Mitochondrial Genetic Diversity among Farmed Stocks of Oreochromis spp. (Perciformes, Cichlidae) in Madagascar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling, Sequencing and International Repositories
2.2. Mitochondrial Lineage Delimitation and Genetic Diversity
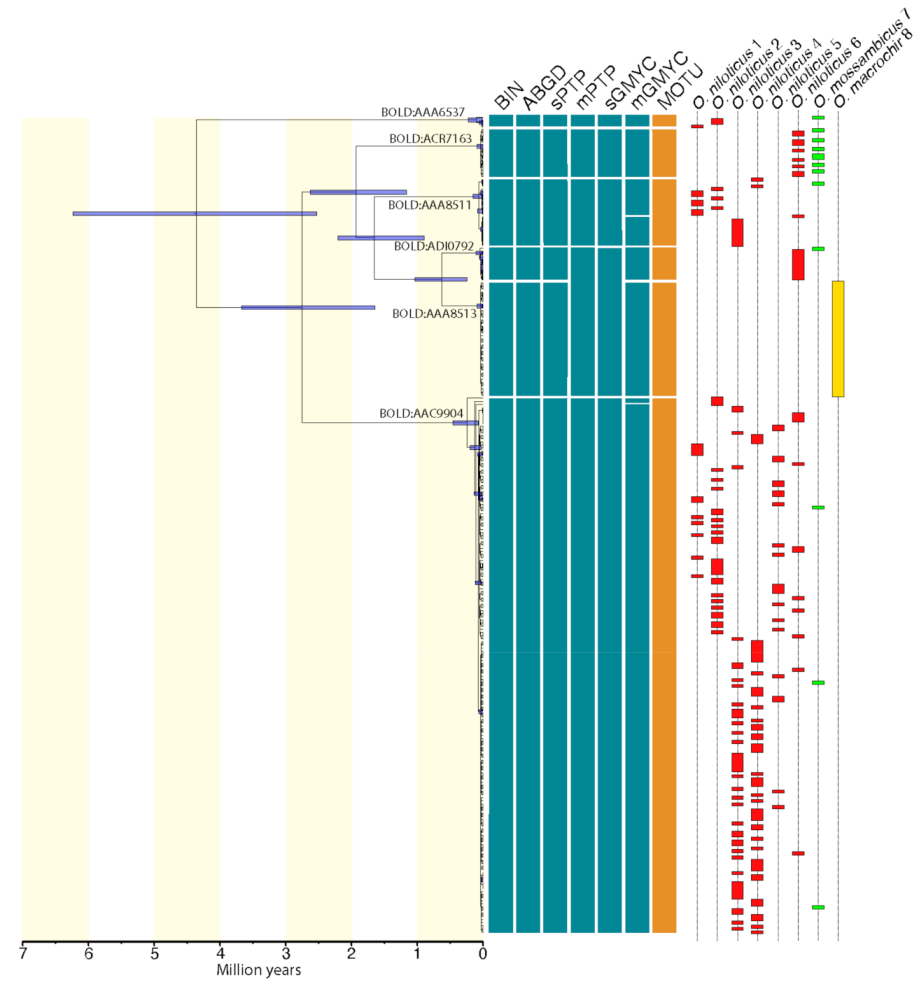
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; De Palma, A.; Ferrier, S.; Hill, S.L.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.P. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef]
- Thiault, L.; Mora, C.; Cinner, J.E.; Cheung, W.W.L.; Graham, N.A.J.; Januchowski-Hartley, F.A.; Mouillot, D.; Sumaila, U.R.; Claudet, J. Escaping the perfect storm of simultaneous climate change impacts on agriculture and marine fisheries. Sci. Adv. 2019, 5, eaaw9976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Des Pêches Et De L’aquaculture; FAO: Rome, Italy, 2018; ISBN 9789251306925. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing Climate Change Adaptation Needs for Food Security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Pauly, D. Fishbase. Available online: http://www.fishbase.org (accessed on 14 January 2021).
