Ecology and Conservation of the Laotian langur Trachypithecus laotum in a Protected Area of Laos (Southeast Asia)
Abstract
1. Introduction
- (i)
- What is the density of the Laotian langur groups in the study area? Although scientists primarily use presence and absence data for conservation planning of species even in large landscapes, we can obtain a much better and accurate conservation planning if we include data on individual species abundance or density in the favored habitats [24].
- (ii)
- What is the current group size of these monkeys, and has the average group size changed in the last twenty years in the study area? Although all studies on this species at Phou Hin Poun National Protected Area have been short-term, they have spread since 1994. Hence comparisons of the various collected datasets may show population size and conservation status trends. Given the heavy rates of deforestation and overhunting in Lao PDR [8,9,10,11], we predict that the group size of langur may be smaller nowadays than 20+ years ago, thus revealing an overall declining population.
- (iii)
- Are Laotian langur groups sympatric with other primates, and if so, what are their interspecific interactions? Threatened species may suffer from interspecific competition with closely related species, representing a further threat to their conservation. In addition, Laotian forests are inhabited by a rich diversity of primate species [25]. Thus it is likely that the Laotian langur groups should share their habitat with other primate species.
2. Materials and Methods
2.1. Study Area
2.2. Interviews and Historical Records
2.3. Field Surveys
2.4. Statistical Analyses
3. Results
3.1. Interviews and Historical Records
3.2. Field Surveys
3.3. Observations on Sympatry with Other Primates
3.4. Observations on Threats
4. Discussion
Threats and Conservation Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurance, W.F.; Vasconcelos, H.L.; Lovejoy, T.E. Forest loss and fragmentation in the Amazon: Implications for wildlife conservation. Oryx 2000, 34, 39–45. [Google Scholar] [CrossRef]
- Echeverria, C.; Coomes, D.A.; Hall, M.; Newton, A.C. Spatially explicit models to analyze forest loss and fragmentation between 1976 and 2020 in southern Chile. Ecol. Model. 2008, 212, 439–449. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Zhao, Z.; Zhang, Q.; Su, S. Socioeconomic drivers of forest loss and fragmentation: A comparison between different land use planning schemes and policy implications. Land Use Policy 2016, 54, 58–68. [Google Scholar] [CrossRef]
- Padalia, H.; Ghosh, S.; Reddy, C.S.; Nandy, S.; Singh, S.; Kumar, A.S. Assessment of historical forest cover loss and fragmentation in Asian elephant ranges in India. Environ. Monit. Assess. 2019, 191, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bierregaard, R.O., Jr.; Lovejoy, T.E.; Kapos, V.; dos Santos, A.A.; Hutchings, R.W. The biological dynamics of tropical rainforest fragments. Bioscience 1992, 42, 859–866. [Google Scholar] [CrossRef]
- Laurance, W.F. A crisis in the making: Responses of Amazonian forests to land use and climate change. Trends Ecol. Evol. 1998, 13, 411–415. [Google Scholar] [CrossRef]
- Laurance, W.F. Reflections on the tropical deforestation crisis. Biol. Conserv. 1999, 91, 109–117. [Google Scholar] [CrossRef]
