the daily calling activities of the Undulated Tinamou and the Chaco Chachalaca in the Pantanal Matogrossense (Brazil). Models were fitted independently for each season and species. The daily number of calls was monitored by recording 15 min every hour between 8 June 2015 and 31 May 2016 at three sampling stations. The sign of the effects and relative importance of each (standardized) variable can be inferred from the Estimate values.
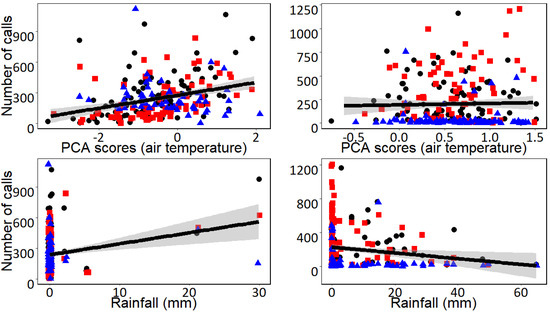
Figure 2.
Relationships between air temperature and daily rainfall (mm) with the daily calling activity of the Undulated Tinamou (number of calls per day) in the Pantanal Matogrossense (Brazil) during the dry (left) and wet seasons (right). Data are shown with different symbols representing the three different monitored stations. Climatic conditions were extracted from a weather station positioned near the sampling stations. Air temperature is represented as the scores of the first Axis of a PCA performed to summarize daily maximum, minimum and mean air temperature. The solid blue line represents the linear regression between daily vocal activity and climatic conditions, and 95% confidence intervals are shown in gray.
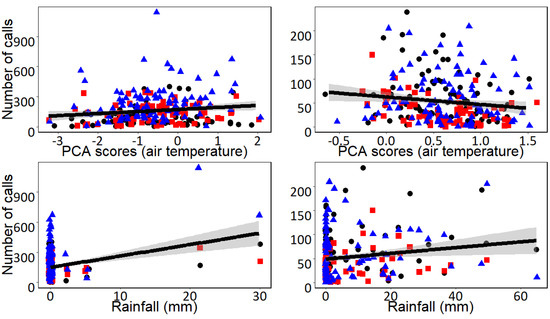
Figure 3.
Relationships between air temperature and daily rainfall (mm) with the daily calling activity of the Chaco Chachalaca (number of calls per day) in the Pantanal Matogrossense (Brazil) during the dry (left) and wet seasons (right). Data are shown with different symbols representing the three different monitored stations. Climatic conditions were extracted from a weather station positioned near the sampling stations. Air temperature is represented as the scores of the first Axis of a PCA performed to summarize daily maximum, minimum and mean air temperature. The solid blue line represents the linear regression between daily vocal activity and climatic conditions, and 95% confidence intervals are shown in gray.
4. Discussion
In this study, we provide evidence that the daily calling activities of the Undulated Tinamou and the Chaco Chachalaca in the Brazilian Pantanal are related to climatic conditions. Moreover, our results show that the effect of climatic conditions on bird calling behavior may differ among species and that these effects might even be contradictory among seasons. To our knowledge, this is the first study to find seasonal variations in the effects of multiple climatic predictors on the calling activity of birds. Daily vocal activity of both focal species was higher during the dry season when compared to the wet season (Figure 1). The peak of annual vocal activity of the Undulated Tinamou (September–October, Pérez-Granados et al. [30]) and the Chaco Chachalaca (August–September, authors’ unpublished data) in the Brazilian Pantanal occurs during the dry season, when both species are breeding. The whole annual pattern of vocal activity of the Undulated Tinamou discussing differences of vocal activity among seasons can be found in Pérez-Granados et al. [30]. In his pioneering descriptive works, Lancaster [51,52] declared that the calling activity of two tinamou species varied widely among successive days and that daily variations did not appear to be related to the presence or absence of females or other males at calling sites or to climatic predictors. Although we monitored a different tinamou species, our results suggest that daily variations in the calling activity of tinamous might be associated with daily weather conditions.