- Kiener, A. Intérêt et perspectives de la pisciculture de la carpe à Madagascar. Bull. Madag. 1958, 8, 693–702. [Google Scholar]
- Kiener, A. Poissons, pêche et pisciculture à Madagascar. Centrotechique For. Trop. Fr. 1963, 405, 599–606. [Google Scholar]
- Therezien, Y. L’introduction de poissons d’eau douce à Madagascar, leur influence sur la modification du biotope. Bull. Français Piscic. 1960, 199, 45–61. [Google Scholar] [CrossRef]
- Duchaufour, H.; Razafimbelo-Andriamifidy, T.; Rakotoarisoa, J.; Ramamonjisoa, B. ; Rakotondravao. Recherche Interdisciplinaire pour le Développement Durable Application à Différentes Thématiques de Territoire et la Biodiversité des Espaces Ruraux Malgaches; Cirad: Antananarivo, Madagascar, 2016. [Google Scholar]
- Bentz, B.; Oswald, M. Respective roles of national institutions and farmers groups in the implementation of an innovation enabling smallholders to reproduce Carp inside their rice fields in betafo (Madagascar). In Proceedings of the Symposium Innovation and Sustainable Development in Agriculture and Food, ISDA 2010, Montpellier, France, 28 June 2010. [Google Scholar]
- Ravakarivelo, M.; Pepey, E.; Benzie, J.; Raminosoa, N.; Rasamoelina, H.; Mikolasek, O.; De Verdal, H. Genetic variation in wild populations and farmed stocks of Nile tilapia (Oreochromis niloticus) in Madagascar. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux 2019, 72, 101. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Hamzah, A.; Tan, S.; Kamaruzzaman, N. Genetic parameters and response to selection for live weight in the GIFT strain of Nile tilapia (Oreochromis niloticus). Aquaculture 2005, 247, 203–210. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Hamzah, A.; Bakar, K.R.A.; Yee, H.Y. Genetic improvement of Nile tilapia (Oreochromis niloticus) with special reference to the work conducted by the WorldFish Center with the GIFT strain. Rev. Aquac. 2011, 3, 27–41. [Google Scholar] [CrossRef]
- Gupta, M.V.; Acosta, B.O. From drawing board to dining table: The success story of the GIFT project. NAGA WorldFish Cent. Q. 2004, 27, 4–14. [Google Scholar]
- Ward, R.D.; Hanner, R.H.; Hebert, P.D.N. The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 2009, 74, 329–356. [Google Scholar] [CrossRef]
- Hubert, N.; Hanner, R.; Holm, E.; Mandrak, N.E.; Taylor, E.; Burridge, M.; Watkinson, D.; Dumont, P.; Curry, A.; Bentzen, P.; et al. Identifying Canadian freshwater fishes through DNA barcodes. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hébert, P.D.N. Universal primers cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Hebert, P.D.N.; deWaard, J.R.; Zakharov, E.; Prosser, S.W.J.; Sones, J.E.; McKeown, J.T.A.; Mantle, B.; La Salle, J. A DNA “barcode blitz”: Rapid digitization and sequencing of a natural history collection. PLoS ONE 2013, 8, e68535. [Google Scholar] [CrossRef]
- Pons, J.; Barraclough, T.G.; Gomez-Zurita, J.; Cardoso, A.; Duran, D.P.; Hazell, S.; Kamoun, S.; Sumlin, W.D.; Vogler, A.P. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 2006, 55, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-rate Poisson Tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, A.; Ling, C.; Ho, S.Y.W.; Zhu, C.-D. Comparison of methods for molecular species delimitation across a range of speciation scenarios. Syst. Biol. 2018, 67, 830–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kekkonen, M.; Mutanen, M.; Kaila, L.; Nieminen, M.; Hebert, P.D.N. Delineating species with DNA barcodes: A case of taxon dependent method performance in moths. PLoS ONE 2015, 10, e0122481. [Google Scholar] [CrossRef] [Green Version]
