The Rabbitfish Siganus virgatus as Key Macroalgae Browser in Coral Reefs of the Gulf of Thailand
Abstract
1. Introduction
2. Materials and Methods
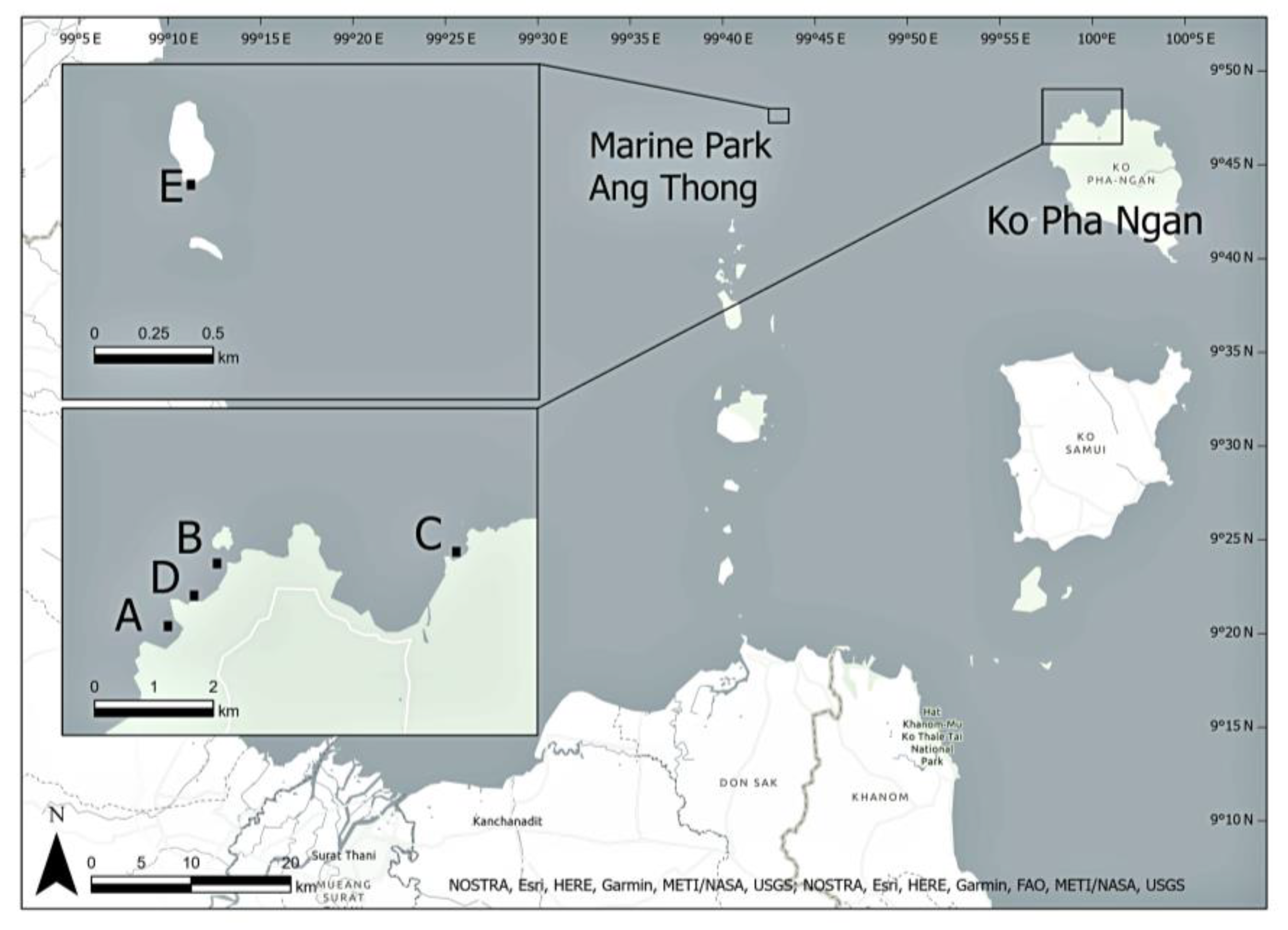
2.1. Study Site
2.2. Fish and Benthic Surveys
2.3. Algae Feeding Assays
2.4. Data Analyses
3. Results
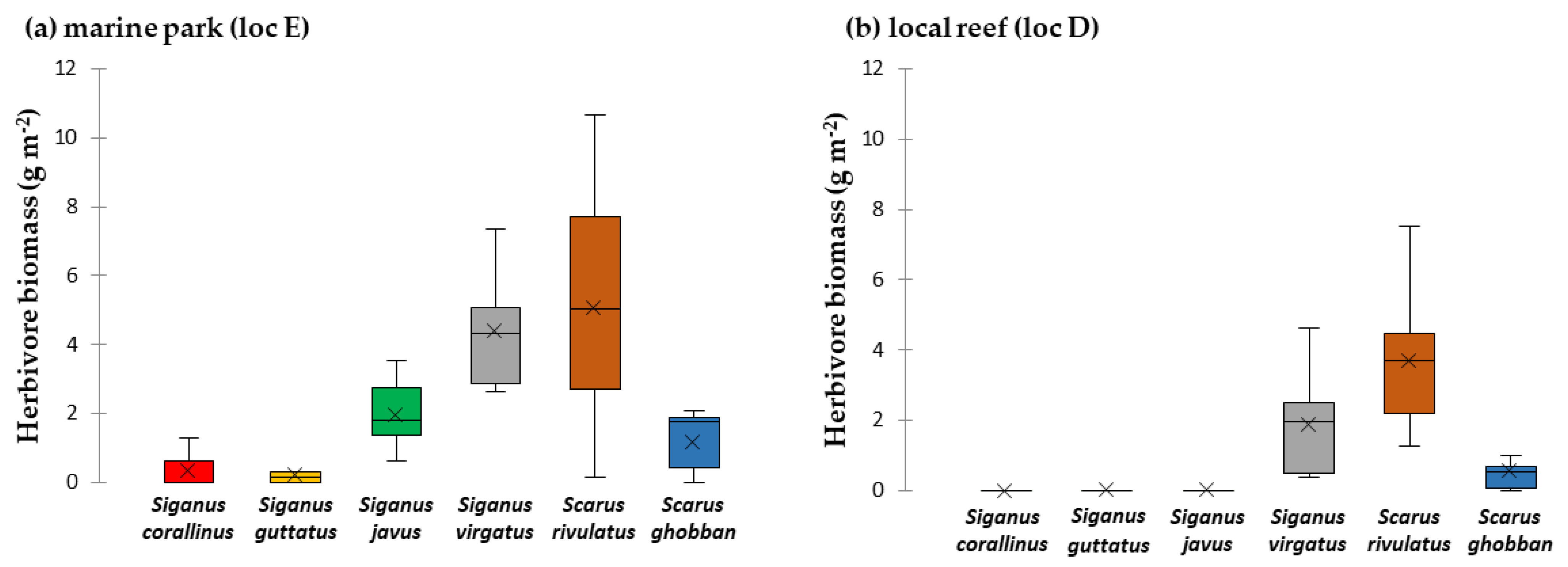
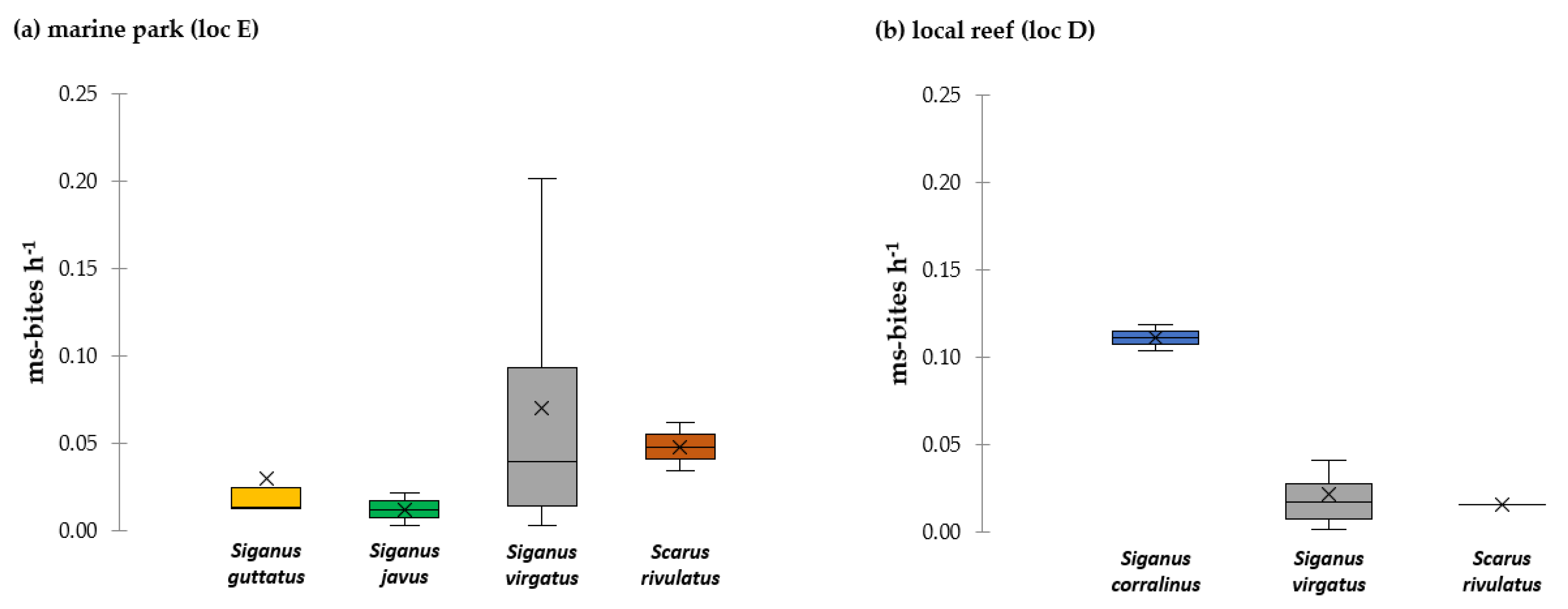
3.1. Key Browsers and Macroalgae Removal in the Fished vs. Protected Reef
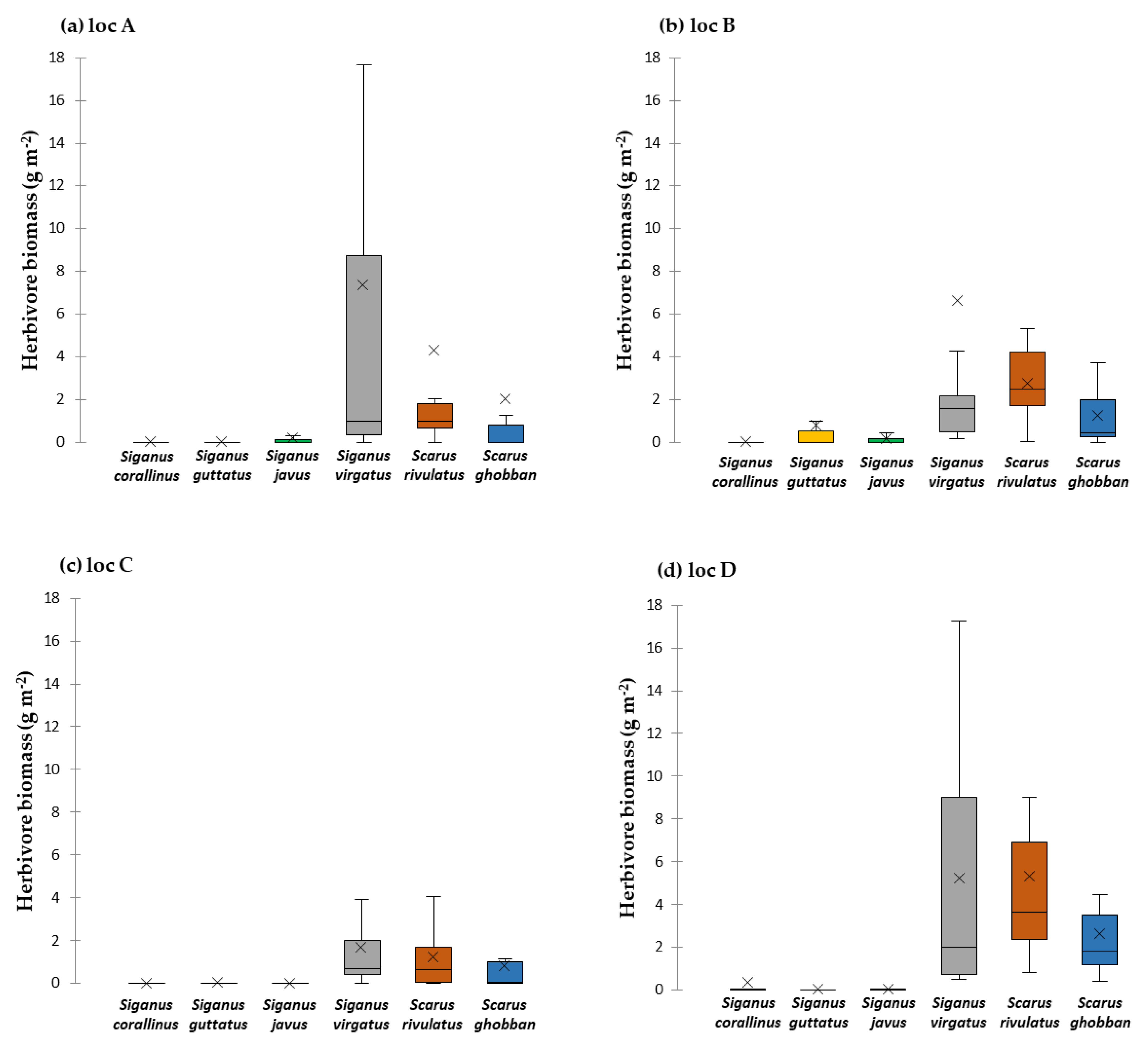
3.2. Key Browsers and Macroalgae Removal in Local Reefs
3.2.1. Single-Choice Assays
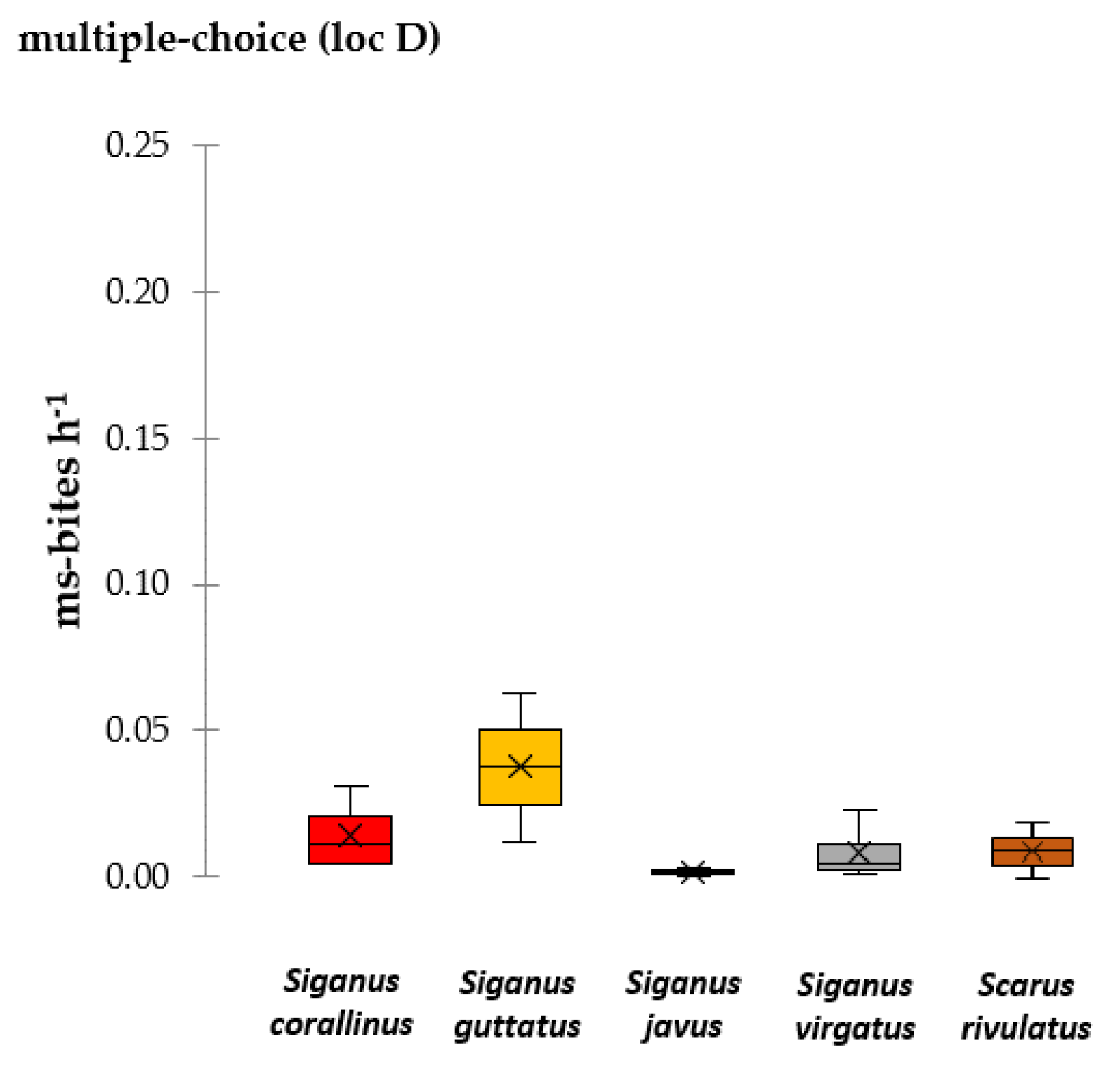
3.2.2. Multiple-Choice Assays
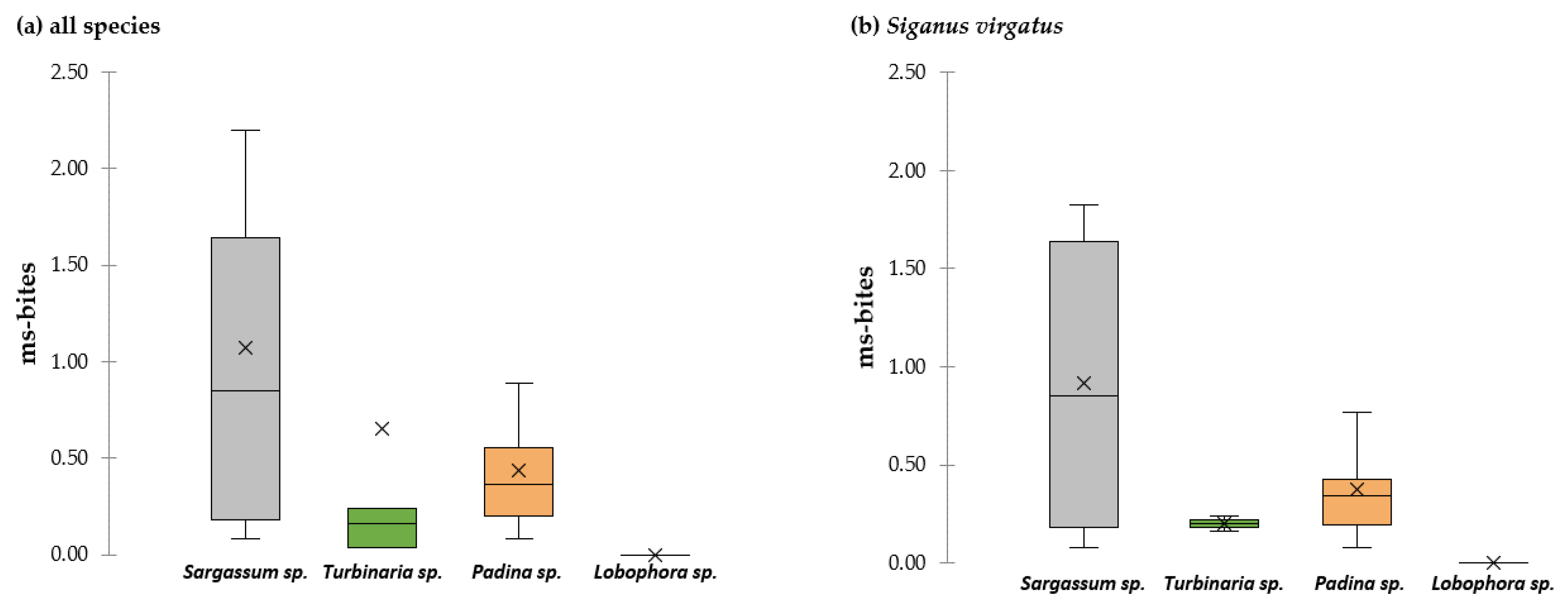
3.2.3. Feeding Selection and Avoidance
3.3. Biomass Removal
4. Discussion
4.1. Identification of Key Browsers and Macroalgae Removal