The impact of minimum air temperature varied among seasons but was constant among species. Contrary to our prediction, the daily calling activity of both focal species was positively related to the air temperature during the wet season. The highest vocal activities occurred at days with higher air temperature. This result is in disagreement with previous studies with other tropical nonpasserines in the study area (see e.g., Pérez-Granados et al. [30]) and a prior study on the closely related Boucard Tinamou (Crypturellus boucardi) in Honduras, where Lancaster [51] suggested a negative impact of air temperature on calling activity of the species. Nonetheless, the positive association between birds’ vocal activity and air temperature is in agreement with a previous study carried out in the study area using an anuran as focal species (Elachistocleis matogrosso, Pérez-Granados et al. [35]). In that study, air temperature was shown to be the most important predictor explaining vocal activity of this frog, with higher vocal activity at hotter days. Although a different taxon was used as model species, we cannot rule out that a similar mechanism might be behind the results found in that study. The pattern found in our study might be related to monthly variations in the vocal activity of both focal species within the dry season. The mean number of calls uttered per day by the Chaco Chachalaca and the Undulated Tinamou during July (first month of the dry season) was 2.5 and three times lower, respectively, than that detected during September (last month of the dry season), when the breeding season of both species begins [30]. Likewise, the average minimum temperature during July was 21.6 °C, while during September it was 25.9 °C. Therefore, the positive relationship found between minimum air temperature and calling rate of the species might be partly related to the change of breeding status of calling birds during the dry season. On the other hand, the daily calling activity of both focal species was not related to the daily minimum air temperature during the wet season. Further research should try to elucidate the mechanisms behind the results found in our study. Likewise, future studies should try to collect measurements of air temperature at hourly scale aiming to perform finer analyses, since a single value per day (as in our analyses) may not be representative of the relationship between birds’ vocal activity and air temperature.
The relationship between the daily calling activity of both species and daily rainfall differed between seasons. Consistent with our predictions, daily rainfall showed a positive relationship with the daily calling activities of the Undulated Tinamou and the Chaco Chachalaca during the dry season, while daily rainfall was negatively correlated with the calling activity of the Undulated Tinamou during the wet season. Rainy days during the dry season were very scarce; indeed, there were only six days with rainfall greater than 0.2 mm. Small variations in daily rainfall during the dry season may have a positive effect on insect populations, which are usually low during the dry season in the tropics [53]. Likewise, daily rainfall may have a positive impact on flowering and fruiting [54]. Therefore, the higher calling activity of both species on rainy days during the dry season might be partly linked to an increase in food availability, which allows birds to dedicate more time to calling activities. The negative relationship between daily rainfall and calling activity of the Undulated Tinamou during the wet season may be related to the large number of rainy days during this season (48 days with daily rainfall higher than 0.2 mm). This result is in agreement with prior studies on tinamous that suggested that they stop calling during periods of heavy rain [52]. During periods of heavy rain, birds may reduce their activity and look for cover [11,19,20]. Likewise, the sound of rain may reduce sound transmission and make bird acoustic communication less efficient [21,22]. Therefore, the lower calling activity of the Undulated Tinamou during the wet season might be due to a change in the species vocal behavior. Water availability during the wet season is very high in the study area, and therefore, an increase in rainfall activity may not support an improvement in habitat conditions (e.g., food availability), contrary to what may occur during the dry season. However, the daily calling activity of the Chaco Chachalaca was not related to daily rainfall during the wet season. Although we do not have a clear explanation for this difference between species, it could be related to the different habitat uses of each species. The Undulated Tinamou is a terrestrial species whose movements, and thus vocal activity, might be negatively influenced during rainy days. However, the Chaco Chachalaca spends a large amount of its time feeding and vocalizing from trees, and therefore, the feeding and vocal activity of the species could be less influenced by rainfall events.