- Kekkonen, M.; Hebert, P.D.N. DNA barcode-based delineation of putative species: Efficient start for taxonomic workflows. Mol. Ecol. Resour. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Shen, Y.; Hubert, N.; Huang, Y.; Wang, X.; Gan, X.; Peng, Z.; He, S. DNA barcoding the ichthyofauna of the Yangtze River: Insights from the molecular inventory of a mega-diverse temperate fauna. Mol. Ecol. Resour. 2019, 19, 1278–1291. [Google Scholar] [CrossRef]
- Sholihah, A.; Delrieu-Trottin, E.; Sukmono, T.; Dahruddin, H.; Risdawati, R.; Elvyra, R.; Wibowo, A.; Kustiati, K.; Busson, F.; Sauri, S.; et al. Disentangling the taxonomy of the subfamily Rasborinae (Cypriniformes, Danionidae) in Sundaland using DNA barcodes. Sci. Rep. 2020, 10, 2818. [Google Scholar] [CrossRef] [Green Version]
- Limmon, G.; Delrieu-Trottin, E.; Patikawa, J.; Rijoly, F.; Dahruddin, H.; Busson, F.; Steinke, D.; Hubert, N. Assessing species diversity of Coral Triangle artisanal fisheries: A DNA barcode reference library for the shore fishes retailed at Ambon harbor (Indonesia). Ecol. Evol. 2020, 10, 3356–3366. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Barraclough, T.G. Delimiting species using single-locus data and the generalized mixed Yule coalescent approach: A revised method and evaluation on simulated data sets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bermingham, E.; McCafferty, S.; Martin, A.P. Fish biogeography and molecular clocks: Perspectives from the Panamanian isthmus. In Molecular Systematics of Fishes; Kocher, T.D., Stepien, C.A., Eds.; CA Academic Press: San Diego, CA, USA, 1997; pp. 113–128. [Google Scholar]
- Ogilvie, H.A.; Bouckaert, R.R.; Drummond, A.J. StarBEAST2 brings faster species tree inference and accurate estimates of substitution rates. Mol. Biol. Evol. 2017, 34, 2101–2114. [Google Scholar] [CrossRef]
- Ho, S.Y.W.; Larson, G. Molecular clocks: When times are a-changin’. TRENDS Genet. 2006, 22, 79–83. [Google Scholar] [CrossRef]
- Paradis, E. pegas: An {R} package for population genetics with an integrated-modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef] [Green Version]
- Nei, M.; Tajima, F. DNA polymorphism detectable by restriction endonucleases. Genetics 1981, 97, 145–163. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: Chichester, NY, USA, 1987; ISBN 0231063210. [Google Scholar]
- Watterson, G.A. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 1975, 7, 256–276. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Kimura, M. A Simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide-sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Brown, S.D.J.; Collins, R.A.; Boyer, S.; Lefort, C.; Malumbres-Olarte, J.; Vink, C.J.; Cruickshank, R.H. Spider: An R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Mol. Ecol. Resour. 2012, 12, 562–565. [Google Scholar] [CrossRef]
