All Populations Matter: Conservation Genomics of Australia’s Iconic Purple Wattle, Acacia purpureopetala
Abstract
:1. Introduction
2. Materials and Methods
2.1. Floristic Data
2.2. Genomic Data
2.2.1. Single Nucleotide Polymorphic (SNP) Data
2.2.2. Genomic Data Analyses
2.3. Environmental Data
2.4. Relationships between Genetic, Environmental, Floristic and Spatial Distances
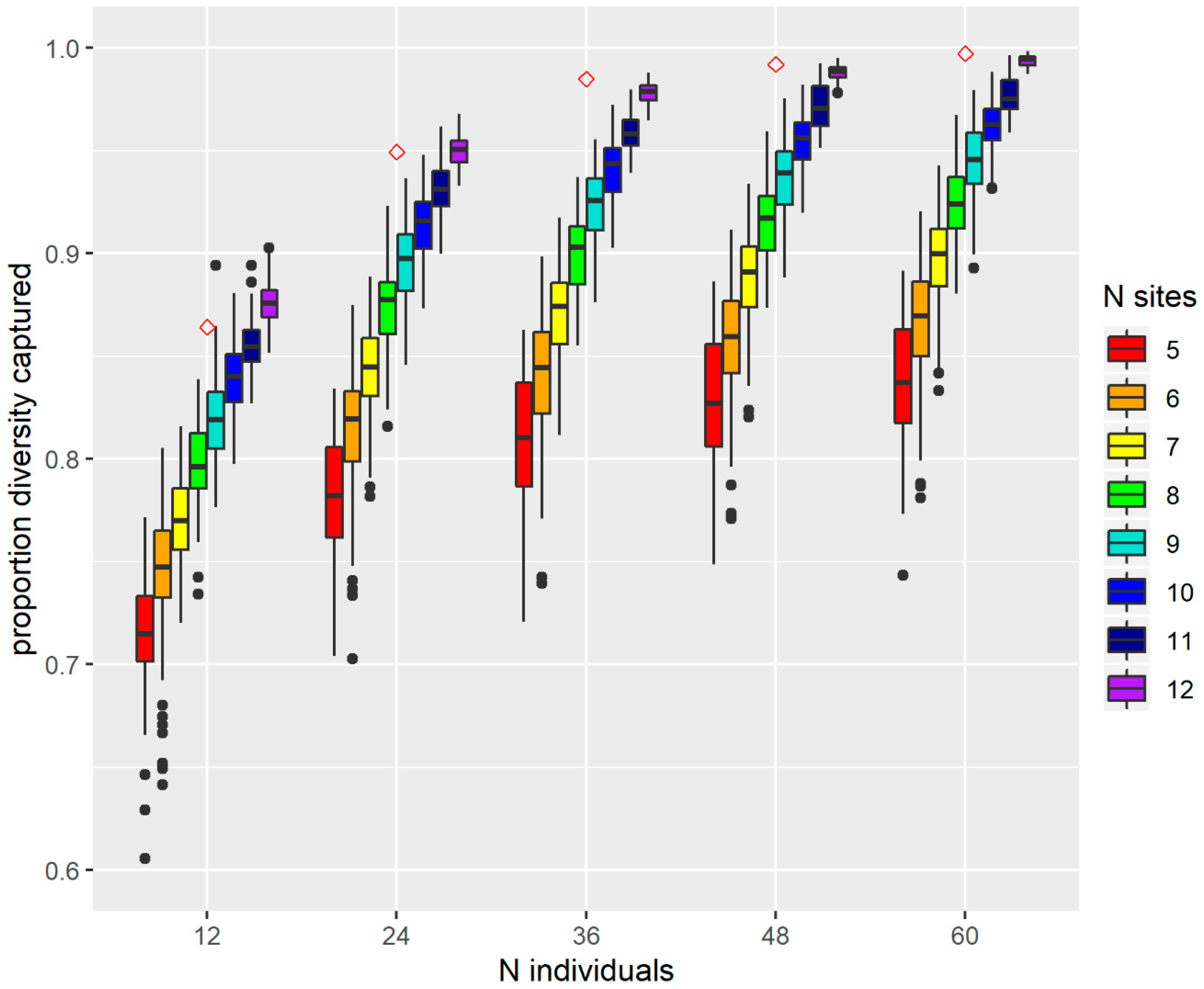
2.5. Optimising Genetic Diversity for A Germplasm Collection
3. Results
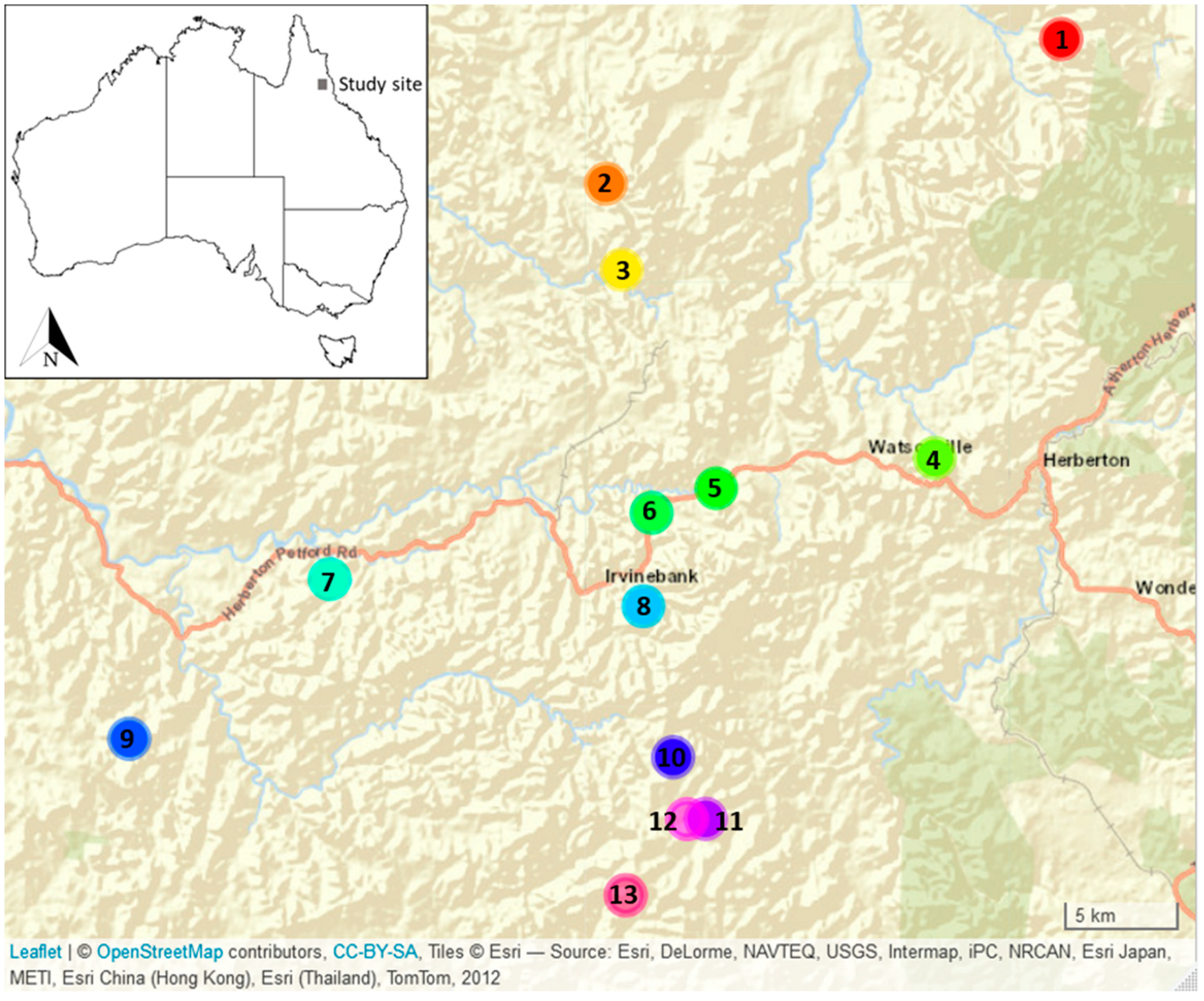
3.1. Floristic Data
3.2. Genotype Data
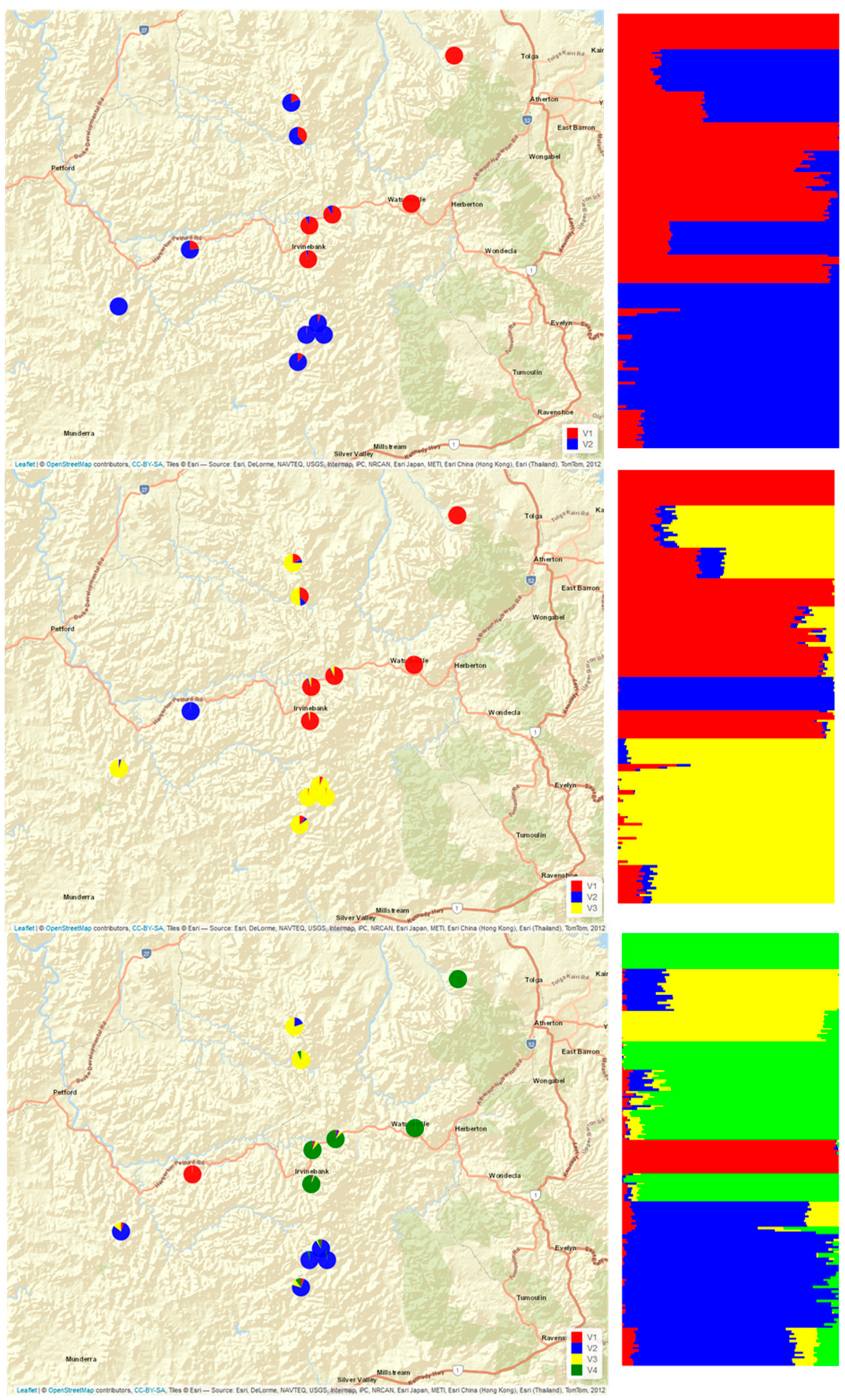
3.2.1. Cluster Analyses
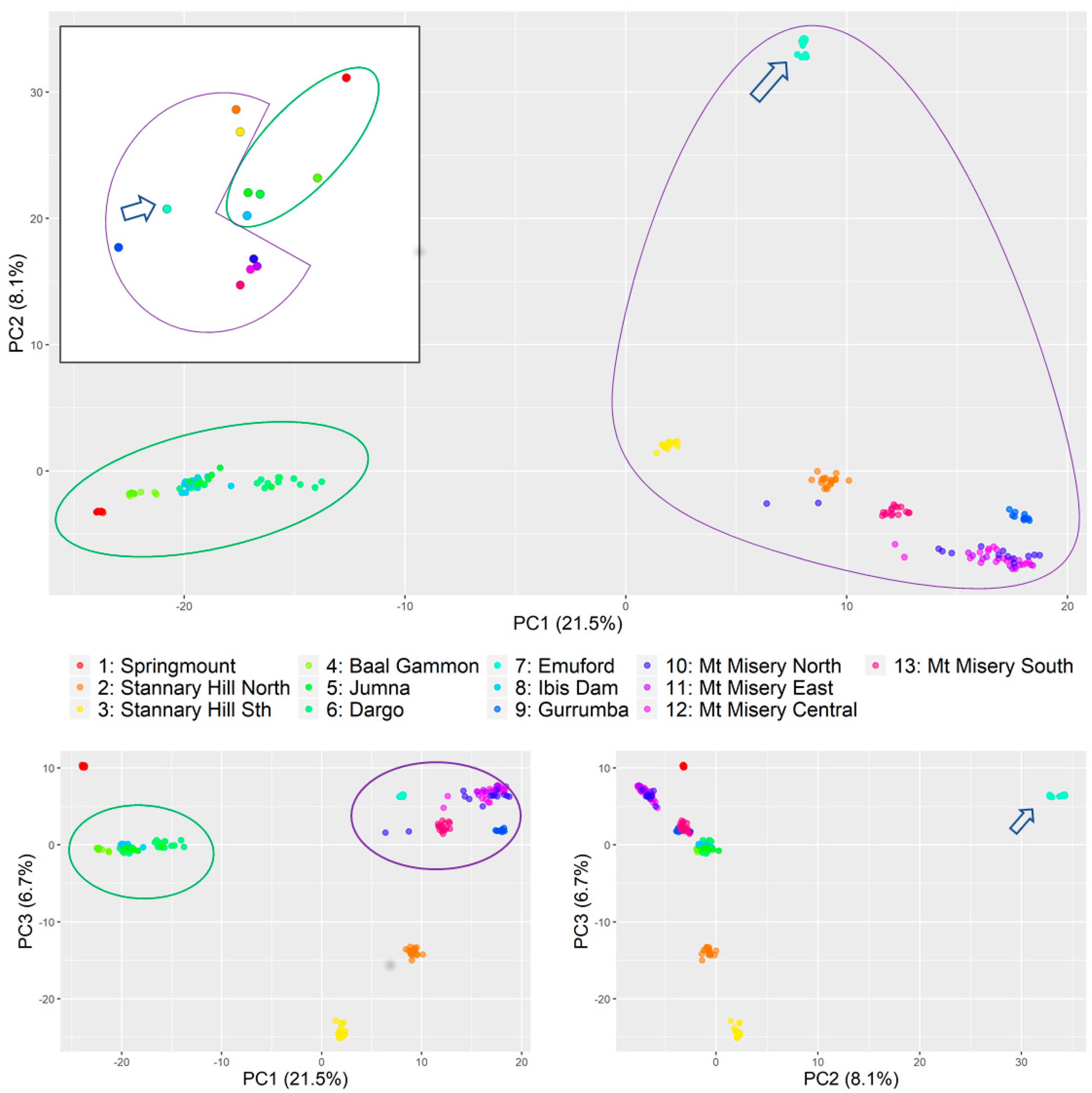
- Principal component analysis (Figure 2) found that most of the variations were explained by the first component (21%), which indicated two strongly differentiated genetic groups (Figure 2, green and purple shapes) with component two also clearly distinguishing a third small cluster consisting only of the individuals collected at Emuford (blue arrow). Principal component three further segregated Stannary Hill North (orange), Stannary Hill South (yellow), and Springmount(red) into distinctive small clusters.
- With the snmf analysis (Figure A1) performed in LEA, K = 2 supported the clustering of all samples from Baal Gammon, Dargo, Jumna, and Springmount as one group and all samples from Mt Misery (Central, East, North, South), Gurrumba and Emuford as the second group, while the samples from Stannary Hill North and South and Emuford were not clearly identified as belonging to either. With K = 3, the three inferred structure groups corresponded to the groups identified on PC1vs PC2 plot. The assignments to K = 4 was congruent with the clusters obtained across the three main principal components.
- The analysis of variance (Table 1) indicated substantial variation between sites (60%) as well as between the two groups (33%) identified with the principal component analysis.
3.2.2. Population Genomic Diversity Estimates
3.3. Relationships between Genomic Data and Ecological Data
3.4. A Collecting Strategy to Optimise Genetic Diversity
4. Discussion
Application of Population Genomics to A Conservation Management Plan for the Purple Wattle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Field Site | Altitude | S1 | S2 | Total | Ap Transect | Ap Site |
|---|---|---|---|---|---|---|
| Springmount | 640 m | 10 | 15 | 19 | 35 | 459 |
| Stannary Hill North | 830 m | 14 | 18 | 22 | 45 | 411 |
| Stannary Hill South | 830 m | 8 | 11 | 15 | 136 | 551 |
| Baal Gammon | 1040 m | 17 | 0 | 17 | 35 | 67 |
| Jumna | 835 m | 8 | 20 | 21 | 63 | 398 |
| Dargo | 795 m | 13 | 23 | 27 | 35 | 310 |
| Emuford | 750 m | 8 | 14 | 16 | 66 | 561 |
| Ibis Dam | 950 m | 7 | 17 | 17 | 12 | 468 |
| Gurrumba | 735 m | 19 | 19 | 32 | 41 | 105 |
| Mount Misery North | 990 m | 19 | 9 | 23 | 63 | 255 |
| Mount MiseryEast | 935 m | 18 | 12 | 23 | 16 | 339 |
| Mount Misery Central | 1030 m | 19 | 11 | 26 | 100 | 168 |
| Mount Misery South | 960 m | 18 | 4 | 21 | 66 | 204 |
References
- Convention on Biological Diversity. Available online: https://www.cbd.int/aichi-targets/ (accessed on 16 February 2021).