4.2. Selection and Avoidance of Macroalgae in Multiple-Choice Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nat. Cell Biol. 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef]
- Mumby, P.J. Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 2009, 28, 761–773. [Google Scholar] [CrossRef]
- Mumby, P.J.; Harborne, A.R. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS ONE 2010, 5, e8657. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Jennings, S.; MacNeil, M.A.; Mouillot, D.; Wilson, S.K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nat. Cell Biol. 2015, 518, 94–97. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Hoey, A.S.; Depczynski, M.; Wismer, S.; Bellwood, D.R. Macroalgae removal on coral reefs: Realised ecosystem functions transcend biogeographic locations. Coral Reefs 2019, 39, 203–214. [Google Scholar] [CrossRef]
- Monitoring functional groups of herbivorous reef fishes as indicators of coral reef resilience. In A Practical Guide for Coral Reef Managers in the Asia Pacific Region, 2009 ed.; Green, A.L., Bellwood, D.R., Eds.; International Union for Conservation of Nature: Gland, Switzerland, 2009; ISBN 283171169X. [Google Scholar]
- Done, T.J. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 1992, 247, 121–132. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef]
- Jouffray, J.-B.; Nyström, M.; Norström, A.V.; Williams, I.D.; Wedding, L.M.; Kittinger, J.N.; Williams, G.J. Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130268. [Google Scholar] [CrossRef]
- Donovan, M.K.; Friedlander, A.M.; Lecky, J.; Jouffray, J.-B.; Williams, G.J.; Wedding, L.M.; Crowder, L.B.; Erickson, A.L.; Graham, N.A.J.; Gove, J.M.; et al. Combining fish and benthic communities into multiple regimes reveals complex reef dynamics. Sci. Rep. 2018, 8, 16943. [Google Scholar] [CrossRef]