The dry season was the period with the highest calling activities in both species and the period during which the daily vocal activities of the Undulated Tinamou and the Chaco Chachalaca were influenced by a larger number of climatic variables. The two analyzed variables were significantly related to the calling production of the Undulated Tinamou and the Chaco Chachalaca during the dry season. However, during the wet season, daily calling activity was significantly related to just daily rainfall in the Undulated Tinamou. Our results suggest that both species were more selective when calling during the dry season, the period with higher calling activity. Calling production is a costly phenomenon [3], and thus birds may choose days with better climatic conditions to save energy during periods of maximum calling activity. However, both species were less selective regarding the timing of calling during the wet season, when vocal output was much reduced.
The daily vocal activity of both focal species varied among some of the acoustic monitoring stations. That variation is likely related to different number of individuals vocalizing around recorders, since it is expected to find a positive association between the number of calls detected in sound recordings and the number of individuals vocalizing around recorders (see review in Pérez-Granados and Traba) [55]. Although we were unable to include the number of individuals vocalizing around recorders as a covariate in the analyses, it should not have influenced our results. The large spatial and temporal scale achieved by applying passive acoustic monitoring could make the inclusion of such a covariate in future studies a challenging perspective. A feasible solution to estimate such covariate, might be to automatically discriminate among individuals based on the different call structure of each individual [56,57], once individual recognition has been tested for the focal species before.
In this paper, we aimed to contribute to the current understanding of the effect of proximate climatic drivers on the daily calling activity of two secretive tropical birds. We described and tested, for the first time, seasonal variation effects of climatic conditions on the daily calling activity of birds. The results varied between species and even between seasons and suggest that further studies assessing the impact of climatic conditions on bird vocal activity should be implemented over multiple annual cycles and using different bird species, since their conclusions about the relationships between bird vocal activity and climatic conditions may differ among seasons, years and species. Future studies should attempt to include information about hormonal concentrations and sex, breeding status, and number of recorded individuals to improve our methodological approach and elucidate the mechanisms behind the associations found in our study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13070319/s1, Figure S1: Spectrogram of a typical Undulated Tinamou (top) and Chaco Chachalaca (bottom) call in the Brazilian Pantanal, Figure S2: Locations of the four acoustic monitoring stations (yellow pins) and of the meteorological station (red square) in Pantanal Matogrossense (Poconé municipality, Mato Grosso, Brazil). The inset shows location of the study area (star) in Brazil. The Cuiabá River is shown in the lower right corner of the image. The image was extracted from Google Earth, 31 March 2016. Scale bar: 2 km, Table S1: Mean (and range) acoustical parameters of the Undulated Tinamou and Chaco Chachalaca call in the Brazilian Pantanal. Acoustical parameters were obtained after measuring 52 Undulated Tinamou calls and 47 Chaco Chachalaca calls. Recordings were collected using a Song Meter SM2 recorder (Wildlife Acoustics), and call measurements were made using Raven Pro 1.5. Intersyllable gap refers to the time lapse between the first and the second syllable, Table S2: Loading factors of the PCA performed with the three air temperature variables.
Author Contributions
Conceived the idea, design, experiment (supervised research, formulated question or hypothesis): C.P.-G., K.-L.S.; Performed the experiments (collected data, conducted the research): K.-L.S.; Wrote the paper (or substantially edited the paper): C.P.-G., K.-L.S.; Developed or designed methods: C.P.-G.; Analyzed the data: C.P.-G.; Contributed substantial materials, resources, or funding: K.-L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES), Finance Code 01; Instituto Nacional de Ciência e Tecnologia em Áreas Úmidas (INAU/UFMT/CNPq); Centro de Pesquisa do Pantanal (CPP); and Brehm Funds for International Bird Conservation (BF), Bonn, Germany.