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Vandewoestijne, S.; Schtickzelle, N.; Baguette, M. Positive correlation between genetic diversity and fitness in a large, well-connected metapopulation. BMC Biol. 2008, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Oostermeijer, J.; Luijten, S.; Nijs, J.D. Integrating demographic and genetic approaches in plant conservation. Biol. Conserv. 2003, 113, 389–398. [Google Scholar] [CrossRef]
- Schäfer, D.; Vincent, H.; Fischer, M.; Kempel, A. The importance of genetic diversity for the translocation of eight threatened plant species into the wild. Glob. Ecol. Conserv. 2020, 24, e01240. [Google Scholar] [CrossRef]
- Khan, S.; Nabi, G.; Ullah, M.W.; Yousaf, M.; Manan, S.; Siddique, R.; Hou, H. Overview on the Role of Advance Genomics in Conservation Biology of Endangered Species. Int. J. Genom. 2016, 2016, 3460416. [Google Scholar] [CrossRef]
- Supple, M.A.; Shapiro, B. Conservation of biodiversity in the genomics era. Genome Biol. 2018, 19, 131. [Google Scholar] [CrossRef]
- Miller, J.T.; Murphy, D.J.; Ho, S.Y.W.; Cantrill, D.J.; Seigler, D. Comparative dating of Acacia: Combining fossils and multiple phylogenies to infer ages of clades with poor fossil records. Aust. J. Bot. 2013, 61, 436–445. [Google Scholar] [CrossRef]
- Crisp, M.D.; Cook, L.G. How Was the Australian Flora Assembled Over the Last 65 Million Years? A Molecular Phylogenetic Perspective. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 303–324. [Google Scholar] [CrossRef]
- Flora of Australia. Available online: https://profiles.ala.org.au/opus/foa/profile/Acacia (accessed on 16 February 2021).
- Species Profile and Threats Database. Available online: http://www.environment.gov.au/cgi-bin/sprat/public/sprat.pl (accessed on 15 March 2021).
- Australian Government, Department of Agriculture, Water and the Environment. Available online: https://www.environment.gov.au/biodiversity/threatened/species/30-plants-by-2020/purple-wattle (accessed on 16 February 2021).
- Kenrick, J.; Knox, R.B. Quantitative Analysis of Self-Incompatibility in Trees of Seven Species of Acacia. J. Hered. 1989, 80, 240–245. [Google Scholar] [CrossRef]
- PlantNET (The NSW Plant Information Network System). Royal Botanic Gardens and Domain Trust, Sydney. Available online: https://plantnet.rbgsyd.nsw.gov.au (accessed on 2 February 2021).
- Entwisle, T.J.; Maslin, B.R.; Cowan, R.S.; Court, A.B. Acacia. In Flora of Victoria; Walsh, N.G., Entwisle, T.J., Eds.; Inkata Press: Melbourne, Australia; Sydney, Australia, 1996; Volume 3, pp. 586–656. [Google Scholar]
- Rossetto, M.; Wilson, P.D.; Bragg, J.; Cohen, J.; Fahey, M.; Yap, J.-Y.S.; Van Der Merwe, M. Perceptions of Similarity Can Mislead Provenancing Strategies—An Example from Five Co-Distributed Acacia Species. Diversity 2020, 12, 306. [Google Scholar] [CrossRef]
- Coates, D.J.; Tischler, G.; McComb, J.A. Genetic variation and the mating system in the rare Acacia sciophanes compared with its common sister species Acacia anfractuosa (Mimosaceae). Conserv. Genet. 2006, 7, 931–944. [Google Scholar] [CrossRef]
- Blyth, C.; Christmas, M.J.; Bickerton, D.C.; Faast, R.; Packer, J.G.; Lowe, A.J.; Breed, M.F. Increased Genetic Diversity via Gene Flow Provides Hope for Acacia whibleyana, an Endangered Wattle Facing Extinction. Diversity 2020, 12, 299. [Google Scholar] [CrossRef]
- Gleed, S.J.; Franklin, D.C. Purple-flowered Wattle (Acacia purpureopetala): Observations and surveys of a threatened plant found only in the Herberton-Irvinebank region. N. Qld. Nat. 2018, 48, 57–68. [Google Scholar]
- Ford, A.; Williams, P.; Collins, E. 2018 Ecological Assessment of Acacia purpureopetala; CSIRO Report to EPBC; CSIRO: Atherton, CA, USA, 2018.