- Jouffray, J.-B.; Wedding, L.M.; Norström, A.V.; Donovan, M.K.; Williams, G.J.; Crowder, L.B.; Erickson, A.L.; Friedlander, A.M.; Graham, N.A.J.; Gove, J.M.; et al. Parsing human and biophysical drivers of coral reef regimes. Proc. R. Soc. B Boil. Sci. 2019, 286, 20182544. [Google Scholar] [CrossRef]
- Kuffner, I.B.; Walters, L.J.; Becerro, M.A.; Paul, V.J.; Ritson-Williams, R.; Beach, K.S. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar. Ecol. Prog. Ser. 2006, 323, 107–117. [Google Scholar] [CrossRef]
- Mumby, P.J.; Steneck, R.S.; Adjeroud, M.; Arnold, S.N. High resilience masks underlying sensitivity to algal phase shifts of Pacific coral reefs. Oikos 2016, 125, 644–655. [Google Scholar] [CrossRef]
- Box, S.; Mumby, P. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser. 2007, 342, 139–149. [Google Scholar] [CrossRef]
- Lirman, D. Competition between macroalgae and corals: Effects of herbivore exclusion and increased algal biomass on coral survivorship and growth. Coral Reefs 2001, 19, 392–399. [Google Scholar] [CrossRef]
- Jompa, J.; McCook, L.J. Effects of competition and herbivory on interactions between a hard coral and a brown alga. J. Exp. Mar. Biol. Ecol. 2002, 271, 25–39. [Google Scholar] [CrossRef]
- Vieira, C.; Payri, C.; De Clerck, O. Overgrowth and killing of corals by the brown alga L obophora hederacea (Dictyotales, Phaeophyceae) on healthy reefs in New Caledonia: A new case of the epizoism syndrome. Psychol. Res. 2015, 63, 152–153. [Google Scholar]
- Birrell, C.L.; McCook, L.J.; Willis, B.L. Effects of algal turfs and sediment on coral settlement. Mar. Pollut. Bull. 2005, 51, 408–414. [Google Scholar] [CrossRef]
