Bryophyte Diversity along an Elevational Gradient on Pico Island (Azores, Portugal)
Abstract
1. Introduction
2. Materials and Methods
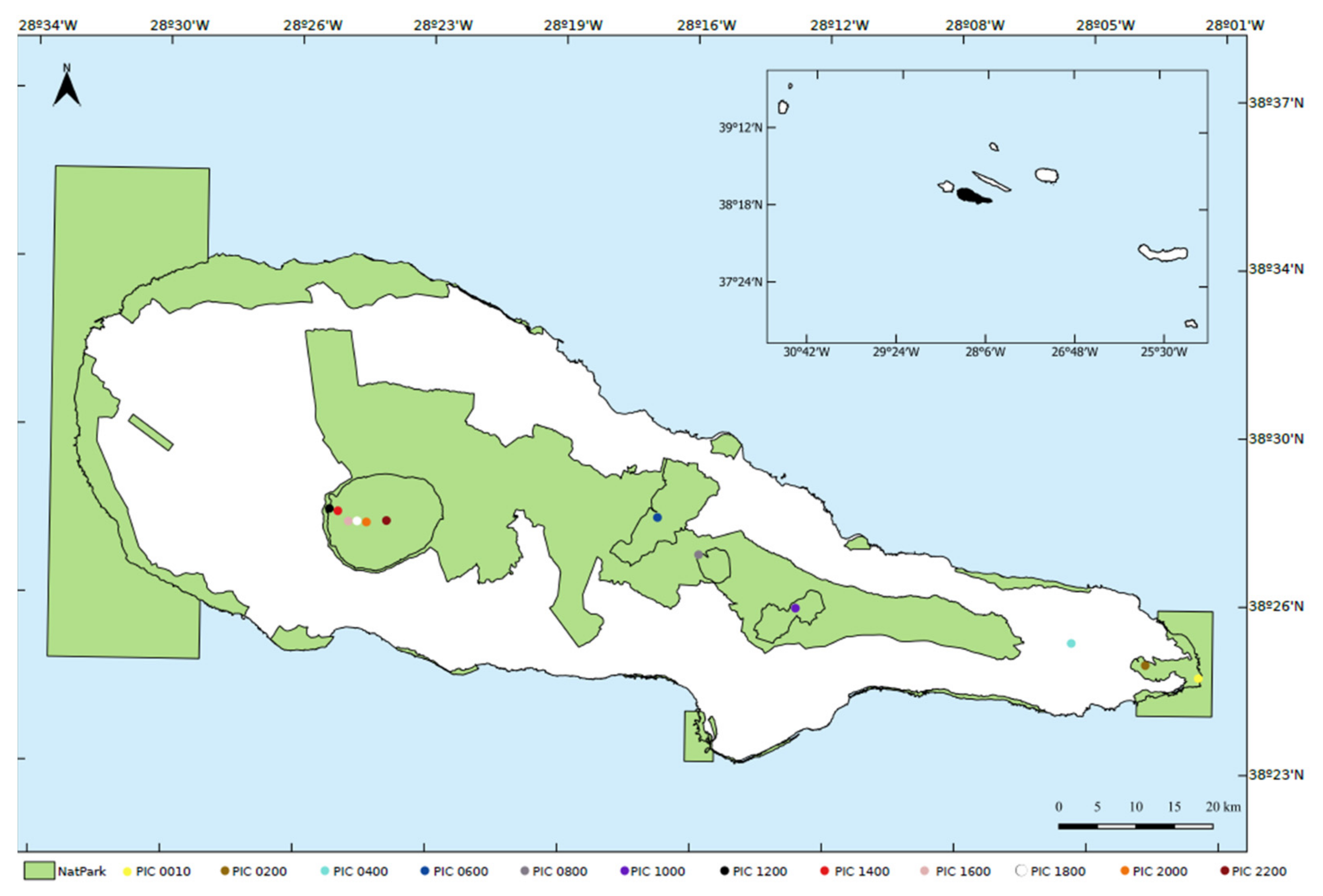
2.1. Study Area
2.2. Sampling Design and Fieldwork
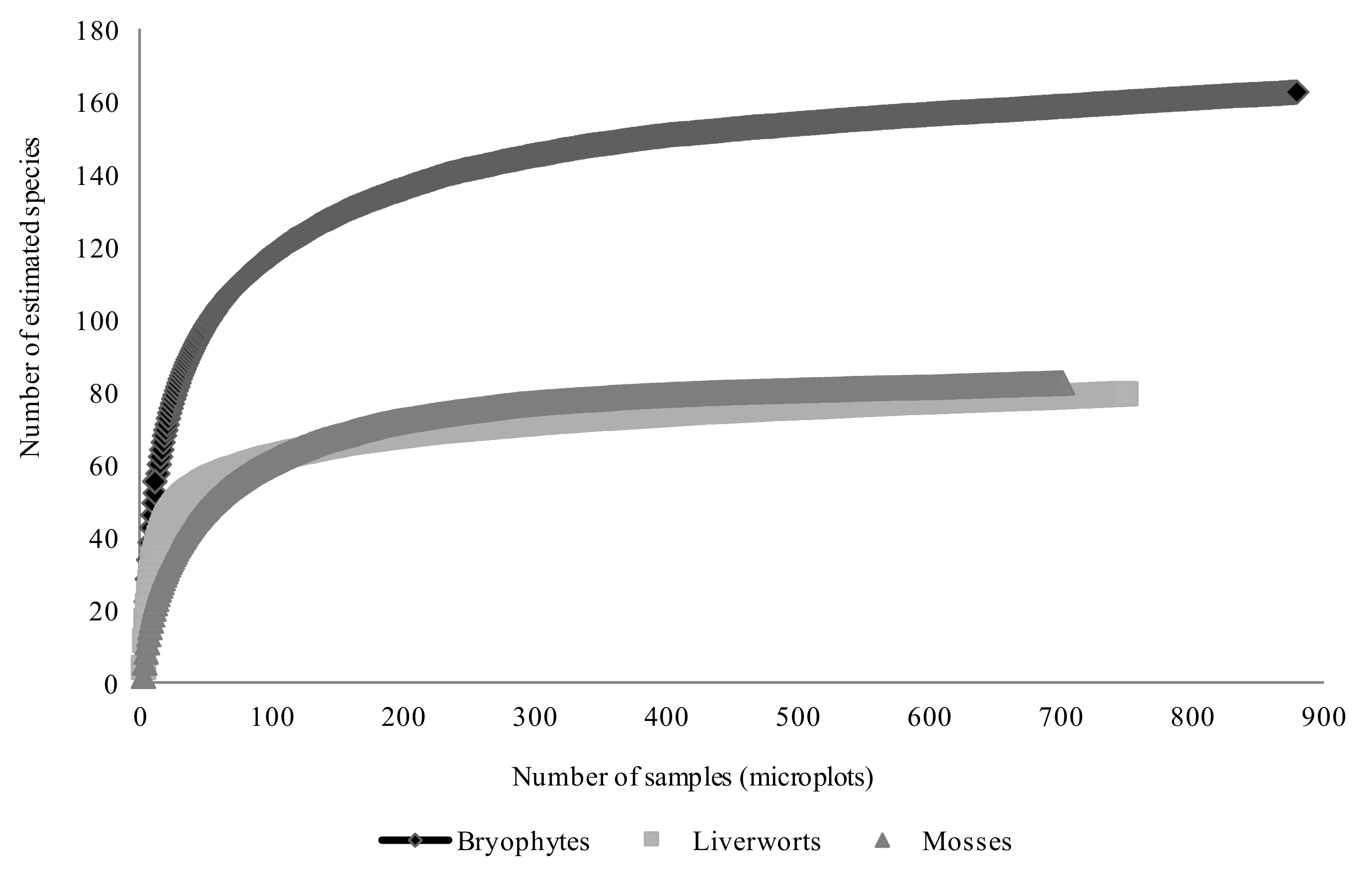
2.3. Species Inventory and Sampling Completeness
2.4. Diversity Measurement
2.5. Substrate Specificity of Species
2.6. Floristic Composition and Ecological Variables
3. Results
3.1. Species Inventory and Sampling Completeness
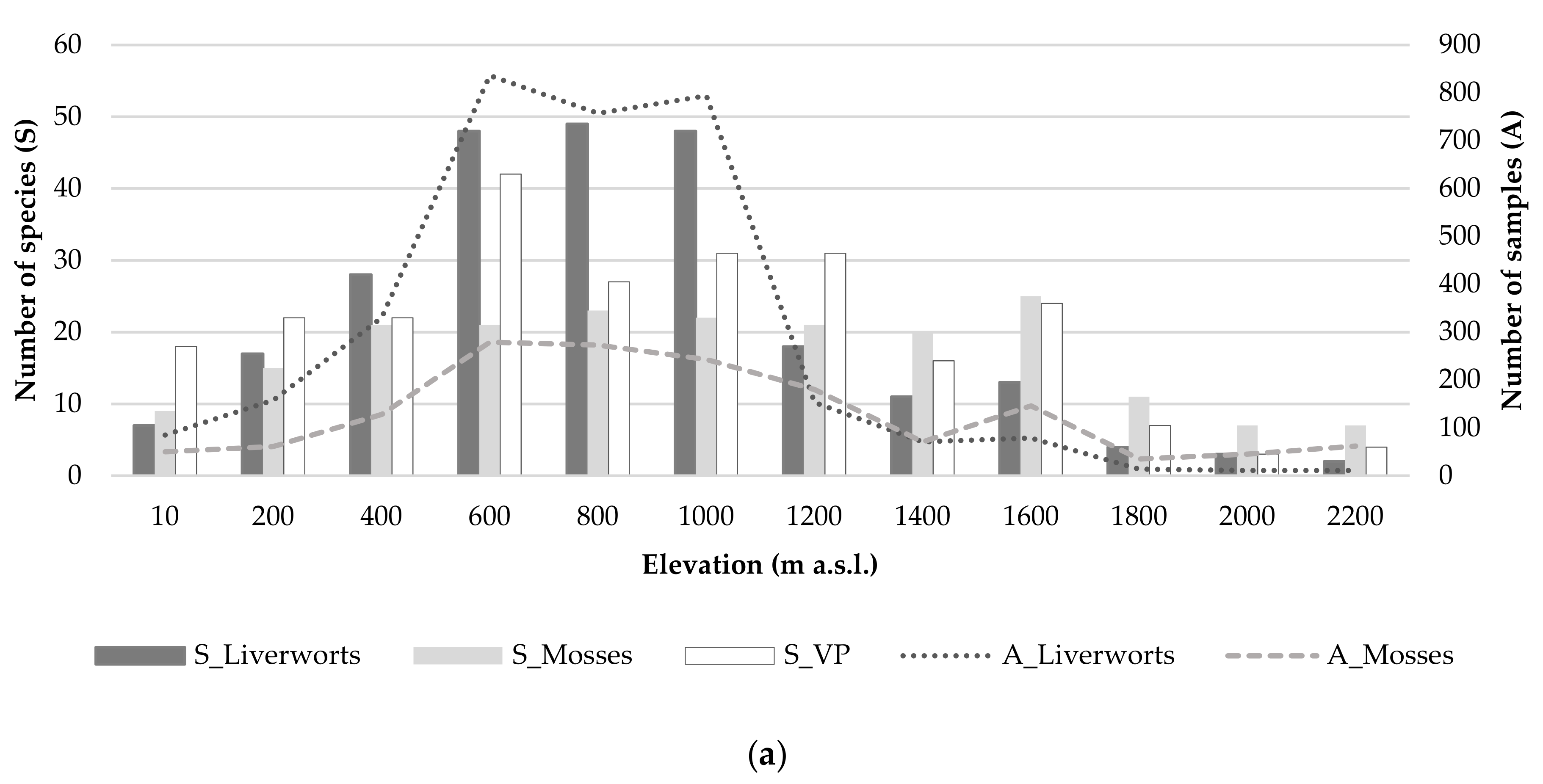
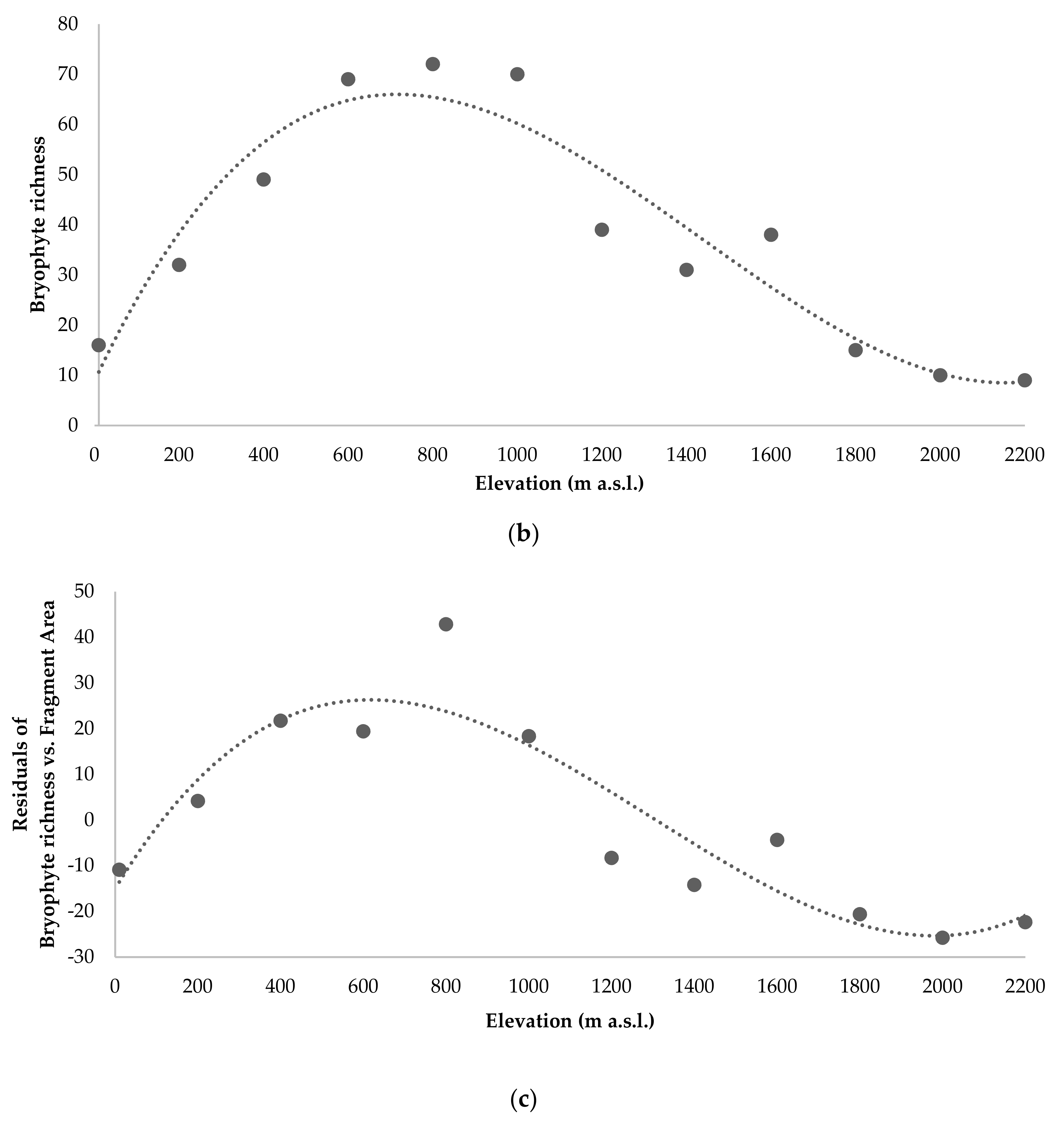
3.2. Species Richness (Alpha Diversity)
3.3. Species Diversity (Hill Numbers)
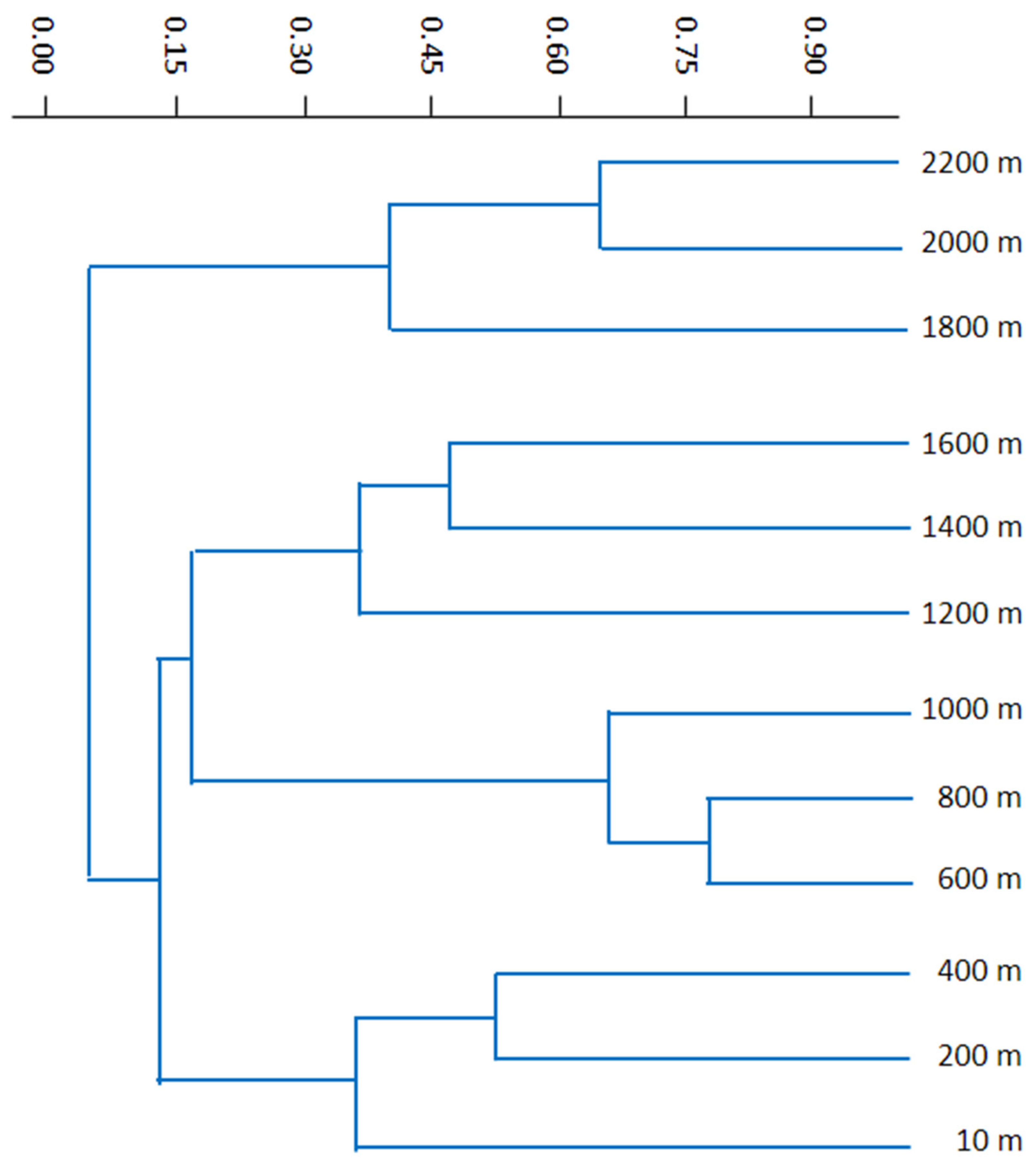
3.4. Similarities and Differences among Elevational Bands and Substrates
3.5. Substrate Specificity of Species
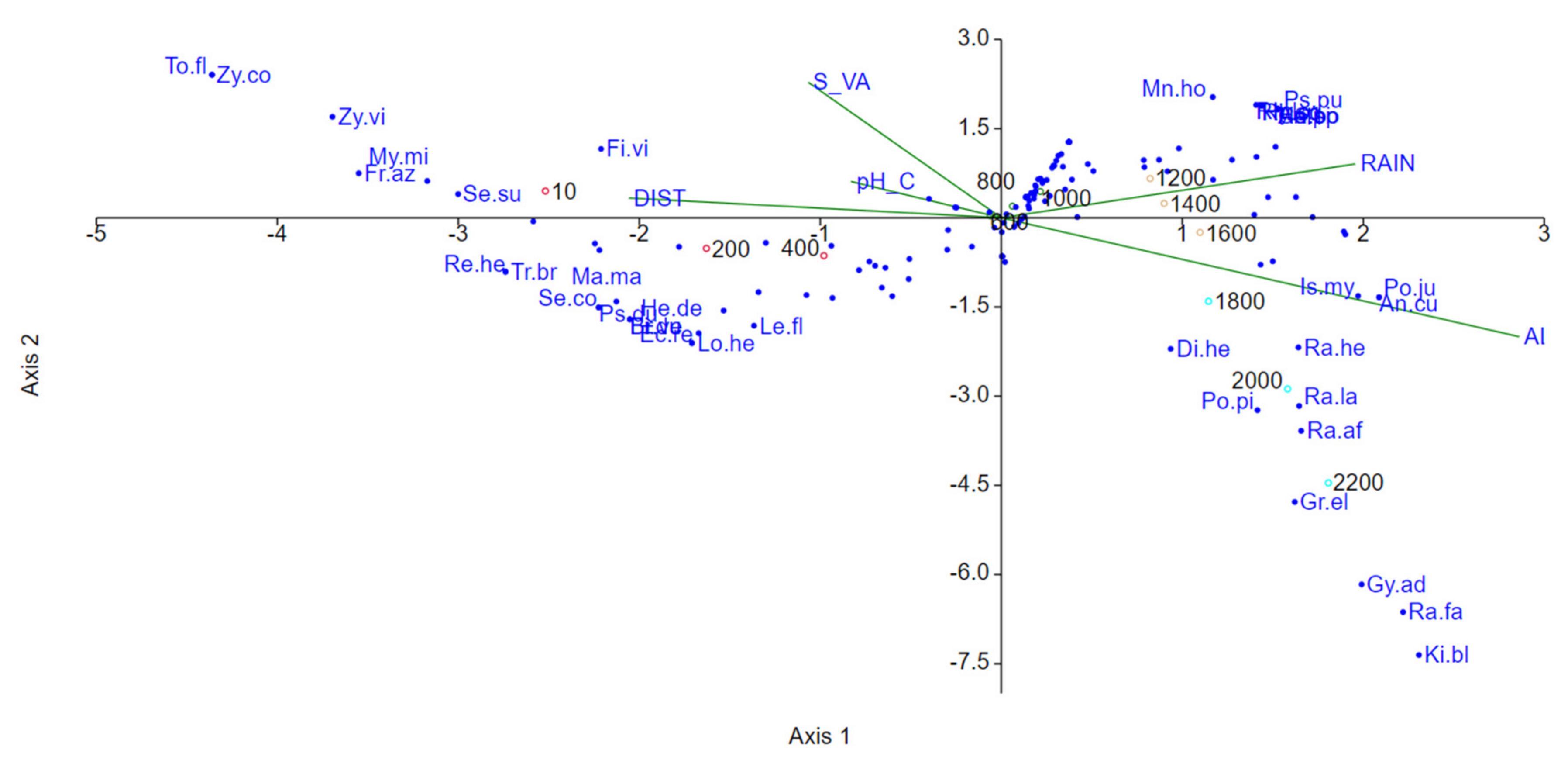
3.6. Ecological Factors Influencing Diversity Patterns
4. Discussion
4.1. Bryophyte Diversity Patterns along Pico Island’ Elevational Transect (Alpha and Beta Diversity)
4.2. Bryophyte Substrate Specificity along the Elevational Transect
4.3. Bryophyte vs. Vascular Plants
4.4. Rare Species: Uniques and Duplicates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| D | Species | Abrev. | 10 m | 200 m | 400 m | 600 m | 800 m | 1000 m | 1200 m | 1400 m | 1600 m | 1800 m | 2000 m | 2200 m |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bryophyta | ||||||||||||||
| Alophosia azorica (Renauld and Cardot) Cardot | Al.az | 3 | 1 | 1 | 5 | |||||||||
| Andoa berthelotiana (Mont.) Ochyra | An.be | 11 | 23 | 32 | 14 | 3 | 1 | 4 | ||||||
| Antitrichia curtipendula (Hedw.) Brid. | An.cu | 15 | ||||||||||||
| Brachytheciastrum velutinum (Hedw.) Ignatov and Huttunen | Br.ve | 1 | 2 | |||||||||||
| Brachythecium mildeanum (Schimp.) Schimp. | Br.mi | 1 | 4 | 1 | ||||||||||
| Campylopus flexuosus (Hedw.) Brid. | Ca.fl | 1 | 2 | 3 | 7 | 1 | 1 | |||||||
| Campylopus fragilis (Brid.) Bruch and Schimp. | Ca.fr | 11 | 3 | |||||||||||
| Campylopus pilifer Brid. | Ca.pi | 4 | 1 | |||||||||||
| Campylopus pyriformis (Schultz) Brid. | Ca.py | 2 | 2 | 1 | 2 | 1 | ||||||||
| Campylopus shawii Wilson | Ca.sh | 1 | 1 | |||||||||||
| Cyclodictyon laetevirens (Hook. and Taylor) Mitt. | Cy.la | 7 | ||||||||||||
| Daltonia lindigiana Hampe | Da.li | 4 | 2 | |||||||||||
| Dicranella heteromalla (Hedw.) Schimp. | Di.he | 1 | ||||||||||||
| Dicranum flagellare Hedw. | Di.fl | 2 | ||||||||||||
| Dicranum scottianum Turner | Di.sc | 1 | 17 | 26 | 34 | 23 | 1 | |||||||
| Diphyscium foliosum (Hedw.) D.Mohr | Di.fo | 1 | 1 | 1 | 1 | |||||||||
| Ditrichum subulatum Hampe | Di.su | 1 | 1 | |||||||||||
| Echinodium renauldii (Cardot) Broth. | Ec.re | 9 | ||||||||||||
| Fissidens bryoides Hedw. | Fi.br | 2 | 1 | |||||||||||
| Fissidens dubius P. Beauv. | Fi.du | 1 | 2 | |||||||||||
| Fissidens serrulatus Brid. | Fi.se | 4 | 1 | 9 | ||||||||||
| Fissidens taxifolius Hedw. | Fi.ta | 7 | 18 | 2 | 1 | 1 | 1 | |||||||
| Fissidens viridulus (Sw.) Wahlenb. | Fi.vi | 4 | 2 | |||||||||||
| Grimmia elongata Kaulf. | Gr.el | 1 | 1 | |||||||||||
| Heterocladium heteropterum (Brid.) Schimp. | He.he | 1 | ||||||||||||
| Heterocladium wulfsbergii I. Hagen | He.wu | 8 | 1 | 2 | ||||||||||
| Hylocomium splendens (Hedw.) Schimp. | Hy.sp | 25 | 8 | |||||||||||
| Hymenoloma crispulum (Hedw.) Ochyra | Hy.cr | 2 | 3 | |||||||||||
| Hypnum cupressiforme Hedw. | Hy.cu | 1 | 6 | 4 | 9 | 4 | 8 | |||||||
| Hypnum uncinulatum Jur. | Hy.un | 3 | 8 | 17 | 81 | 75 | 65 | 53 | 8 | 12 | ||||
| Isothecium interludens Stirt. | Is.in | 1 | 2 | 4 | 20 | 2 | ||||||||
| Isothecium myosuroides Brid. | Is.my | 18 | 4 | |||||||||||
| Isothecium prolixum (Mitt.) M. Stech, Sim-Sim, Tangney and D. Quandt | Is.pr | 13 | 29 | 34 | 8 | 1 | 2 | |||||||
| Kiaeria blyttii (Bruch and Schimp.) Broth. | Ki.bl | 4 | ||||||||||||
| Leucobryum glaucum (Hedw.) Ångstr. | Le.gl | 3 | 1 | 2 | ||||||||||