- Timmins, R.J. Notes on Wildlife and Habitats in Khammouan Limestone National Biodiversity Conservation Area, Khammouan Province, Lao PDR 1997; Centre for Protected Areas and Watershed Management/The Wildlife Conservation Society Lao Program: Vientiane, Lao People’s Democratic Republic, 1997. [Google Scholar]
- Rigg, J.D. Forests, marketization, livelihoods and the poor in the Lao PDR. Land Degrad. Dev. 2006, 17, 123–133. [Google Scholar] [CrossRef]
- McNamara, S.; Erskine, P.D.; Lamb, D.; Chantalangsy, L.; Boyle, S. Primary tree species diversity in secondary fallow forests of Laos. For. Ecol. Manag. 2012, 281, 93–99. [Google Scholar] [CrossRef]
- Phompila, C.; Lewis, M.; Ostendorf, B.; Clarke, K. Forest cover changes in Lao tropical forests: Physical and socio-economic factors are the most important drivers. Land 2017, 6, 23. [Google Scholar] [CrossRef]
- Lamb, D. Deforestation and its consequences in the asia-pacific region. In Regreening the Bare Hills; Springer: Dordrecht, Germany, 2011; pp. 1–39. [Google Scholar]
- Long, B.; Gray, T.; Lynam, A.; Seng, T.; Laurance, W.; Scotson, L.; Ripple, W. Reversing ‘empty forest syndrome’ in Southeast Asia. Natl. Geogr. Voices 2017, 8, 1–6. [Google Scholar]
- Pruvot, M.; Khammavong, K.; Milavong, P.; Philavong, C.; Reinharz, D.; Mayxay, M.; Theppangna, W. Toward a quantification of risks at the nexus of conservation and health: The case of bushmeat markets in Lao PDR. Sci. Total Environm. 2019, 676, 732–745. [Google Scholar] [CrossRef]
- Zuklin, T.; Maury, N.; Sitthivong, S.; Pham, T.V.; Le Duc, O.; Bordes, C.; Leprince, B.; Ducotterd, C.; Oanh, L.V.; Vilay, P.; et al. The “empty forest syndrome” and the herpetofaunal communities in Laos (South-Eastern Asia). Russ. J. Herpetol. 2021, in press. [Google Scholar]
- Johnson, A.; Singh, S.; Duangdala, M.; Hedemark, M. The western black crested gibbon Nomascus concolor in Laos: New records and conservation status. Oryx 2005, 39, 311–317. [Google Scholar] [CrossRef]
- Sacklokham, S.; Dufumier, M. Land-tenure policy, deforestation, and agricultural development in Lao PDR: The case of the Vientiane plain. Moussons. Rech. En Sci. Hum. Sur L’asie Du Sud-Est 2006, 9, 189–207. [Google Scholar] [CrossRef]
- Suwannarong, K.; Chapman, R.S.; Lantican, C.; Michaelides, T.; Zimicki, S. Hunting, food preparation, and consumption of rodents in Lao PDR. PLoS ONE 2015, 10, e0133150. [Google Scholar] [CrossRef]
- Groves, C. Trachypithecus. In Mammal. Species of the World. A Taxonomic and Geographic Reference, 3rd ed.; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 175–178. ISBN 0-8018-8221-4. [Google Scholar]
- Timmins, R.J.; Boonratana, R. Trachypithecus laotum. In The IUCN Red List of Threatened Species; The International Union for Conservation of Nature: Gland, Switzerland, 2008; E.T 22044A9350930; Available online: www.iucnredlist.org (accessed on 21 March 2021).
- Steinmetz, R.; Timmins, R.J.; Duckworth, J.W. Distribution and conservation status of the Lao leaf monkey (Trachypithecus (Fr.) Laotum). Int. J. Primatol. 2011, 32, 587–604. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species, 2020–2021. IUCN Website. Available online: Iucnredlist.org (accessed on 27 April 2021).
- Coudrat, C.N.Z.; Nadler, T.; Phiapalath, P.; Duckworth, J.W. Trachypithecus laotum. In The IUCN Red List of Threatened Species; The International Union for Conservation of Nature: Gland, Switzerland, 2020; E.T 22044A1795913; Available online: www.iucnredlist.org (accessed on 21 March 2021).