Institutional Review Board Statement
The study was carried out according to Brazilian laws and the SISBIO permit (KLS No. 39095).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the SESC Pantanal, Mato Grosso, for permission to conduct research on their property and their logistical help with our fieldwork. We also thank Ana Silvia Tissiani for her technical support and two anonymous reviewers whose comments helped to improve the manuscript. We wish to thank José de Souza Nogueira from the Post-Graduate Program in Physics, UFMT, who kindly provided the weather data for the time period addressed in our study. This study is part of the biodiversity monitoring project “Sounds of the Pantanal–The Pantanal Automated Audiovisual Biodiversity Monitoring Program” of INAU, Cuiabá, Mato Grosso, Brazil, which was conducted under SISBIO permit no. 39095 (KLS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marler, P. Bird calls: Their potential for behavioral neurobiology. Ann. N. Y. Acad. Sci. 2004, 1016, 31–44. [Google Scholar] [CrossRef]
- Farnsworth, A. Flight calls and their value for future ornithological studies and conservation research. Auk 2005, 122, 733–746. [Google Scholar] [CrossRef]
- Catchpole, C.K.; Slater, P.J. Bird Song: Biological Themes and Variations, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; p. 335. ISBN 978-0-521-87242-3. [Google Scholar]
- Sandoval, L.; Méndez, C.; Mennill, D.J. Vocal behaviour of White-eared Ground-sparrows (Melozone leucotis) during the breeding season: Repertoires, diel variation, behavioural contexts, and individual distinctiveness. J. Ornithol. 2016, 157, 1–12. [Google Scholar] [CrossRef]
- Staicer, C.A.; Spector, D.A.; Horn, A.G. The dawn chorus and other diel patterns in acoustic signaling. In Ecology and Evolution of Acoustic Communication in Birds; Kroodsma, D.E., Miller, E.H., Eds.; Cornell University Press: New York, NY, USA, 1996; pp. 426–453. [Google Scholar]
- Staicer, C.A. Honest advertisement of pairing status: Evidence from a tropical resident wood-warbler. Anim. Behav. 1996, 51, 375–390. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Osiejuk, T.S.; López-Iborra, G.M. Dawn chorus interpretation differs when using songs or calls: The Dupont’s Lark Chersophilus duponti case. PeerJ 2018, 6, e5241. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, V.; Korner, P.; Naguib, M. Nocturnal and diurnal singing activity in the nightingale: Correlations with mating status and breeding cycle. Anim. Behav. 2002, 64, 939–944. [Google Scholar] [CrossRef]
- Rowan, W. Experiments in bird migration, I. Manipulation of the reproductive cycle: Seasonal histological changes in the gonads. In Proceedings of the Boston Society of Natural History, Boston, MA, USA, 1 January 1929; Volume 39, pp. 115–208. [Google Scholar]
- Ball, G.F.; Hulse, S.H. Birdsong. Am. Psychol. 1998, 53, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.S. Effect of time and day on bird activity. Stud. Avian Biol. 1981, 6, 275–282. [Google Scholar]
- Boncoraglio, G.; Saino, N. Habitat structure and the evolution of bird song: A meta-analysis of the evidence for the acoustic adaptation hypothesis. Funct. Ecol. 2007, 21, 134–142. [Google Scholar] [CrossRef]
- York, J.E.; Young, A.J.; Radford, A.N. Singing in the moonlight: Dawn song performance of a diurnal bird varies with lunar phase. Biol. Lett. 2014, 10, 20130970. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L. Monitoring the annual vocal activity of two enigmatic nocturnal Neotropical birds: The Common Potoo (Nyctibius griseus) and the Great Potoo (Nyctibius grandis). J. Ornithol. 2020, 161, 1129–1141. [Google Scholar] [CrossRef]