| Leucobryum juniperoideum (Brid.) Müll. Hal. | Le.ju | 4 | 1 | 1 | ||||||||||
| Mnium hornum Hedw. | Mn.ho | 1 | ||||||||||||
| Myurium hochstetteri (Schimp.) Kindb. | My.ho | 4 | 36 | 28 | 27 | 1 | 2 | 2 | ||||||
| Plagiomnium undulatum (Hedw.) T.J. Kop. | Pl.un | 4 | ||||||||||||
| Polytrichum commune Hedw. | Po.co | 12 | 12 | 7 | ||||||||||
| Polytrichum formosum Hedw. | Po.fo | 1 | 2 | |||||||||||
| Polytrichum juniperinum Hedw. | Po.ju | 1 | ||||||||||||
| Polytrichum piliferum Hedw. | Po.pi | 1 | 3 | 9 | 5 | |||||||||
| Pseudorhynchostegiella duriaei (Mont.) Ignatov and Vanderp. | Ps.du | 1 | 1 | |||||||||||
| Pseudoscleropodium purum (Hedw.) M. Fleisch. | Ps.pu | 4 | ||||||||||||
| Pseudotaxiphyllum laetevirens (Dixon and Luisier ex F. Koppe and Düll) Hedenäs | Ps.la | 1 | 1 | 3 | 7 | 2 | ||||||||
| Ptychostomum torquescens (Bruch and Schimp.) Ros and Mazimpaka | Pt.to | 2 | 1 | 2 | ||||||||||
| Racomitrium affine (F. Weber and D. Mohr) Lindb. | Ra.af | 2 | 7 | 10 | 18 | 20 | ||||||||
| Racomitrium fasciculare (Hedw.) Brid. | Ra.fa | 1 | 12 | |||||||||||
| Racomitrium heterostichum (Hedw.) Brid. | Ra.he | 1 | 1 | 1 | ||||||||||
| Racomitrium lanuginosum (Hedw.) Brid. | Ra.la | 5 | 5 | 3 | 5 | 14 | 19 | |||||||
| Rhynchostegiella curviseta (Brid.) Limpr. | Rh.cu | 9 | 8 | 6 | 1 | 2 | ||||||||
| Rhynchostegium confertum (Dicks.) Schimp. | Rh.co | 1 | ||||||||||||
| Rhytidiadelphus loreus (Hedw.) Warnst. | Rh.lo | 2 | 1 | |||||||||||
| Rhytidiadelphus squarrosus (Hedw.) Warnst. | Rh.sq | 3 | 1 | |||||||||||
| Sciuro-hypnum plumosum (Hedw.) Ignatov and Huttunen | Sc.pl | 1 | ||||||||||||
| Sematophyllum substrumulosum (Hampe) E. Britton | Se.su | 9 | 8 | 3 | 2 | |||||||||
| Serpoleskea confervoides (Brid.) Schimp. | Se.co | 2 | 2 | |||||||||||
| Sphagnum palustre L. | Sp.pa | 2 | ||||||||||||
| Tetrastichium fontanum (Mitt.) Cardot | Te.fo | 10 | 1 | 5 | ||||||||||
| Tetrastichium virens (Cardot) S.P. Churchill | Te.vi | 20 | 12 | 4 | ||||||||||
| Thamnobryum alopecurum (Hedw.) Gangulee | Th.al | 3 | ||||||||||||
| Thamnobryum maderense (Kindb.) Hedenäs | Th.ma | 1 | 6 | 1 | 3 | |||||||||
| Thuidium tamariscinum (Hedw.) Schimp. | Th.ta | 57 | 51 | 22 | 3 | 13 | ||||||||
| Tortella flavovirens (Bruch) Broth. | To.fl | 15 | ||||||||||||
| Trichostomum brachydontium Bruch | Tr.br | 5 | ||||||||||||
| Ulota calvescens Wilson | Ul.ca | 1 | 2 | |||||||||||
| Ulota crispa (Hedw.) Brid. | Ul.cr | 15 | 28 | |||||||||||
| Zygodon conoideus (Dicks.) Hook. and Taylor | Zy.co | 1 | ||||||||||||
| Zygodon viridissimus (Dicks.) Brid. | Zy.vi | 7 | 1 | 1 | ||||||||||
| Marchantiophyta | ||||||||||||||
| Aneura pinguis (L.) Dumort. | An.pi | 2 | ||||||||||||
| Bazzania azorica H. Buch and Perss. | Ba.az | 3 | 2 | 32 | ||||||||||
| Blepharostoma trichophyllum (L.) Dumort. | Bl.tr | 1 | 3 | |||||||||||
| Calypogeia arguta Nees and Mont. | Ca.ar | 1 | 1 | 1 | 2 | 1 | ||||||||
| Calypogeia azorica Bischl. | Ca.az | 1 | ||||||||||||
| Calypogeia fissa (L.) Raddi | Ca.fi | 8 | 12 | 26 | 11 | 2 | 8 | 13 | ||||||
| Calypogeia muelleriana (Schiffn.) Müll. Frib. | Ca.mu | 1 | 2 | 9 | ||||||||||
| Calypogeia sphagnicola (Arnell and J. Perss.) Warnst. and Loeske | Ca.sp | 1 | ||||||||||||
| Cephalozia bicuspidata (L.) Dumort. | Ce.bi | 2 | 6 | 3 | ||||||||||
| Cheilolejeunea cedercreutzii (H. Buch and Perss.) Grolle | Ch.ce | 4 | 5 | 22 | ||||||||||
| Cololejeunea azorica V. Allorge and Jovet-Ast | Co.az | 1 | ||||||||||||
| Cololejeunea microscopica (Taylor) Schiffn. | Co.mi | 5 | 39 | 47 | 59 | 1 | ||||||||
| Cololejeunea sintenisii (Steph.) Pócs | Co.si | 9 | 13 | 1 | ||||||||||
| Colura calyptrifolia (Hook.) Dumort. | Co.ca | 5 | 8 | 8 | 15 | 1 | ||||||||
| Diplophyllum albicans (L.) Dumort. | Di.al | 1 | 11 | 20 | 16 | 6 | 5 | |||||||
| Drepanolejeunea hamatifolia (Hook.) Schiffn. | Dr.ha | 1 | 53 | 31 | 43 | 12 | ||||||||
| Frullania acicularis Hentschel and von Konrat | Fr.ac | 4 | 4 | 48 | 47 | 2 | 1 | 7 | 1 | |||||