- Maslin, B.R. Acacia purpureopetala. In Flora of Australia Volume 11A, Mimosaceae, Acacia Part 1; Orchard, A.E., Wilson, A.J.G., Eds.; CSIRO Publishing: Melbourne, Australia, 2001; p. 449. [Google Scholar]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef]
- Gilpin, M. The genetic effective size of a metapopulation. Biol. J. Linn. Soc. 1991, 42, 165–175. [Google Scholar] [CrossRef]
- Ouborg, N.J.; Vergeer, P.; Mix, C. The rough edges of the conservation genetics paradigm for plants. J. Ecol. 2006, 94, 1233–1248. [Google Scholar] [CrossRef]
- Young, A.; Boyle, T.; Brown, T. The population genetic consequences of habitat fragmentation for plants. Trends Ecol. Evol. 1996, 11, 413–418. [Google Scholar] [CrossRef]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nat. Cell Biol. 2000, 405, 243–253. [Google Scholar] [CrossRef]
- Whitlock, R.; Hipperson, H.; Thompson, D.; Butlin, R.; Burke, T. Consequences of in-situ strategies for the conservation of plant genetic diversity. Biol. Conserv. 2016, 203, 134–142. [Google Scholar] [CrossRef]
- Acacia purpureopetala SPRAT Profile. Available online: http://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=61156pl (accessed on 17 May 2017).
- Cheng, J.; Karambelkar, B.; Xie, Y. Leaflet: Create Interactive Web Maps with the JavaScript ‘Leaflet’ Library. R package Version 2.0.2. 2018. Available online: https://CRAN.R-project.org/package=leaflet (accessed on 17 March 2021).
- Neldner, V.J.; Wilson, B.A.; Dilleward, H.A.; Ryan, T.S.; Butler, D.W. Methodology for Survey and Mapping of Regional Ecosystems and Vegetation Communities in Queensland; Version 4; Queensland Herbarium, Queensland Department of Science, Information Technology and Innovation: Brisbane, Australia, 2017. Available online: https://publications.qld.gov.au/dataset/redd/resource/6dee78ab-c12c-4692-9842-b7257c2511e4 (accessed on 1 July 2018).
- Sansaloni, C.P.; Petroli, C.D.; Carling, J.; Hudson, C.J.; Steane, D.; Myburg, A.; Grattapaglia, D.; Vaillancourt, R.; Kilian, A. A high-density Diversity Arrays Technology (DArT) microarray for genome-wide genotyping in Eucalyptus. Plant Methods 2010, 6, 16. [Google Scholar] [CrossRef]
- Rossetto, M.; Bragg, J.; Kilian, A.; McPherson, H.; Van Der Merwe, M.; Wilson, P.D. Restore and Renew: A genomics-era framework for species provenance delimitation. Restor. Ecol. 2019, 27, 538–548. [Google Scholar] [CrossRef]
- Smith, A.L.; Hodkinson, T.R.; Villellas, J.; Catford, J.A.; Csergő, A.M.; Blomberg, S.P.; Crone, E.E.; Ehrlén, J.; Garcia, M.B.; Laine, A.-L.; et al. Global gene flow releases invasive plants from environmental constraints on genetic diversity. Proc. Natl. Acad. Sci. USA 2020, 117, 4218–4227. [Google Scholar] [CrossRef]
- Rutherford, S.; Van Der Merwe, M.; Wilson, P.G.; Kooyman, R.M.; Rossetto, M. Managing the risk of genetic swamping of a rare and restricted tree. Conserv. Genet. 2019, 20, 1113–1131. [Google Scholar] [CrossRef]
- Bragg, J.G.; Cuneo, P.; Sherieff, A.; Rossetto, M. Optimizing the genetic composition of a translocation population: Incorporating constraints and conflicting objectives. Mol. Ecol. Resour. 2020, 20, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Frichot, E.; François, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Keenan, K.; McGinnity, P.; Cross, T.F.; Crozier, W.W.; Prodöhl, P.A. Diversity: An R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol. Evol. 2013, 4, 782–788. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef]
- Weir, B.S.; Hill, W.G. Estimating F-Statistics. Annu. Rev. Genet. 2002, 36, 721–750. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Chelsa. Available online: https://chelsa-climate.org (accessed on 12 October 2020).
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. Bioclim: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Diver. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- CSIRO Data Access Portal. Available online: https://data.csiro.au/dap (accessed on 12 March 2021).
- Hijmans, R.J. 2020 Raster: Geographic Data Analysis and Modeling, R Package Version 3.4-5; 2020. Available online: https://rdrr.io/cran/raster/ (accessed on 11 March 2021).
- Ferrier, S.; Manion, G.; Elith, J.; Richardson, K. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 2007, 13, 252–264. [Google Scholar] [CrossRef]
- Pebesma, E.J.; Bivand, R.S. Classes and methods for spatial data in R. R News 2005, 5, 9–13. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package Version 2.5-5. 2019. Available online: https://rdrr.io/cran/vegan/ (accessed on 5 December 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Australia, 2018; Available online: https://www.R-project.org (accessed on 10 September 2020).