- Veloz, S.; Salas, L.; Altman, B.; Alexander, J.; Jongsomjit, D.; Elliott, N.; Ballard, G. Improving effectiveness of systematic conservation planning with density data. Conserv. Biol. 2015, 29, 1217–1227. [Google Scholar] [CrossRef]
- Hamada, Y.; Malaivijitnond, S.; Kingsada, P.; Bounnam, P. The distribution and present status of primates in the northern region of Lao PDR. Trop. Nat. Hist. 2007, 7, 161–191. [Google Scholar]
- Brakels, P. Summary Report of the Zoning Activities in Phou Hin Poun National Protected Area; IUCN Lao PDR: Vientiane, Lao People’s Democratic Republic, 2019. [Google Scholar]
- Phiapalath, P. Brief Report of Lao Langur Trachypithecus laotum Survey in Phou Hin Poun National Protected Area, Khammouane Province; Lao Wildlife Conservation Association: Vientiane, Lao PDR, 2010. [Google Scholar]
- Brockelman, W.Y.; Ali, R. Methods of surveying and sampling forest primate populations. In Primate Conservation in the Tropical Forest; Marsh, C.W., Mittermeier, R.A., Eds.; Alan R. Liss: New York, NY, USA, 1987; pp. 23–62. [Google Scholar]
- Frankfurt Zoological Society in Vietnam. Biodiversity Survey of the Macaque, Langur and Douq Monkey in and around the Phong Nha–Ke Bang National Park, Quang Binh, Vietnam; Vietnam-Germany Development Cooperation: Hanoi, Vietnam, 2011. [Google Scholar]
- Fiore, R.R. A Survey of Indochinese Silvered Langurs (Trachypithecus germaini) in Phu Quoc National Park, Vietnam. Ph.D. Thesis, University of Colorado at Boulder, Boulder, CO, USA, 2015. [Google Scholar]
- Ha, N.M. Some observations on the Hatinh Langur, Trachypithecus laotum hatinhensis (Dao 1970), in north central Vietnam. Primate Conserv. 2006, 21, 149–154. [Google Scholar] [CrossRef]
- Pham, N.; Do, T.; Truong, V.L. Preliminary survey for Hatinh langur in north central Vietnam. Asian Primates 1996, 6, 13–17. [Google Scholar]
- Haus, T.; Vogt, M.; Forster, B. Observations on the Hatinh langur (Trachypithecus hatinhensis) during point and line transect sampling in the Phong Nha–Ke Bang National Park, Central Vietnam. Vietnam. J. Primatol. 2009, 3, 17–27. [Google Scholar]