| Frullania azorica Sim-Sim, Sérgio, Mues and Kraut | Fr.az | 1 | 1 | |||||||||||
| Frullania microphylla (Gottsche) Pearson | Fr.mi | 24 | 39 | 29 | 20 | 4 | ||||||||
| Frullania tamarisci (L.) Dumort. | Fr.ta | 2 | 8 | 16 | 51 | 30 | 81 | 9 | 12 | 11 | 5 | |||
| Frullania teneriffae (F. Weber) Nees | Fr.te | 26 | 28 | 48 | 66 | 9 | 24 | 2 | ||||||
| Fuscocephaloziopsis crassifolia (Lindenb. and Gottsche) Váňa and L. Söderstr. | Fu.cr | 7 | 10 | 14 | 1 | 2 | ||||||||
| Geocalyx graveolens (Schrad.) Nees | Ge.gr | 1 | ||||||||||||
| Gymnomitrion adustum Nees | Gy.ad | 3 | 10 | |||||||||||
| Harpalejeunea molleri (Steph.) Grolle | Ha.mo | 8 | 36 | 14 | 4 | |||||||||
| Herbertus azoricus (Steph.) P.W. Richards | He.az | 2 | ||||||||||||
| Herbertus borealis Crundw. | He.bo | 1 | ||||||||||||
| Heteroscyphus denticulatus (Mitt.) Schiffn. | He.de | 1 | 20 | 1 | ||||||||||
| Jubula hutchinsiae (Hook.) Dumort. | Ju.hu | 8 | 15 | 12 | ||||||||||
| Lejeunea eckloniana Lindenb. | Le.ec | 5 | 1 | |||||||||||
| Lejeunea flava (Sw.) Nees | Le.fl | 4 | 1 | |||||||||||
| Lejeunea hibernica Bischl., H.A. Mill. and Bonner ex Grolle | Le.hi | 2 | 1 | 4 | ||||||||||
| Lejeunea lamacerina (Steph.) Schiffn. | Le.la | 15 | 35 | 60 | 75 | 59 | 20 | |||||||
| Lejeunea patens Lindb. | Le.pa | 1 | 10 | 4 | ||||||||||
| Lepidozia cupressina (Sw.) Lindenb. | Le.cu | 9 | 18 | 25 | ||||||||||
| Lepidozia reptans (L.) Dumort. | Le.re | 1 | 9 | 2 | ||||||||||
| Leptoscyphus porphyrius (Nees) Grolle | Le.po | 7 | 8 | 33 | ||||||||||
| Lophocolea coadunata (Sw.) Mont. | Lo.co | 3 | 2 | 4 | ||||||||||
| Lophocolea fragrans (Moris and De Not.) Gottsche, Lindenb. and Nees | Lo.fr | 1 | 28 | 9 | 25 | 3 | ||||||||
| Lophocolea heterophylla (Schrad.) Dumort. | Lo.he | 1 | ||||||||||||
| Marchantia polymorpha L. | Ma.po | 1 | ||||||||||||
| Marchesinia mackaii (Hook.) Gray | Ma.ma | 33 | 28 | 4 | ||||||||||
| Marsupella sparsifolia (Lindb.) Dumort. | Ma.sp | 5 | 4 | 1 | 1 | 3 | 1 | |||||||
| Metzgeria furcata (L.) Corda | Me.fu | 2 | 22 | 58 | 9 | 16 | 2 | |||||||
| Mnioloma fuscum (Lehm.) R.M. Schust. | Mn.fu | 13 | 29 | 21 | ||||||||||
| Myriocoleopsis minutissima (Sm.) R.L. Zhu, Y. Yu and Pócs | My.mi | 34 | 13 | 16 | 2 | 1 | ||||||||
| Nardia scalaris Gray | Na.sc | 2 | ||||||||||||
| Nowellia curvifolia (Dicks.) Mitt. | No.cu | 13 | 7 | 3 | ||||||||||
| Odontoschisma denudatum (Mart.) Dumort. | Od.de | 1 | ||||||||||||
| Pallavicinia lyellii (Hook.) Gray | Pa.ly | 3 | ||||||||||||
| Pellia epiphylla (L.) Corda | Pe.ep | 2 | 1 | 9 | ||||||||||
| Plagiochila bifaria (Sw.) Lindenb. | Pl.bi | 3 | 6 | 73 | 45 | 45 | ||||||||
| Plagiochila exigua (Taylor) Taylor | Pl.ex | 19 | 15 | 13 | ||||||||||
| Plagiochila punctata (Taylor) Taylor | Pl.pu | 2 | 5 | 12 | 33 | 6 | ||||||||
| Plagiochila retrorsa Gottsche | Pl.re | 2 | 1 | |||||||||||
| Porella canariensis (F. Weber) Underw. | Po.ca | 1 | 12 | 7 | 8 | 3 | ||||||||
| Porella obtusata (Taylor) Trevis. | Po.ob | 1 | 13 | 7 | 2 | |||||||||
| Pseudomarsupidium decipiens (Hook.) Grolle | Ps.de | 15 | 15 | 32 | ||||||||||
| Radula aquilegia (Hook. f. and Taylor) Gottsche, Lindenb. and Nees | Ra.aq | 1 | 75 | 75 | 70 | 2 | 2 | |||||||
| Radula carringtonii J.B. Jack | Ra.ca | 3 | 19 | 39 | 33 | 17 | 1 | |||||||
| Radula complanata (L.) Dumort. | Ra.co | 2 | 2 | |||||||||||
| Radula holtii Spruce | Ra.ho | 5 | 6 | 6 | ||||||||||
| Radula wichurae Steph. | Ra.wi | 6 | 8 | 12 | 9 | 7 | 2 | 1 | 2 | |||||
| Reboulia hemisphaerica (L.) Raddi | Re.he | 1 | ||||||||||||
| Riccardia chamedryfolia (With.) Grolle | Ri.ch | 4 | 4 | |||||||||||
| Riccardia multifida (L.) Gray | Ri.mu | 8 | 3 | 3 | ||||||||||
| Saccogyna viticulosa (L.) Dumort. | Sa.vi | 2 | 6 | 16 | 37 | 15 | ||||||||
| Scapania gracilis Lindb. | Sc.gr | 10 | 16 | 33 | 13 | |||||||||
| Scapania scandica (Arnell and H. Buch) Macvicar | Sc.sc | 3 | ||||||||||||