- Fitzpatrick, M.C.; Mokany, K.; Manion, G.; Lisk, M.; Ferrier, S.; Diego Nieto-Lugilde, D. GDM: Generalized Dissimilarity Modeling. R Package Version 1.4.2.1. 2021. Available online: https://CRAN.R-project.org/package=gdm (accessed on 11 March 2021).
- Jasonbragg/OptGenMix. Available online: https://github.com/jasongbragg/OptGenMix (accessed on 15 October 2020).
- Kirkpatrick, S.; Gelatt, C.D.; Vecchi, M.P. Optimization by Simulated Annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Analysis of Gene Diversity in Subdivided Populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, M.M. Research Centre for Ecosystem Resilience; Royal Botanic Garden Sydney: Sydney, Australia, 2021; unpublished work.
- Bragg, J.G.; Yap, J.S.; Wilson, T.; Lee, E.; Rossetto, M. Conserving the genetic diversity of condemned populations: Optimizing collections and translocation. Evol. Appl. 2021. [Google Scholar] [CrossRef]
- Lande, R. Genetics and demography in biological conservation. Science 1988, 241, 1455–1460. [Google Scholar] [CrossRef]
- Pannell, J.R.; Voillemot, M. Evolution and Ecology of Plant Mating Systems; Wiley: Hoboken, NJ, USA, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Keller, L.F. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
- Whitehead, M.R.; Lanfear, R.; Mitchell, R.J.; Karron, J.D. Plant Mating Systems Often Vary Widely Among Populations. Front. Ecol. Evol. 2018, 6, 38. [Google Scholar] [CrossRef]
- Levin, D.A. Dispersal versus Gene Flow in Plants. Ann. Mol. Bot. Gard. 1981, 68, 233. [Google Scholar] [CrossRef]
- Doligez, A.; Baril, C.; Joly, H.I. Fine-scale spatial genetic structure with nonuniform distribution of individuals. Genetics 1998, 148, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Balloux, F.; Lehmann, L.; De Meeûs, T. The population genetics of clonal and partially clonal diploids. Genetics 2003, 164, 1635–1644. [Google Scholar]
- Kenrick, J.; Knox, R.B. Function of the Polyad in Reproduction of Acacia. Ann. Bot. 1982, 50, 721–727. [Google Scholar] [CrossRef]
- Baker, H.G. Self-Compatibility and Establishment after ’Long-Distance’ Dispersal. Evolution 1955, 9, 347. [Google Scholar] [CrossRef]
- Charlesworth, B.; Charlesworth, D. The genetic basis of inbreeding depression. Genet. Res. 1999, 74, 329–340. [Google Scholar] [CrossRef]
- Frankham, R.; Gilligan, D.M.; Morris, D.; Briscoe, D.A. Inbreeding and extinction: Effects of purging. Conserv. Genet. 2001, 2, 279–284. [Google Scholar] [CrossRef]
- Griffith, M.P.; Clase, T.; Toribio, P.; Piñeyro, Y.E.; Jimenez, F.; Gratacos, X.; Sanchez, V.; Meerow, A.; Meyer, A.; Kramer, A.; et al. Can a Botanic Garden Metacollection Better Conserve Wild Plant Diversity? A Case Study Comparing Pooled Collections with an Ideal Sampling Model. Int. J. Plant Sci. 2020, 181, 485–496. [Google Scholar] [CrossRef]
- Griffith, M.P.; Barber, G.; Tucker Lima, J.; Barros, M.; Calonje, C.; Noblick, L.R.; Calonje, M.; Magellan, T.; Dosmann, M.; Thibault, T.; et al. Plant Collection “Half-life:” Can Botanic Gardens Weather the Climate? Curat. Mus. J. 2017, 60, 395–410. [Google Scholar] [CrossRef]
- Krishnan, S.; Ranker, T.A.; Davis, A.P.; Rakotomalala, J.J. The study of genetic diversity patterns of Coffea commersoniana, an endangered coffee species from Madagascar: A model for conservation of other littoral forest species. Tree Genet. Genomes 2013, 9, 179–187. [Google Scholar] [CrossRef]
- Griffith, M.P.; Calonje, M.; Meerow, A.W.; Tut, F.; Kramer, A.T.; Hird, A.; Magellan, T.M.; Husby, C.E. Can a Botanic Garden Cycad Collection Capture the Genetic Diversity in a Wild Population? Int. J. Plant Sci. 2015, 176, 1–10. [Google Scholar] [CrossRef]