- Harding, L.E. Trachypithecus delacouri (Primates: Cercopithecidae). Mamm. Species 2011, 43, 118–128. [Google Scholar] [CrossRef]
- Lhendup, S.; Tshering, U.; Tenzin, J. Population structure and habitat use of golden langur (Trachypithecus geei) in Royal Manas National Park, Bhutan. Int. J. Fauna Biol. Stud. 2018, 5, 97–101. [Google Scholar]
- Phiapalath, P.; Bousa, A.; Paul Insua-Cao, P. The Status and Distribution of Gibbons in Phou Hin Poun National Protected Area; Fauna & Flora International/IUCN Lao PDR: Vientiane, Lao PDR, 2012. [Google Scholar]
- Brakels, P. The Status and Distribution of Gibbons in Phou Hin Poun National Protected Area; Fauna & Flora International/IUCN Lao PDR: Vientiane, Lao PDR, 2019. [Google Scholar]
- Zhou, Q.; Wei, H.; Huang, Z.; Huang, C. Diet of the Assamese macaque Macaca assamensis in limestone habitats of Nonggang, China. Curr. Zool. 2011, 57, 18–25. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, C.; Tang, C.; Huang, L.; Tang, H.; Ma, G.; Zhou, Q. Dietary adaptations of Assamese macaques (Macaca assamensis) in limestone forests in Southwest China. Am. J. Primatol. 2015, 77, 171–185. [Google Scholar] [CrossRef]
- Schülke, O.; Pesek, D.; Whitman, B.J.; Ostner, J. Ecology of Assamese macaques (Macaca assamensis) at Phu Khieo Wildlife Sanctuary, Thailand. J. Wildl. Thail. 2011, 18, 1–15. [Google Scholar]
- Koirala, S.; Chalise, M.K.; Katuwal, H.B.; Gaire, R.; Pandey, B.; Ogawa, H. Diet and activity of Macaca assamensis in wild and semi-provisioned groups in Shivapuri Nagarjun National Park, Nepal. Folia Primatol. 2017, 88, 57–74. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Huang, Z.H.; Wei, H.; Huang, C.M. Variations in diet composition of sympatric Trachypithecus francoisi and Macaca assamensis in the limestone habitats of Nonggang, China. Zool. Res. 2018, 39, 284–291. [Google Scholar] [CrossRef]
- Brakels, P.; Somdachit, T. Record of cats from Phou Hin Poun National Protected Area. IUCN Lao PDR. Cat News 2020, 71. [Google Scholar]

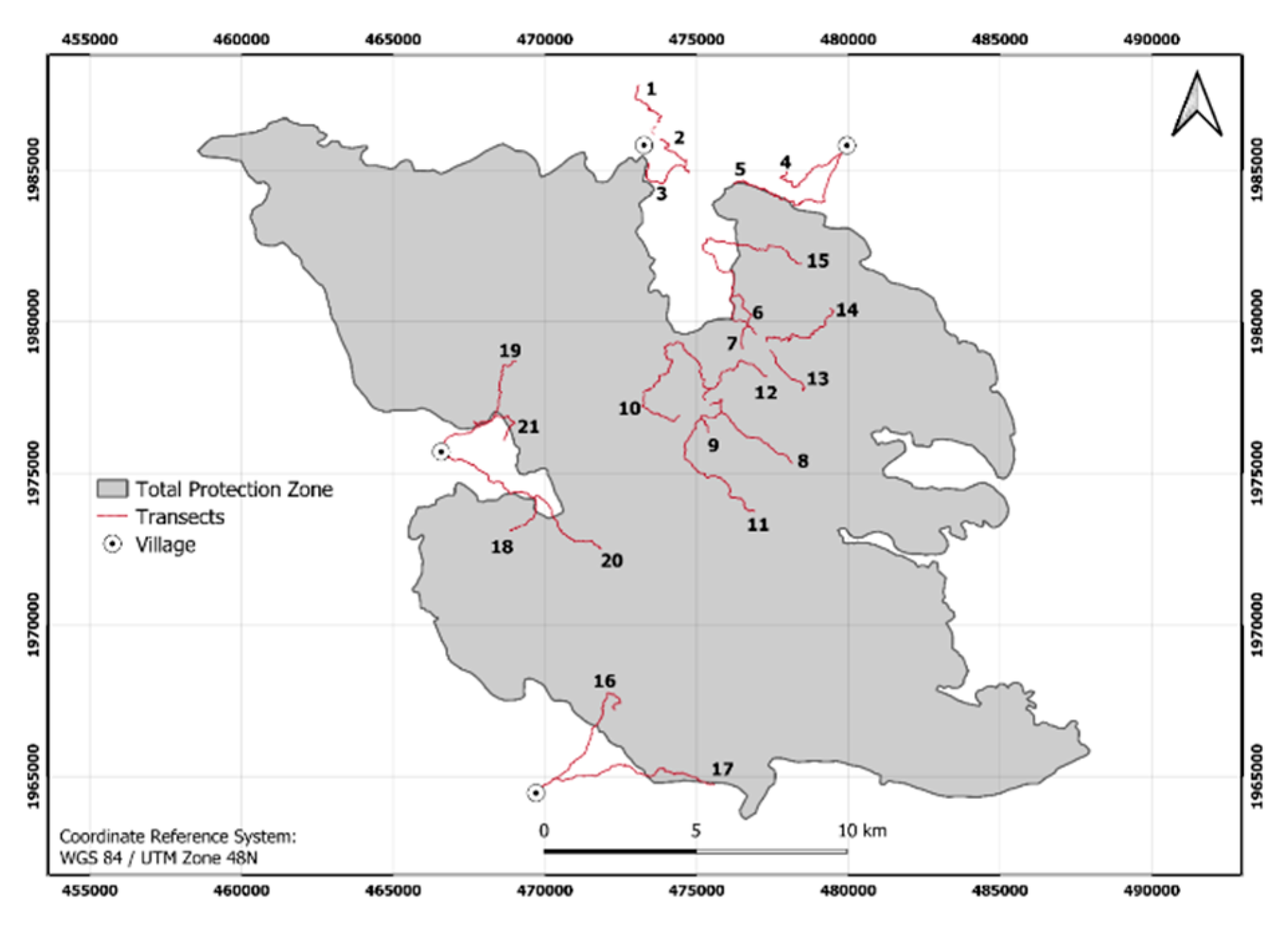
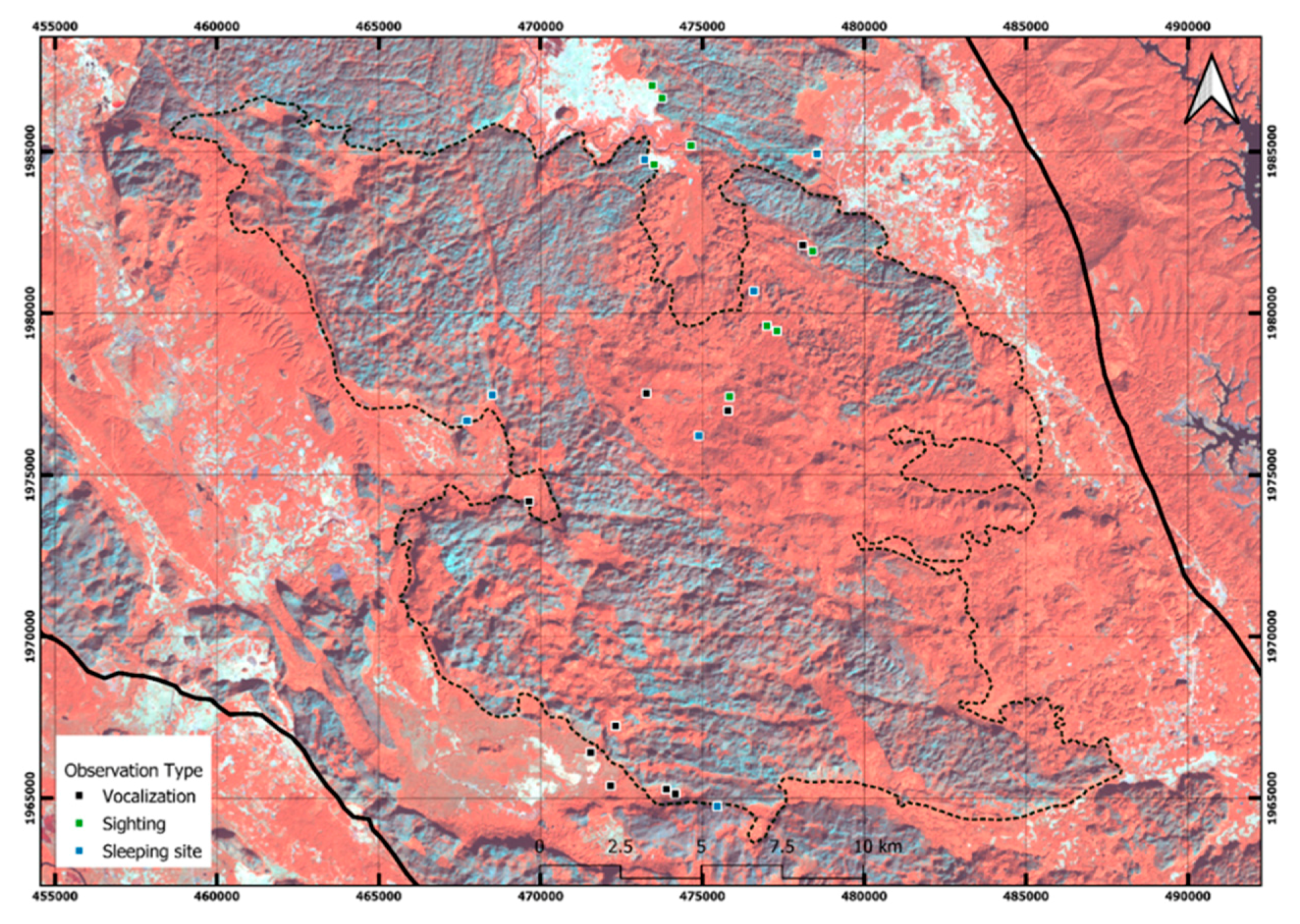


| District | Village | Occurrence | Threats | Population | Interviewees |