- Ford, A.G.P.; Bullen, T.R.; Pang, L.; Genner, M.J.; Bills, R.; Flouri, T.; Ngatunga, B.P.; Rüber, L.; Schliewen, U.K.; Seehausen, O. Molecular phylogeny of Oreochromis (Cichlidae: Oreochromini) reveals mito-nuclear discordance and multiple colonisation of adverse aquatic environments. Mol. Phylogenet. Evol. 2019, 136, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Nyingi, D.W.; Agnèse, J. Recent introgressive hybridization revealed by exclusive mtDNA transfer from Oreochromis leucostictus (Trewavas, 1933) to Oreochromis niloticus (Linnaeus, 1758) in Lake Baringo, Kenya. J. Fish Biol. 2007, 70, 148–154. [Google Scholar] [CrossRef]
- D’Amato, M.E.; Esterhuyse, M.M.; Van Der Waal, B.C.W.; Brink, D.; Volckaert, F.A.M. Hybridization and phylogeography of the Mozambique tilapia Oreochromis mossambicus in southern Africa evidenced by mitochondrial and microsatellite DNA genotyping. Conserv. Genet. 2007, 8, 475–488. [Google Scholar] [CrossRef]
- Gregg, R.E.; Howard, J.H.; Snhonhiwa, F. Introgressive hybridization of tilapias in Zimbabwe. J. Fish Biol. 1998, 52, 1–10. [Google Scholar] [CrossRef]
- Mojekwu, T.O.; Cunningham, M.J.; Bills, R.I.; Pretorius, P.C.; Hoareau, T.B. Utility of DNA barcoding in native Oreochromis species. J. Fish Biol. 2021, 98, 498–506. [Google Scholar] [CrossRef]
- Firmat, C.; Alibert, P.; Losseau, M.; Baroiller, J.-F.; Schliewen, U.K. Successive invasion-mediated interspecific hybridizations and population structure in the endangered cichlid Oreochromis mossambicus. PLoS ONE 2013, 8, e63880. [Google Scholar] [CrossRef]
- Seehausen, O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004, 19, 198–207. [Google Scholar] [CrossRef]
- Selz, O.M.; Seehausen, O. Interspecific hybridization can generate functional novelty in cichlid fish. Proc. R. Soc. B 2019, 286, 20191621. [Google Scholar] [CrossRef] [Green Version]
- Englebrecht, C.; Freyhof, J.; Nolte, A.; Rassman, K.; Schliewen, U.; Tautz, D. Phylogeography of the bullhead Cottus gobio (Pisces: Teleostei: Cottidae) suggests a pre-Pleistocene origin of the major central European populations. Mol. Ecol. 2000, 9, 709–722. [Google Scholar] [CrossRef]
- Fricke, R.; Mahafina, J.; Behivoke, F.; Jaonalison, H.; Léopold, M.; Ponton, D. Annotated checklist of the fishes of Madagascar, southwestern Indian Ocean, with 158 new records. FishTaxa 2018, 3, 1–432. [Google Scholar]
- Oswald, M.R.; Ravakarivelo, M.; Mikolasek, O.; Rasamoelina, H.; de Verdal, H.; Bentz, B.; Pepey, E.; Al, E. Combining a comprehensive approach to fish-farming systems with assessment of their genetics—From planning to realization. In Actes Projet FSP PARRUR, Recherche Interdisciplinaire pour le Développement Durable et la Biodiversité des Espaces Ruraux Malgaches. Application à Différentes Thématiques de Territoire; Parrur, Ed.; Cirad: Antananarivo, MG, USA, 2016; pp. 219–267. [Google Scholar]
- Borrell, Y.J.; Pineda, H.; McCarthy, I.; Vazquez, E.; Sanchez, J.A.; Lizana, G.B. Correlations between fitness and heterozygosity at allozyme and microsatellite loci in the Atlantic salmon, Salmo salar L. Heredity 2004, 92, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieutenant-Gosselin, M.; Bernatchez, L. Local heterozygosity-fitness correlations with global positive effects on fitness in threespine stickleback. Evolution 2006, 60, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Borrell, Y.J.; Carleos, C.E.; Sánchez, J.A.; Vázquez, E.; Gallego, V.; Asturiano, J.F.; Blanco, G. Heterozygosity–fitness correlations in the gilthead sea bream Sparus aurata using microsatellite loci from unknown and gene-rich genomic locations. J. Fish Biol. 2011, 79, 1111–1129. [Google Scholar] [CrossRef]