- Potvin, D.A.; Parris, K.M.; Mulder, R.A. Geographically pervasive effects of urban noise on frequency and syllable rate of songs and calls in silvereyes (Zosterops lateralis). Proc. R. Soc. Lond. B Biol. Sci. 2011, 278, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.J.; Wilson, D.R.; Mennill, D.J. Anthropogenic light is associated with increased vocal activity by nocturnally migrating birds. Condor 2016, 118, 338–344. [Google Scholar] [CrossRef]
- Møller, A.P. When climate change affects where birds sing. Behav. Ecol. 2010, 22, 212–217. [Google Scholar] [CrossRef]
- Bas, Y.; Devictor, V.; Moussus, J.P.; Jiguet, F. Accounting for weather and time-of-day parameters when analysing count data from monitoring programs. Biodivers. Conserv. 2008, 17, 3403–3416. [Google Scholar] [CrossRef]
- Keast, A. Temporal vocalisation patterns in members of a Eucalypt forest bird community: The effects of weather on song production. Emu 1994, 94, 172–180. [Google Scholar] [CrossRef]
- Zuberogoitia, I.; Burgos, G.; González-Oreja, J.A.; Morant, J.; Martínez, J.E.; Zabala Albizua, J. Factors affecting spontaneous vocal activity of Tawny Owls Strix aluco and implications for surveying large areas. Ibis 2019, 161, 495–503. [Google Scholar] [CrossRef]
- Ryan, M.J.; Brenowitz, E.A. The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am. Nat. 1985, 126, 87–100. [Google Scholar] [CrossRef]
- Lengagne, T.; Slater, P.J. The effects of rain on acoustic communication: Tawny owls have good reason for calling less in wet weather. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 2121–2125. [Google Scholar] [CrossRef]
- Digby, A.; Towsey, M.; Bell, B.D.; Teal, P.D. Temporal and environmental influences on the vocal behaviour of a nocturnal bird. J. Avian Biol. 2014, 45, 591–599. [Google Scholar] [CrossRef]
- Curio, E. Beiträge zur Populationsökologie des Trauerschnäppers (Ficedula hypoleuca Pallas). Zool. Jarhbücher 1959, 87, 185–230. [Google Scholar]
- Garson, P.J.; Hunter, J.R.M.L. Effects of temperature and time of year on the singing behaviour of wrens Troglodytes and great tits Parus major. Ibis 1979, 121, 481–487. [Google Scholar] [CrossRef]
- Strain, J.G.; Mumme, R.L. Effects of food supplementation, song playback, and temperature on vocal territorial behaviour of Carolina Wrens. Auk 1988, 105, 11–16. [Google Scholar] [CrossRef]
- Riebel, K.; Odom, K.J.; Langmore, N.E.; Hall, M.L. New insights from female bird song: Towards an integrated approach to studying male and female communication roles. Biol. Lett. 2019, 15, 20190059. [Google Scholar] [CrossRef]
- Topp, S.M.; Mennill, D.J. Seasonal variation in the duetting behaviour of rufous-and-white wrens (Thryothorus rufalbus). Behav. Ecol. Sociobiol. 2008, 62, 1107–1117. [Google Scholar] [CrossRef]
- Odom, K.J.; Omland, K.E.; McCaffrey, D.R.; Monroe, M.K.; Christhilf, J.L.; Roberts, N.S.; Logue, D.M. Typical males and unconventional females: Songs and singing behaviors of a tropical, duetting oriole in the breeding and non-breeding season. Front. Ecol. Evol. 2016, 4, 14. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L.; Marques, M.I. Vocal behavior of the Undulated Tinamou (Crypturellus undulatus) over an annual cycle in the Brazilian Pantanal: New ecological information. Biotropica 2020, 52, 165–171. [Google Scholar] [CrossRef]
- Hau, M.; Perfito, N.; Moore, I.T. Timing of breeding in tropical birds: Mechanisms and evolutionary implications. Ornitol. Neotrop. 2008, 19, 39–59. [Google Scholar]
- Demko, A.D.; Mennill, D.J. Rufous-capped Warblers Basileuterus rufifrons show seasonal, temporal and annual variation in song use. Ibis 2019, 161, 481–494. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L. Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi). Diversity 2020, 12, 402. [Google Scholar] [CrossRef]
- Mennill, D.J. Variation in the vocal behavior of common loons (Gavia immer): Insights from landscape-level recordings. Waterbirds 2014, 37, 26–36. [Google Scholar] [CrossRef][Green Version]