|---|---|---|---|---|---|
| Hinboun | Ban Pheepaeng | T. laotum, N. siki, Macaque indet. | Hunting/poaching | Decreased | DAFO staffs |
| Ban Huana | T. laotum, N. siki, Macaque indet. | Hunting/poaching | Unknown | DAFO staffs | |
| Ban Buamlou | T. laotum, N. siki, Macaque indet. | Hunting/poaching | Unknown | DAFO staffs; villagers | |
| Ban Kuankacha | T. laotum, N. siki, Macaque indet. | Hunting/poaching/mining | Decreased | Villagers | |
| Ban Bo Neng | T. laotum, Macaque indet. | Hunting/poaching | Decreased | Villagers | |
| Khounkham | Ban Konglor | T. laotum, N. siki, Macaque indet. | Hunting/poaching/logging | Decreased | DAFO staffs; villagers |
| Ban Or | T. laotum | Hunting | Decreased | DAFO staffs | |
| Ban Kateup | T. laotum, N. siki, Macaque indet., serow sp. | Hunting | Unknown | Villagers | |
| Nakai | Ban Natan | T. laotum. Macaque indet. | Poaching | Decreased | DAFO staffs; villagers |
| Ban Kuam Sam | T. laotum | Unknown | Unknown | DAFO staffs | |
| Ban Vanghin | T. laotum | Unknown | Unknown | DAFO staffs | |
| Yommalath | Ban Kuanphan | T. ebenus, Macaque indet., maybe T. laotum | Hunting | Unknown | DAFO staffs |
| Thakhek | Ban Doi | T. laotum, N. siki, maybe T. ebenus | Hunting | Decreased | DAFO staffs |
| Ban Phalem | T. laotum, N. siki, maybe T. ebenus | Hunting | Decreased | DAFO staffs |
| Location | Lat and Long | Date | Habitat | Group Size | Type |
|---|---|---|---|---|---|
| Un-named | 17°58′104°49′ | 21 December 1994 | Open karst vegetation | 1+ | Vis |
| Un-named | 18°02′104°47′ | 21 December 1994 | Open karst vegetation | 1+ | Vis |
| Near Ban Vang dao | 18°05′104°32′ | 16 May 1995 | Open karst vegetation | 8+ | Vis |
| Near Ban Vang dao | 18°03′104°31′ | 17 May 1995 | n/k | n/k | Voc |
| North of Khua Din | 17°51′104°51 | 6 February 1996 | SEF | 4+ | Vis |
| Khua Din | 17°50′104°50′ | 6 February 1996 | n/k | probably several groups | Voc |
| South of Khua Din | 17°49′104°50′ | 7 February 1996 | SEF | 8+ | Vis |
| South of Ban Ak | 17°52′104°52′ | 8 February 1996 | Karst ridge | n/k | Vis |
| South of Ban Ak | 17°52′104°53′ | 9 February 1996 | n/k | n/k (probably several groups) | Voc |