| Telaranea azorica (H.Buch and Perss.) Pócs | Te.az | 5 | ||||||||||||
| Telaranea europaea J.J. Engel and G.L. Merr. | Te.eu | 6 | 10 | 13 | 15 | 3 | 1 | |||||||
References
- Hallingbäck, T.; Hodgetts, N. Mosses, Liverworts, and Hornworts. Status Survey and Conservation Action Plan for Bryophytes; IUCN/SSC Bryophyte Specialist Group, Ed.; IUCN/SSC Bryophyte Specialist Group: Gland, Switzerland; Cambridge, UK, 2000; ISBN 2-8317-0466-9. [Google Scholar]
- Andrew, N.R.; Rodgerson, L. Variation in invertebrate—Bryophyte community structure at different spatial scales along altitudinal gradients. J. Biogeogr. 2003, 30, 731–746. [Google Scholar] [CrossRef]
- Sandvik, S.M.; Heegaard, E. Effects of Simulated Environmental Changes on Growth and Growth Form in a Late Snowbed Population of Pohlia wahlenbergii (Web. et Mohr) Andr. Arctic Antarct. Alp. Res. 2003, 35, 341–348. [Google Scholar] [CrossRef]
- Tuba, Z.; Slack, N.G.; Stark, L.R. Bryophyte Ecology and Climate Change; Tuba, Z., Slack, N.G., Stark, L.R., Eds.; Cambridge University Press: New York, NY, USA, 2011; ISBN 9780521767637. [Google Scholar]
- Alpert, P. Distribution quantified by microtopography in an assemblage of saxicolous mosses. Vegetatio 1985, 64, 131–139. [Google Scholar] [CrossRef]
- McCune, B. Gradients in epiphyte biomass in three Pseudotsuga-Tsuga forests of different ages in Western Oregon and Washington. Bryologist 1993, 96, 405–411. [Google Scholar] [CrossRef]
- Gabriel, R.; Bates, J.W. Bryophyte community composition and habitat specificity in the natural forests of Terceira, Azores. Plant Ecol. 2005, 177, 125–144. [Google Scholar] [CrossRef]
- Ashton, D.H. Ecology of bryophytic communities in mature Eucalyptus regnans F Muell forest at Wallaby Creek, Victoria. Aust. J. Bot. 1986, 34, 107–129. [Google Scholar] [CrossRef]
- Alpert, P. Microtopography as habitat structure for mosses on rocks. In Habitat Structure: The Physical Arrangement of Objects in Space; Springer: Dordrecht, The Netherlands, 1991; pp. 120–140. ISBN 978-94-010-5363-1. [Google Scholar]
- Medina, N.G.; Albertos, B.; Lara, F.; Mazimpaka, V.; Garilleti, R.; Draper, D.; Hortal, J. Species richness of epiphytic bryophytes: Drivers across scales on the edge of the Mediterranean. Ecography 2014, 37, 80–93. [Google Scholar] [CrossRef]
- Brown, J.H. Macroecology; University of Chicago Press: Chicago, IL, USA, 1995; ISBN 9780226076157. [Google Scholar]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Tusiime, F.M.; Byarujali, S.M.; Bates, J.W. Diversity and distribution of bryophytes in three forest types of Bwindi Impenetrable National Park, Uganda. Afr. J. Ecol. 2007, 45, 79–87. [Google Scholar] [CrossRef]
- Bruun, H.H.; Moen, J.; Virtanen, R.; Grytnes, J.-A.; Oksanen, L.; Angerbjörn, A. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Veg. Sci. 2006, 17, 37–46. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Wilding, N.; Kluge, J.; Descamps-Julien, B.; Bardat, J.; Chuah-Petiot, M.; Strasberg, D.; Hedderson, T.A.J. Bryophyte diversity and range size distribution along two altitudinal gradients: Continent vs. island. Acta Oecologica 2012, 42, 58–65. [Google Scholar] [CrossRef]
- Sun, S.; Wu, Y.; Wang, G.; Zhou, J.; Yu, D.; Bing, H.; Luo, J. Bryophyte species richness and composition along an altitudinal gradient in Gongga Mountain, China. PLoS ONE 2013, 8, e58131. [Google Scholar] [CrossRef]
- Dias, E. Vegetação Natural dos Açores. Ecologia e Sintaxonomia das Florestas Naturais. Master’s Thesis, University of the Azores, Angra do Heroísmo, Portugal, 1996. [Google Scholar]
- Azevedo, E.B.; Rodrigues, M.C.; Fernandes, J.F. O Clima dos Açores. Climatologia: Introdução. In Atlas Básico dos Açores; Forjaz, V.H., Ed.; Observatório Vulcanológico e Geotérmico dos Açores: Ponta Delgada, Portugal, 2004. [Google Scholar]
- Veneklaas, E.J.; Zagt, R.J.; Leerdam, A.; Ek, R.; Broekhoven, A.J.; Genderen, M. Hydrological properties of the epiphyte mass of a montane tropical rain forest, Colombia. Vegetatio 1990, 89, 183–192. [Google Scholar] [CrossRef]