- Pérez-Granados, C.; Schuchmann, K.-L.; Marques, M.I. Vocal activity of the Ferruginous Pygmy-Owl (Glaucidium brasilianum) is strongly correlated with moon phase and nocturnal temperature. Ethol. Ecol. Evol. 2020, 33, 62–72. [Google Scholar] [CrossRef]
- Cabot, J.; Christie, D.A.; Jutglar, F.; Sharpe, C.J. Undulated Tinamou (Crypturellus undulatus), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Cabot, J. Family Tinamidae (Tinamous). In Handbook of the Birds of the World. Volume 1: Ostrich to Duck; del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1992; pp. 112–138. [Google Scholar]
- Davies, S.J.J.F. Ratites and Tinamous; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- del Hoyo, J.; Kirwan, G.M. Chaco Chachalaca (Ortalis canicollis), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Junk, W.J.; Da Cunha, C.N.; Wantzen, K.M.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Heckman, C.W. The Pantanal of Poconé; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998. [Google Scholar]
- de Deus, F.F.; Arieira, J.; Schuchmann, K.-L.; Tissiani, A.S.O.; Marques, M.I. Avian beta-diversity in a Neotropical wetland: Effects of flooding and vegetation. Wetlands 2020, 40, 1513–1527. [Google Scholar] [CrossRef]
- Rempel, R.S.; Francis, C.M.; Robinson, J.N.; Campbell, M. Comparison of audio recording system performance for detecting and monitoring songbirds. J. Field Ornithol. 2013, 84, 86–97. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Bota, G.; Giralt, D.; Albarracín, J.; Traba, J. Cost-effective assessment of five audio recording systems for wildlife monitoring: Differences between recording distances and singing direction. Ardeola 2019, 66, 311–325. [Google Scholar] [CrossRef]
- Bioacoustics Research Program. Raven Pro: Interactive Sound Analysis Software (Version 1.5). [Computer software]; The Cornell Lab of Ornithology: Ithaca, NY, USA, 2014; Available online: https://www.birds.cornell.edu/raven (accessed on 18 May 2020).
- Knight, E.; Hannah, K.; Foley, G.; Scott, C.; Brigham, R.; Bayne, E. Recommendations for acoustic recognizer performance assessment with application to five common automated signal recognition programs. Avian Conserv. Ecol. 2017, 12, 14. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L. Nocturnal vocal behavior of the diurnal Undulated Tinamou Crypturellus undulatus is associated with temperature and moon phase. Ibis 2020, 163, 684–694. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Harrison, X.A. A comparison of observation-level random effect and Beta-Binomial models for modelling overdispersion in Binomial data in ecology & evolution. PeerJ 2015, 3, e1114. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org (accessed on 12 July 2021).
- Lancaster, D.A. Life history of the Boucard tinamou in British Honduras. Part I: Distribution and general behavior. Condor 1964, 66, 165–181. [Google Scholar] [CrossRef]
- Lancaster, D.A. Biology of the Brushland Tinamou, Nothoprocta cinerascens. Bull. Am. Mus. Nat. Hist. 1964, 127, 269–314. [Google Scholar]
- Wolda, H. Seasonal fluctuations in rainfall, food and abundance of tropical insects. J. Anim. Ecol. 1978, 47, 369–381. [Google Scholar] [CrossRef]
- Van Schaik, C.P.; Terborgh, J.W.; Wright, S.J. The phenology of tropical forests, adaptative significante, and consequences for primary consumers. Ann. Rev. Ecol. Syst. 1993, 24, 353–377. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Traba, J. Estimating bird density using passive acoustic monitoring: A review of methods and suggestions for further research. Ibis 2021, 163, 765–783. [Google Scholar] [CrossRef]
- Ehnes, M.; Foote, J.R. Comparison of autonomous and manual recording methods for discrimination of individually distinctive ovenbird songs. Bioacoustics 2015, 24, 111–121. [Google Scholar] [CrossRef]
- Dent, J.M.; Molles, L.E. Call-based identification as a potential tool for monitoring Great spotted kiwi. Emu 2016, 116, 315–322. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).