| Near Nam Hinboun | 18°01′104°26 | 31 January 1998 | Karst above degraded SEF | 9 | Vis |
| Tham Pai | 17°52′104°52′ | 15 March 1998 | MDF | 15–25 | Vis |
| Tham Pai | 17°51′104°52 | 16 March1998 | MDF near cliff | n/k | Voc |
| Tham Pai | 17°52′104°52′ | 21 March 1998 | SEF on ridge | n/k | Voc |
| Khua Din | 17°50′104°50′ | 18 March 1998 | SEF near cliff | 15–20 | Vis |
| Khua Din | 17°50′104°50′ | 20 March 1998 | SEF not near cliff | 15–20 | Vis |
| Khua Din | 17°50′104°50′ | 19 March 1998 | SEF | Probably 2 group | Vis |
| Khouan Huy | 17°42′104°48′ | 2 April 1998 | Boulders at cave mouth | 2+ (heads not seen) | Vis |
| Khouan Huy | 17°41′104°49′ | 1 April 1998 | Cliff face vegetation | n/k | Voc |
| Zone 1_Camp 1 | 2010 | 5 calls; 2 groups (8 individuals) | Vis | ||
| Zone 1_Camp 2 | 2010 | 2 calls, 1 group (4 individuals) | Vis + Voc | ||
| Zone 1_Camp 3 | 2010 | 4 calls, 1 group (5 individuals) | Vis + Voc | ||
| Zone 2_Camp 4 | 2010 | 2 calls, 1 group (3 individuals) | Vis + Voc | ||
| Zone 2_Camp 5 | 2010 | 1 call | Voc | ||
| Zone 2_Camp 6 | 2010 | 1 call | Voc | ||
| Zone 3_Camp 7 | 2010 | 2 calls | Voc | ||
| Zone 3_Camp 8 | 2010 | 2 calls | Voc | ||
| Zone 3_Camp 9 | 2010 | 1 call, 1 group (4 individuals) | Vis + Voc | ||
| Zone 3_Camp 10 | 2010 | 2 calls | Voc | ||
| Zone 3_Camp 11 | 2010 | 1 call, 1 group (6 individuals) | Vis + Voc | ||
| Zone 3_Camp 12 | 2010 | 1 call | Voc | ||
| Zone 3_Camp 13 | 2010 | 2 calls, 1 group (6 individuals) | Vis + Voc | ||
| Zone 4_Camp 14 | 2010 | 1 call | Voc | ||
| Zone 4_ Camp 15 | 2010 | 2 calls, 1 group (8 individuals) | Vis + Voc | ||
| Zone 4_ Camp 16 | 2010 | 2 calls, 2 groups (12 individuals) | Vis + Voc | ||
| Zone 4_Camp 17 | 2010 | 1 call, 1 group (6 individuals) | Vis + Voc | ||
| Zone 4_Camp 18 | 2010 | 1 call, 1 group (5 individuals) | Vis + Voc | ||
| Zone 5_Camp 19 | 2010 | 3 call, 3 groups (21 individuals) | Vis + Voc | ||
| Zone 5_Camp 20 | 2010 | 3 call, 3 groups (15 individuals) | Vis + Voc |
| Sectors (Total Effort) | Frequency of Encountered Groups | No. of Individuals | Encounter Rates (Groups Per km Walked) |