- Sjögren, E. Epiphyllous bryophytes in the Azores Islands. Arquipel. Life Mar. Sci. 1997, 15, 1–49. [Google Scholar]
- Sjögren, E. Bryophyte vegetation in the Azores islands. Mem. Soc. Broteriana 1978, 26, 1–273. [Google Scholar]
- Sjögren, E. Azorean Bryophyte Communities—A revision of differential species. Arquipel. Life Mar. Sci. 2003, 20A, 1–29. [Google Scholar]
- Pharo, E.J.; Beattie, A.J. The association between substrate variability and Bryophyte and Lichen diversity in Eastern Australian Forests. Bryologist 2002, 105, 11–26. [Google Scholar] [CrossRef]
- Russel, J.L. Some notes on the cryptogamic vegetation of Fayal, Azores. Proccedings Essex Inst. 1862, 2, 134–137. [Google Scholar]
- Trelease, W. Botanical Observations on the Azores; Annual Report of the Missouri Botanical Garden; Missouri Botanical Garden: St. Louis, MO, USA, 1897; Volume 8, pp. 77–220. [Google Scholar]
- Richards, P.W. A collection of bryophytes from the Azores. Ann. Bryol. 1937, 9, 131–138. [Google Scholar]
- Allorge, P.; Allorge, V. Hépatiques recoltées par P. et V. Allorge aux îles Açores en 1937. Rev. Bryol. Lichenol. 1950, 19, 90–118. [Google Scholar]
- Allorge, P.; Allorge, V. Mousses recoltées par P. et V. Allorge aux îles Açores en 1937. Rev. Bryol. Lichenol. 1952, 21, 50–95. [Google Scholar]
- González-Mancebo, J.M.; Losada-Lima, A.; Hérnandez-Garcia, C.D. A contribution to the floristic knwoledge of caves on the Azores. Mémoires Biospéologie 1991, 17, 219–226. [Google Scholar]
- Crundwell, A.C.; Greven, H.C.; Stern, R.C. Some additions to the bryophyte flora of the Azores. J. Bryol. 1994, 18, 329–337. [Google Scholar] [CrossRef]
- Homem, N.F.M. Biodiversidade de Briófitos nas Florestas Naturais dos Açores—Ilhas Terceira e Pico. Master’s Thesis, Universidade dos Açores, Angra do Heroísmo, Portugal, 2005. [Google Scholar]
- Eggers, J. Artenliste der Moose Makaronesiens. Cryptogam. Bryol. Lichenol. 1982, 3, 283–335. [Google Scholar]
- Sjögren, E. Distribution of Azorean bryophytes up to 1999, their island distribution and information on their presence elsewhere, including Madeira and the Canary Islands. In Boletim do Museu Municipal do Funchal (História Natural); Museum of Natural History of Funchal: Funchal, Portugal, 2001; pp. 1–89. [Google Scholar]
- Gabriel, R.; Sjögren, E.; Schumacker, R.; Sérgio, C.; Frahm, J.; Sousa, E. Lista dos Briófitos (Bryophyta). In A List of the Terrestrial and Marine Biota from the Azores; Princípia Editora Lda: Cascais, Portugal, 2010; pp. 117–130. [Google Scholar]
- Borges, P.A.V.; Costa, A.; Cunha, R.; Gabriel, R.; Gonçalves, V.; Martins, A.; Melo, I.; Parente, M.; Raposeiro, P.; Rodrigues, P.; et al. A List of the Terrestrial and Marine Biota from the Azores; Princípia: Cascais, Portugal, 2010; ISBN 9789898131751. [Google Scholar]
- Sutherland, W.J.; Freckleton, R.P.; Godfray, H.C.J.; Beissinger, S.R.; Benton, T.; Cameron, D.D.; Carmel, Y.; Coomes, D.A.; Coulson, T.; Emmerson, M.C.; et al. Identification of 100 fundamental ecological questions. J. Ecol. 2013, 101, 58–67. [Google Scholar] [CrossRef]
- Patiño, J.; Mateo, R.G.; Zanatta, F.; Marquet, A.; Aranda, S.C.; Borges, P.A.V.; Dirkse, G.; Gabriel, R.; Gonzalez-Mancebo, J.M.; Guisan, A.; et al. Climate threat on the Macaronesian endemic bryophyte flora. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- França, Z.; Cruz, J.V.; Nunes, J.C.; Forjaz, V.H. Geologia dos Açores: Uma perpectiva actual. Açoreana 2003, 10, 11–140. [Google Scholar]
- Forjaz, V.H. Atlas básico dos Açores; OVGA: Ponta Delgada, Portugal, 2004. [Google Scholar]
- Cruz, J.V.; Pereira, R.; Moreira, A. Carta de Ocupação do Solo da Região Autónoma dos Açores; Secretaria Regional do Ambiente e do Mar, Direcção Regional do Ordenamento do Território e dos Recursos Hídricos: Ponta Delgada, Portugal, 2007.