| N | K2P Distance | O. niloticus (Freq.) | O. mossambicus (Freq.) | O. macrochir (Freq.) | O. aureus (Freq.) | O. urolepis (Freq.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BIN | Present Study | BOLD | Within (Max) | Between (Min) | Present Study | BOLD | Present Study | BOLD | Present Study | BOLD | Present Study | BOLD | Present Study | BOLD |
| BOLD:AAA6537 | 4 | 304 | 0.004 | 0.065 | 0.75 | 0.33 | 0.25 | 0.0001 | - | - | - | 0.3 | - | - |
| BOLD:AAA8511 | 22 | 234 | 0.004 | 0.035 | 0.9995 | 0.14 | 0.0005 | 0.52 | - | - | - | - | - | - |
| BOLD:AAA8513 | 37 | 18 | 0 | 0.016 | - | - | - | - | 1 | 0.17 | - | - | - | - |
| BOLD:AAC9904 | 172 | 417 | 0.007 | 0.050 | 0.9998 | 0.73 | 0.0002 | 0.0001 | - | - | - | - | - | - |
| BOLD:ACR7163 | 16 | 24 | 0 | 0.036 | 0.56 | - | 0.44 | 0.01 | - | - | - | - | - | 0.55 |
| BOLD:ADI0792 | 11 | 28 | 0 | 0.016 | 0.9 | - | 0.1 | 0.29 | - | - | - | - | - | - |
| Species | Strain | Locality | Site | N | h | Hd | π | θw | D | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| O. niloticus | GIFT | Matera | 1 | 16 | 3 | 0.575 | 0.031 | 18.082 | 0.434 | 0.664 |
| O. niloticus | GIFT | Tamatave | 2 | 31 | 8 | 0.539 | 0.018 | 15.519 | −1.262 | 0.207 |
| O. niloticus | Local | Ampefy | 3 | 58 | 7 | 0.622 | 0.016 | 8.209 | 0.554 | 0.579 |
| O. niloticus | JICA | Mahajunga | 4 | 46 | 4 | 0.243 | <0.001 | 0.455 | −1.481 | 0.127 |
| O. niloticus | Local | Fenerive | 5 | 30 | 3 | 0.393 | 0.008 | 8.582 | −1.719 | 0.086 |
| O. niloticus | Local | Brickaville | 6 | 31 | 6 | 0.785 | 0.037 | 14.268 | 2.086 | 0.037 |
| O. mossambicus | Local | Fenerive | 7 | 13 | 6 | 0.718 | 0.037 | 23.52 | 0.067 | 0.947 |
| O. macrochir | Local | Milasoa | 8 | 37 | 2 | 0.054 | <0.001 | 0.240 | −1.131 | 0.258 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubert, N.; Pepey, E.; Mortillaro, J.-M.; Steinke, D.; Andria-Mananjara, D.E.; de Verdal, H. Mitochondrial Genetic Diversity among Farmed Stocks of Oreochromis spp. (Perciformes, Cichlidae) in Madagascar. Diversity 2021, 13, 281. https://doi.org/10.3390/d13070281
Hubert N, Pepey E, Mortillaro J-M, Steinke D, Andria-Mananjara DE, de Verdal H. Mitochondrial Genetic Diversity among Farmed Stocks of Oreochromis spp. (Perciformes, Cichlidae) in Madagascar. Diversity. 2021; 13(7):281. https://doi.org/10.3390/d13070281
Chicago/Turabian StyleHubert, Nicolas, Elodie Pepey, Jean-Michel Mortillaro, Dirk Steinke, Diana Edithe Andria-Mananjara, and Hugues de Verdal. 2021. "Mitochondrial Genetic Diversity among Farmed Stocks of Oreochromis spp. (Perciformes, Cichlidae) in Madagascar" Diversity 13, no. 7: 281. https://doi.org/10.3390/d13070281
APA StyleHubert, N., Pepey, E., Mortillaro, J.-M., Steinke, D., Andria-Mananjara, D. E., & de Verdal, H. (2021). Mitochondrial Genetic Diversity among Farmed Stocks of Oreochromis spp. (Perciformes, Cichlidae) in Madagascar. Diversity, 13(7), 281. https://doi.org/10.3390/d13070281