|---|---|---|---|
| Konglor-Natan (14.9 km) | 5 | 17 | 0.34 |
| Konglor (32.8 km) | 7 | 22 | 0.21 |
| Buamlou (5 km) | 5 | 6 | 1.00 |
| Kuankacha (11.4 km) | 2 | 2 | 0.18 |
| Group No. | Location | Coordinate (GPS) WGS 84/UTM Zone 48 N | Adults | Subadults | Juveniles | Infants | Group Size | |
|---|---|---|---|---|---|---|---|---|
| Northing | Easting | |||||||
| 1 | Tham Kuay | 473,753 | 1,986,651 | 2 | 0 | 0 | 0 | 2 |
| 2 | Tham Huator | 473,457 | 1,987,041 | 3 | 2 | 0 | 0 | 5 |
| 3 | Near Konglor cave | 474,646 | 1,985,180 | 3 | 1 | 0 | 0 | 4 |
| 4 | Poung Ta Tid Pha | 473,512 | 1,984,602 | 3 | 0 | 0 | 0 | 3 |
| 5 | Tham Huator | 473,753 | 1,986,651 | 2 | 1 | 0 | 0 | 3 |
| 6 | Pha Soung | 476,995 | 1,979,610 | 2 | 2 | 0 | 0 | 4 |
| 7 | Ang Nam Ta Ngon | 475,844 | 1,977,413 | 4 | 2 | 0 | 0 | 6 |
| 8 | Ang Khee Ther | 477,304 | 1,979,456 | 2 | 1 | 0 | 0 | 3 |
| 9 | Kuan Dik | 478,413 | 1,981,923 | 3 | 1 | 1 | 0 | 5 |
| Total | 24 | 10 | 1 | 0 | 35 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souwideth, J.; Phiapalath, P.; Thanh, H.D.; Brakels, P.; Van, T.P.; Luiselli, L. Ecology and Conservation of the Laotian langur Trachypithecus laotum in a Protected Area of Laos (Southeast Asia). Diversity 2021, 13, 231. https://doi.org/10.3390/d13060231
Souwideth J, Phiapalath P, Thanh HD, Brakels P, Van TP, Luiselli L. Ecology and Conservation of the Laotian langur Trachypithecus laotum in a Protected Area of Laos (Southeast Asia). Diversity. 2021; 13(6):231. https://doi.org/10.3390/d13060231
Chicago/Turabian StyleSouwideth, Johnny, Phaivanh Phiapalath, Hai Dong Thanh, Peter Brakels, Thong Pham Van, and Luca Luiselli. 2021. "Ecology and Conservation of the Laotian langur Trachypithecus laotum in a Protected Area of Laos (Southeast Asia)" Diversity 13, no. 6: 231. https://doi.org/10.3390/d13060231
APA StyleSouwideth, J., Phiapalath, P., Thanh, H. D., Brakels, P., Van, T. P., & Luiselli, L. (2021). Ecology and Conservation of the Laotian langur Trachypithecus laotum in a Protected Area of Laos (Southeast Asia). Diversity, 13(6), 231. https://doi.org/10.3390/d13060231