- Elias, R.B.; Gil, A.; Silva, L.; Fernández-Palacios, J.M.; Azevedo, E.B.; Reis, F. Natural zonal vegetation of the Azores Islands: Characterization and potential distribution. Phytocoenologia 2016, 46, 107–123. [Google Scholar] [CrossRef]
- Coelho, M.; Elias, R.; Kluge, J.; Pereira, F.; Henriques, D.; Aranda, S.; Borges, P.; Ah-Peng, C.; Gabriel, R. Long-term monitoring across elevational gradients (II): Vascular plants on Pico Island (Azores) transect. Arquipel. Life Mar. Sci. 2016, 33, 21–44. [Google Scholar]
- Borges, P.A.V.; Cardoso, P.; Kreft, H.; Whittaker, R.J.; Fattorini, S.; Emerson, B.C.; Gil, A.; Gillespie, R.G.; Matthews, T.J.; Santos, A.M.C.; et al. Global Island Monitoring Scheme (GIMS): A proposal for the long-term coordinated survey and monitoring of native island forest biota. Biodivers. Conserv. 2018, 27, 2567–2586. [Google Scholar] [CrossRef]
- Gabriel, R.; Coelho, M.; Henriques, D.; Borges, P.; Elias, R.; Kluge, J.; Ah-Peng, C. Long-term monitoring across elevational gradients to assess ecological hypothesis: A description of standardized sampling methods in oceanic islands and first results. Arquipel. Life Mar. Sci. 2014, 31, 45–67. [Google Scholar]
- ISO 10390: 2005 Soil Quality—Determination of pH; International Organization for Standardization: Geneva, Switzerland, 2005.
- Silva, L.; Moura, M.; Schaefer, H.; Rumsey, F.; Dias, E.F. List of vascular plants (Tracheobionta). In A List of The Terrestrial and Marine Biota from the Azores; Princípia, Ed.; Princípia: Cascais, Portugal, 2010; pp. 117–146. [Google Scholar]
- Azevedo, E.B. Modelação do Clima Insular à Escala Local. Modelo CIELO Aplicado à ilha Terceira. Ph.D. Thesis, University of the Azores, Angra do Heroísmo, Portugal, 1996. [Google Scholar]
- Azevedo, E.B.; Pereira, L.S.; Itier, B. Simulation of local climate in islands environments using a GIS interated model. In Proceedings of the Emerging Technologies for Sustainable land Use and Water Management, Lausanne, Switzerland, 1–3 September 1999. [Google Scholar]
- Azevedo, E.B.; Pereira, L.S.; Itier, B. Modelling the local climate in island environments: Water balance applications. Agric. Water Manag. 1999, 40, 393–403. [Google Scholar] [CrossRef]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics, 2nd ed.; Arnold, E., Ed.; Elsevier Science: London, UK, 1990; ISBN 9780123869104. [Google Scholar]
- Cardoso, P.; Rigal, F.; Fattorini, S.; Terzopoulou, S.; Borges, P.A. Integrating Landscape Disturbance and Indicator Species in Conservation Studies. PLoS ONE 2013, 8, e63294. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 2nd ed.; Springer: Wien, Austria, 1951. [Google Scholar]
- Hodgetts, N.; Lockhart, N. Checklist and Country Status of E Uropean Bryophytes—Update 2020. Irish Wildlife Manuals. 2020. Available online: https://www.npws.ie/sites/default/files/publications/pdf/IWM123.pdf (accessed on 7 April 2021).
- Hammer, Ø. PAST—Paleontological Statistics, Version 4.05—Reference Manual; Natural History Museum, University of Oslo: Oslo, Norway, 2021. [Google Scholar]
- Colwell, R.K.; Rahbek, C.; Gotelli, N. The mid-domain effect and species richness patterns: What have we learned so far? Am. Nat. 2004, 163, E1–E23. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science Ltd.: Malden, MA, USA, 2004; ISBN 0-632-05633-9. [Google Scholar]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L.; Devries, P.; Walla, T.; Greeney, H.; Chao, A.; Ricotta, C. Partitioning diversity for conservation analyses. Divers. Distrib. 2010, 16, 65–76. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Harper Collins Publishers: New York, NY, USA, 1999. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of planktonic foraminifera in deep-sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2000; ISBN 0632054778. [Google Scholar]
- Lloyd, M. Mean crowding. J. Anim. Ecol. 1967, 36, 1–30. [Google Scholar] [CrossRef]
- Henriques, D.S.G.; Borges, P.A.V.; Gabriel, R. Regional processes drive bryophyte diversity and community composition in a small oceanic island. Community Ecol. 2017, 18, 193–202. [Google Scholar] [CrossRef]
- Cardoso, P.; Borges, P.A.V.; Gaspar, C. Biotic integrity of the arthropod communities in the natural forests of Azores. Biodivers. Conserv. 2007, 16, 2883–2901. [Google Scholar] [CrossRef][Green Version]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 9780444892492. [Google Scholar]
- Ellis, L.T.; Ah-Peng, C.; Aranda, S.C.; Bednarek-Ochyra, H.; Borovichev, E.A.; Cykowska-Marzencka, B.; Duarte, M.C.; Enroth, J.; Erzberger, P.; Fedosov, V.; et al. New national and regional bryophyte records, 45. J. Bryol. 2015, 37, 308–329. [Google Scholar] [CrossRef]
- Sjögren, E. Bryophytes (Musci) unexpectedly rare or absent in the Azores. Arquipélago. Life Mar. Sci. 2006, 23A, 1–17. [Google Scholar]
- Coelho, M.; Henriques, D.; Ah-Peng, C. Espécies raras de briófitos ao longo do gradiente altitudinal de floresta nativa na ilha do Pico (Açores): O caso de Echinodium renauldii (Cardot) Broth. In Proceedings of the Ciência nos Açores—Que Futuro? Ponta Delgada, Portugal, 7–8 June 2013; pp. 129–134. [Google Scholar]
- Henriques, D.S.; Elias, R.B.; Coelho, M.C.; Hérnandez, R.H.; Pereira, F.; Gabriel, R. Long-term monitoring across elevational gradients (III): Vascular plants on Terceira Island (Azores) transect. Arquipel. Life Mar. Sci. 2017, 34, 1–20. [Google Scholar]
- Hernández-Hernández, R.; Borges, P.A.V.; Gabriel, R.; Rigal, F.; Ah-Peng, C.; González-Mancebo, J.M. Scaling α- and β-diversity: Bryophytes along an elevational gradient on a subtropical oceanic Island (La Palma, Canary Islands). J. Veg. Sci. 2017. [Google Scholar] [CrossRef]
- Marline, L. Diversity and Biogeography of Madagascan Bryophytes with an Analysis of Taxic and Functional Diversity Along an Elevational Gradient in Marojejy National Park. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2018. [Google Scholar]
- Dansereau, P. Études Macaronésiennes III. La zonation altitudinale. Nat. Can. 1966, 93, 779–795. [Google Scholar]
- Purvis, O.W.; Smith, C.W.; James, P.W. Studies in the lichens of the Azores. Part 2—Lichens of the upper slopes of Pico mountain. A comparison between the lichen floras of the Azores, Madeira and the Canary Islands at high altitudes. Arquipel. Life Mar. Sci. 1994, 12A, 35–50. [Google Scholar]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Marline, L.; Andriamiarisoa, R.L.; Bardat, J.; Chuah-Petiot, M.; Hedderson, T.A.J.; Reeb, C.; Strasberg, D.; Wilding, N.; Ah-Peng, C. Checklist of the Bryophytes of Madagascar. Cryptogam. Bryol. 2012, 33, 199–255. [Google Scholar] [CrossRef]
- Van Reenen, G.B.A.; Gradstein, S.R. Studies on Colombian Cryptogams XX—A transect analysis of the bryophyte vegetation along an altitudinal gradient on the Sierra Nevada de Santa Marta, Colombia. Acta Bot. Neerl. 1983, 32, 1–5. [Google Scholar] [CrossRef]
- Wolf, J.H.D. Diversity Patterns and Biomass of Epiphytic Bryophytes and Lichens Along an Altitudinal Gradient in the Northern Andes. Ann. Missouri Bot. Gard. 1993, 80, 928–960. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Chuah-Petiot, M.; Descamps-Julien, B.; Bardat, J.; Stamenoff, P.; Strasberg, D. Bryophyte diversity and distribution along an altitudinal gradient on a lava flow in La Réunion. Divers. Distrib. 2007, 13, 654–662. [Google Scholar] [CrossRef]
- Madruga, J.S. Andossolos dos Açores. Contributo Para o Seu Estudo. Ph.D. Thesis, University of the Azores, Angra do Heroísmo, Portugal, 1986. [Google Scholar]
- Gabriel, R. Ecophysiology of Azorean Forest Bryophytes. Ph.D. Thesis, Imperial College of Science, Technology and Medicine, University of London, London, UK, 2000. [Google Scholar]
- Borges, P.A.V.; Gabriel, R. Predicting Extinctions on Oceanic Islands: Arthropods and Bryophytes; Universidade dos Açores: Ponta Delgada, Portugal, 2009. [Google Scholar]
- Zartman, C.E.; Amaral, J.A.; Figueiredo, J.N.; de Sales Dambros, C. Drought Impacts Survivorship and Reproductive Strategies of an Epiphyllous Leafy Liverwort in Central Amazonia. Biotropica 2015, 47, 172–178. [Google Scholar] [CrossRef]
- Malombe, I.; Matheka, K.W.; Pócs, T.; Patiño, J. Edge effect on epiphyllous bryophytes in Taita Hills fragmented afromontane forests. J. Bryol. 2016, 38, 33–46. [Google Scholar] [CrossRef]
- Glime, J.M.; Pócs, T. Tropics: Epiphylls. In Bryophyte Ecology; Ebook Sponsored by Michigan Technological University and the International Association of Bryologists; 2018; Volume 4, pp. 8-6-1–8-6-54. [Google Scholar]
- Hill, M.O.; Preston, C.D.; Bosanquet, S.D.S.; Roy, D.B. BRYOATT—Attributes of British and Irish Mosses, Liverworts and Hornworts; Centre for Ecology and Hydrology: Norwich, UK, 2007. [Google Scholar]
- Pharo, E.J.; Beattie, A.J. Bryophyte and lichen diversity: A comparative study. Austral Ecol. 1997, 22, 151–162. [Google Scholar] [CrossRef]
- Elias, R.B. Ecologia das Florestas de Juniperus dos Açores. Ph.D. Thesis, University of Azores, Angra do Heroísmo, Portugal, 2007. [Google Scholar]
- Gabriel, R.; Homem, N.; Couto, A.B.; Aranda, S.C.; Borges, P.A.V. Azorean bryophytes: A preliminary review of rarity patterns. Açoreana 2011, 144, 149–206. [Google Scholar]
- Gabriel, R.; Sim-Sim, M.; Hodgetts, N.; Martins, A. Echinodium Renauldii. The IUCN Red List of Threatened Species 2019: E.T39167A87712737; IUCN: Cambridge, UK, 2019; Available online: https://www.iucnredlist.org (accessed on 7 April 2021).