- Wood, J.; Ballou, J.D.; Callicrate, T.; Fant, J.B.; Griffith, M.P.; Kramer, A.T.; Lacy, R.C.; Meyer, A.; Sullivan, S.; Traylor-Holzer, K.; et al. Applying the zoo model to conservation of threatened exceptional plant species. Conserv. Biol. 2020, 34, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Resende-Moreira, L.C.; Carvalho, C.S.; Lanes, E.C.M.; Ortiz-Vera, M.P.; Viana, P.L.; Jaffé, R. Range-wide neutral and adaptive genetic structure of an endemic herb from Amazonian Savannas. AoB Plants 2020, 12, plaa003. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Strader, A.A.; Murphy, V.; Nghiem-Phu, L.; Calonje, M.; Griffith, M.P. Palma Corcho: A Case Study in Botanic Garden Conservation Horticulture and Economics. HortTechnology 2011, 21, 474–481. [Google Scholar] [CrossRef]
- Rossetto, M.; Yap, J.-Y.S.; Lemmon, J.; Bain, D.; Bragg, J.; Hogbin, P.; Gallagher, R.; Rutherford, S.; Summerell, B.; Wilson, T.C. A conservation genomics workflow to guide practical management actions. Glob. Ecol. Conserv. 2021, 26, e01492. [Google Scholar] [CrossRef]
| Level of Hierarchy | Partitioning | D.F | SS | % of Variation | Phi Φ |
|---|---|---|---|---|---|
| Field sites | Between sites | 11 | 184,582.2 | 60 | 0.599 |
| Within sites | 172 | 121,641.8 | 40 | ||
| Total | 183 | 306,224.0 | 100 | ||
| Among groups (based on PCA) | Between group | 1 | 68,093.04 | 33 | 0.44626 |
| Between sites | 9 | 91,066.82 | 30 | 0.627 | |
| Within sites | 159 | 123,423.4 | 37 | ||
| Total | 169 | 282,583.3 | 100 | 0.327 |
| Site Number | Site Name | Sample Size | Size | Ar | Ho | He | Fis |
|---|---|---|---|---|---|---|---|
| 1 | Springmount | 15 | 14.75 | 1.04 | 0.02 | 0.02 | 0.10 |
| 2 | Stannary Hill North | 18 | 17.44 | 1.32 | 0.06 | 0.14 | 0.50 |
| 3 | Stannary Hill South | 13 | 12.56 | 1.17 | 0.03 | 0.07 | 0.45 |
| 4 | Baal Gammon | 12 | 11.66 | 1.14 | 0.04 | 0.06 | 0.27 |
| 5 | Jumna | 6 | 5.77 | 1.29 | 0.06 | 0.12 | 0.42 |
| 6 | Dargo | 24 | 23.17 | 1.41 | 0.05 | 0.18 | 0.69 |
| 7 | Emuford | 14 | 13.69 | 1.05 | 0.01 | 0.02 | 0.23 |
| 8 | Ibis Dam | 12 | 11.58 | 1.30 | 0.06 | 0.12 | 0.45 |
| 9 | Gurrumba | 11 | 10.67 | 1.18 | 0.04 | 0.08 | 0.40 |
| 10 | Mt Misery North | 14 | 13.59 | 1.39 | 0.06 | 0.16 | 0.54 |
| 11 | Mt Misery East | 10 | 9.68 | 1.30 | 0.07 | 0.12 | 0.39 |
| 12 | Mt Misery Central | 19 | 18.38 | 1.41 | 0.09 | 0.16 | 0.38 |
| 13 | Mt Misery South | 16 | 15.37 | 1.33 | 0.06 | 0.14 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Merwe, M.M.; Yap, J.-Y.S.; Wilson, P.D.; Murphy, H.T.; Ford, A. All Populations Matter: Conservation Genomics of Australia’s Iconic Purple Wattle, Acacia purpureopetala. Diversity 2021, 13, 139. https://doi.org/10.3390/d13040139
van der Merwe MM, Yap J-YS, Wilson PD, Murphy HT, Ford A. All Populations Matter: Conservation Genomics of Australia’s Iconic Purple Wattle, Acacia purpureopetala. Diversity. 2021; 13(4):139. https://doi.org/10.3390/d13040139
Chicago/Turabian Stylevan der Merwe, Marlien M., Jia-Yee S. Yap, Peter D. Wilson, Helen T. Murphy, and Andrew Ford. 2021. "All Populations Matter: Conservation Genomics of Australia’s Iconic Purple Wattle, Acacia purpureopetala" Diversity 13, no. 4: 139. https://doi.org/10.3390/d13040139
APA Stylevan der Merwe, M. M., Yap, J.-Y. S., Wilson, P. D., Murphy, H. T., & Ford, A. (2021). All Populations Matter: Conservation Genomics of Australia’s Iconic Purple Wattle, Acacia purpureopetala. Diversity, 13(4), 139. https://doi.org/10.3390/d13040139