- Ferreira, M.T.; Cardoso, P.; Borges, P.A.V.; Gabriel, R.; de Azevedo, E.B.; Reis, F.; Araujo, M.B.; Elias, R.B. Effects of climate change on the distribution of indigenous species in oceanic islands (Azores). Clim. Chang. 2016, 138, 603–615. [Google Scholar] [CrossRef]
- Vergílio, M.; Fonseca, C.; Calado, H.; Borges, P.A.V.; Elias, R.B.; Gabriel, R.; Martins, A.; Azevedo, E.; Cardoso, P. Assessing the efficiency of protected areas to represent biodiversity: A small island case study. Environ. Conserv. 2016, 43, 337–349. [Google Scholar] [CrossRef]
- ABP—Azores Biodiversity Portal. Available online: http://azoresbioportal.uac.pt/ (accessed on 1 October 2017).
- Gaston, K.J.; Fuller, R.A. Commonness, population depletion and conservation biology. Trends Ecol. Evol. 2008, 23, 14–19. [Google Scholar] [CrossRef]
- Gaston, K.J. Ecology. Valuing common species. Science 2010, 327, 154–155. [Google Scholar] [CrossRef]
- Loppi, S. Lichens as sentinels for air pollution at remote alpine areas (Italy). Environ. Sci. Pollut. Res. 2014, 21, 2563–2571. [Google Scholar] [CrossRef]
- Sim-Sim, M.; Carvalho, S.; Fontinha, S.; Lobo, C.; Esquível, M.G.; Figueiredo, A.C. Diversity of Bryophytes—Useful indicators of Madeira Laurel forest conservation. In EFI Proceedings No. 51 Monitoring and Indicators of Forest Biodiversity in Europe—From Ideas to Operationality; European Forest Institute: Joensuu, Finland, 2005; pp. 247–257. [Google Scholar]

| Substrate Elevation (m a.s.l.) | RU | TE | HU | LI | EP | LF | Total |
|---|---|---|---|---|---|---|---|
| 10 | 18 | 3 | 18 | 25 | 64 | ||
| 200 | 16 | 5 | 18 | 34 | 73 | ||
| 400 | 18 | 11 | 6 | 18 | 40 | 93 | |
| 600 | 15 | 18 | 17 | 54 | 17 | 121 | |
| 800 | 4 | 18 | 18 | 54 | 18 | 112 | |
| 1000 | 3 | 5 | 18 | 18 | 48 | 18 | 110 |
| 1200 | 6 | 12 | 12 | 13 | 36 | 9 | 88 |
| 1400 | 8 | 12 | 12 | 3 | 18 | 53 | |
| 1600 | 17 | 10 | 12 | 17 | 22 | 78 | |
| 1800 | 18 | 2 | 2 | 7 | 29 | ||
| 2000 | 18 | 1 | 7 | 26 | |||
| 2200 | 18 | 12 | 1 | 31 | |||
| Total | 155 | 65 | 117 | 141 | 338 | 62 | 878 |
| Elevation (m a.s.l.) | 10 | 200 | 400 | 600 | 800 | 1000 | 1200 | 1400 | 1600 | 1800 | 2000 | 2200 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRYOPHYTES | |||||||||||||
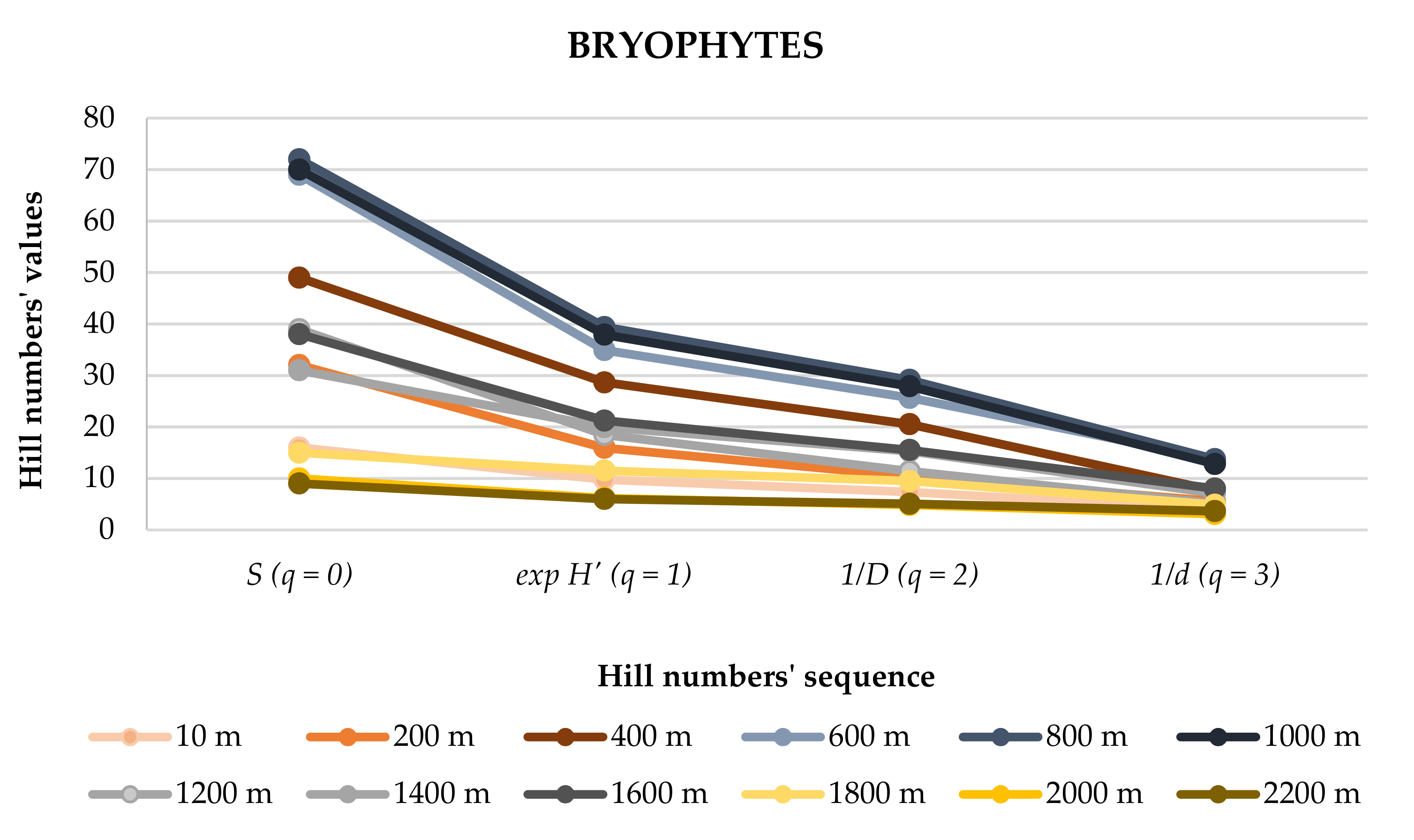
| S (q = 0) | 16 | 32 | 49 | 69 | 72 | 70 | 39 | 31 | 38 | 15 | 10 | 9 | 141 |
| H’ | 2.28 | 2.77 | 3.36 | 3.55 | 3.67 | 3.64 | 2.92 | 2.99 | 3.06 | 2.45 | 1.82 | 1.79 | 4.11 |
| exp H’ (q = 1) | 9.75 | 15.94 | 28.65 | 34.92 | 39.41 | 37.92 | 18.45 | 19.97 | 21.23 | 11.52 | 6.18 | 6.01 | 61.13 |
| D | 0.14 | 0.10 | 0.05 | 0.04 | 0.03 | 0.04 | 0.09 | 0.07 | 0.06 | 0.11 | 0.21 | 0.20 | 0.02 |
| 1/D (q = 2) | 7.38 | 10.41 | 20.53 | 25.64 | 29.14 | 27.90 | 11.40 | 15.28 | 15.52 | 9.49 | 4.84 | 5.08 | 40.48 |
| d | 0.25 | 0.18 | 0.13 | 0.07 | 0.07 | 0.08 | 0.20 | 0.14 | 0.12 | 0.20 | 0.32 | 0.27 | 0.07 |
| 1/d (q = 3) | 3.97 | 5.56 | 7.62 | 13.76 | 13.73 | 12.79 | 5.11 | 7.10 | 8.04 | 4.90 | 3.11 | 3.65 | 15.11 |
| LIVERWORTS | |||||||||||||
| S (q = 0) | 7 | 17 | 28 | 48 | 49 | 48 | 18 | 11 | 13 | 4 | 3 | 2 | 71 |
| H’ | 1.48 | 2.10 | 2.85 | 3.28 | 3.39 | 3.31 | 2.07 | 2.05 | 1.97 | 1.20 | 1.07 | 0.30 | 3.59 |
| exp H’ (q = 1) | 4.37 | 8.15 | 17.32 | 26.49 | 29.61 | 27.38 | 7.93 | 7.73 | 7.16 | 3.31 | 2.91 | 1.36 | 36.09 |
| D | 0.28 | 0.17 | 0.08 | 0.05 | 0.04 | 0.05 | 0.22 | 0.16 | 0.18 | 0.34 | 0.36 | 0.83 | 0.04 |
| 1/D (q = 2) | 3.60 | 5.97 | 12.87 | 19.63 | 22.55 | 20.68 | 4.57 | 6.32 | 5.41 | 2.97 | 2.81 | 1.20 | 26.73 |
| d | 0.40 | 0.25 | 0.18 | 0.09 | 0.10 | 0.10 | 0.43 | 0.28 | 0.30 | 0.43 | 0.45 | 0.91 | 0.08 |
| 1/d (q = 3) | 2.50 | 4.03 | 5.48 | 11.15 | 10.09 | 9.79 | 2.34 | 3.55 | 3.29 | 2.33 | 2.20 | 1.10 | 12.48 |
| MOSSES | |||||||||||||
| S (q = 0) | 9 | 15 | 21 | 21 | 23 | 22 | 21 | 20 | 25 | 11 | 7 | 7 | 70 |
| H’ | 1.86 | 2.39 | 2.53 | 2.13 | 2.29 | 2.38 | 2.36 | 2.56 | 2.65 | 2.11 | 1.39 | 1.56 | 3.27 |
| exp H’ (q = 1) | 6.42 | 10.95 | 12.57 | 8.44 | 9.82 | 10.76 | 10.55 | 12.89 | 14.09 | 8.21 | 4.02 | 4.75 | 26.26 |
| D | 0.19 | 0.11 | 0.11 | 0.17 | 0.15 | 0.14 | 0.14 | 0.10 | 0.10 | 0.15 | 0.30 | 0.25 | 0.07 |
| 1/D (q = 2) | 5.39 | 9.18 | 9.30 | 6.04 | 6.78 | 7.33 | 7.03 | 9.64 | 10.11 | 6.55 | 3.35 | 4.05 | 13.80 |
| d | 0.30 | 0.17 | 0.18 | 0.29 | 0.27 | 0.27 | 0.29 | 0.18 | 0.19 | 0.29 | 0.40 | 0.32 | 0.20 |
| 1/d (q = 3) | 3.33 | 6.00 | 5.56 | 3.44 | 3.64 | 3.74 | 3.40 | 5.46 | 5.21 | 3.50 | 2.50 | 3.10 | 4.88 |
| RU | TE | HU | LI | EP | LF | Total | |
|---|---|---|---|---|---|---|---|
| BRYOPHYTES | |||||||
| S (q = 0) | 76 | 61 | 74 | 70 | 84 | 38 | 141 |
| H’ | 3.79 | 3.75 | 3.84 | 3.73 | 3.68 | 2.89 | 4.11 |
| exp H’ (q = 1) | 44.43 | 42.63 | 46.73 | 41.58 | 39.74 | 18.07 | 61.13 |
| D | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.08 | 0.02 |
| 1/D (q = 2) | 29.85 | 32.69 | 33.58 | 30.70 | 28.90 | 12.84 | 40.48 |
| d | 0.08 | 0.07 | 0.07 | 0.09 | 0.09 | 0.14 | 0.07 |
| 1/d (q = 3) | 11.92 | 13.80 | 14.33 | 11.39 | 11.44 | 7.35 | 15.11 |
| LIVERWORTS | |||||||
| S (q = 0) | 32 | 29 | 38 | 47 | 51 | 32 | 71 |
| H’ | 2.90 | 3.03 | 3.20 | 3.41 | 3.37 | 2.72 | 3.59 |
| exp H’ (q = 1) | 18.15 | 20.69 | 24.63 | 30.21 | 29.12 | 15.24 | 36.09 |
| D | 0.08 | 0.06 | 0.05 | 0.04 | 0.04 | 0.09 | 0.04 |
| 1/D (q = 2) | 12.44 | 16.17 | 18.78 | 24.19 | 22.78 | 11.07 | 26.73 |
| d | 0.19 | 0.14 | 0.10 | 0.08 | 0.08 | 0.15 | 0.08 |
| 1/d (q = 3) | 5.39 | 7.20 | 9.81 | 12.05 | 12.72 | 6.64 | 12.48 |
| MOSSES | |||||||
| S (q = 0) | 47 | 33 | 37 | 23 | 33 | 6 | 70 |
| H’ | 3.31 | 3.12 | 3.16 | 2.44 | 2.36 | 1.20 | 3.28 |
| exp H’ (q = 1) | 27.34 | 22.54 | 23.49 | 11.41 | 10.62 | 3.31 | 26.58 |
| D | 0.06 | 0.06 | 0.07 | 0.14 | 0.15 | 0.45 | 0.07 |
| 1/D (q = 2) | 17.59 | 16.63 | 15.34 | 7.24 | 6.53 | 2.23 | 13.80 |
| d | 0.15 | 0.11 | 0.18 | 0.30 | 0.32 | 0.65 | 0.20 |
| 1/d (q = 3) | 6.53 | 8.80 | 5.54 | 3.36 | 3.12 | 1.54 | 4.88 |
| Altitudinal Bands | Bryophytes (n = 141) | Liverworts (n = 71) | Mosses (n = 70) |
|---|---|---|---|
| Low–Middle | 0.56 | 0.58 | 0.52 |
| Low–Medium High | 0.39 | 0.49 | 0.32 |
| Low–High | 0.10 | 0.06 | 0.13 |
| Middle–Medium High | 0.41 | 0.44 | 0.38 |
| Middle–High | 0.12 | 0.09 | 0.16 |
| Medium High–High | 0.41 | 0.29 | 0.47 |
| Altitudinal Bands | Bryophytes (n = 141) | Liverworts (n = 71) | Mosses (n = 70) |
|---|---|---|---|
| EP–HU | 0.54 | 0.63 | 0.43 |
| EP–LF | 0.59 | 0.72 | 0.31 |
| EP–LI | 0.74 | 0.82 | 0.61 |
| EP–RU | 0.53 | 0.53 | 0.52 |
| EP–TE | 0.51 | 0.53 | 0.49 |
| HU–LF | 0.46 | 0.60 | 0.24 |
| HU–LI | 0.60 | 0.66 | 0.51 |
| HU–RU | 0.67 | 0.69 | 0.65 |
| HU–TE | 0.70 | 0.66 | 0.74 |
| LF–LI | 0.63 | 0.71 | 0.41 |
| LF–RU | 0.37 | 0.53 | 0.16 |
| LF–TE | 0.38 | 0.46 | 0.26 |
| LI–RU | 0.52 | 0.58 | 0.45 |
| LI–TE | 0.56 | 0.55 | 0.58 |
| RU–TE | 0.66 | 0.69 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.C.M.; Gabriel, R.; Hespanhol, H.; Borges, P.A.V.; Ah-Peng, C. Bryophyte Diversity along an Elevational Gradient on Pico Island (Azores, Portugal). Diversity 2021, 13, 162. https://doi.org/10.3390/d13040162
Coelho MCM, Gabriel R, Hespanhol H, Borges PAV, Ah-Peng C. Bryophyte Diversity along an Elevational Gradient on Pico Island (Azores, Portugal). Diversity. 2021; 13(4):162. https://doi.org/10.3390/d13040162
Chicago/Turabian StyleCoelho, Márcia C. M., Rosalina Gabriel, Helena Hespanhol, Paulo A. V. Borges, and Claudine Ah-Peng. 2021. "Bryophyte Diversity along an Elevational Gradient on Pico Island (Azores, Portugal)" Diversity 13, no. 4: 162. https://doi.org/10.3390/d13040162
APA StyleCoelho, M. C. M., Gabriel, R., Hespanhol, H., Borges, P. A. V., & Ah-Peng, C. (2021). Bryophyte Diversity along an Elevational Gradient on Pico Island (Azores, Portugal). Diversity, 13(4), 162. https://doi.org/10.3390/d13040162