- Arnold, S.N.; Steneck, R.S.; Mumby, P.J. Running the gauntlet: Inhibitory effects of algal turfs on the processes of coral recruitment. Mar. Ecol. Prog. Ser. 2010, 414, 91–105. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Hoey, A.S. Sleeping functional group drives coral-reef recovery. Curr. Biol. 2006, 16, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Simpson, T. Large-scale bleaching of corals on the Great Barrier Reef. Ecology 2018, 99, 501. [Google Scholar] [CrossRef]
- Mantyka, C.S.; Bellwood, D.R. Macroalgal grazing selectivity among herbivorous coral reef fishes. Mar. Ecol. Prog. Ser. 2007, 352, 177–185. [Google Scholar] [CrossRef]
- Bennett, S.; Bellwood, D. Latitudinal variation in macroalgal consumption by fishes on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2011, 426, 241–252. [Google Scholar] [CrossRef][Green Version]
- Vergés, A.; Bennett, S.; Bellwood, D.R. Diversity among macroalgae-consuming fishes on coral reefs: A transcontinental comparison. PLoS ONE 2012, 7, e45543. [Google Scholar] [CrossRef]
- Puk, L.D.; Ferse, S.C.A.; Wild, C. Patterns and trends in coral reef macroalgae browsing: A review of browsing herbivorous fishes of the Indo-Pacific. Rev. Fish Biol. Fish. 2015, 26, 53–70. [Google Scholar] [CrossRef]
- Lefèvre, C.D.; Bellwood, D.R. Temporal variation in coral reef ecosystem processes: Herbivory of macroalgae by fishes. Mar. Ecol. Prog. Ser. 2011, 422, 239–251. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Streit, R.P.; Brandl, S.J.; Tebbett, S.B. The meaning of the term ‘function’in ecology: A coral reef perspective. Funct. Ecol. 2019, 33, 948–961. [Google Scholar]
- Rasher, D.B.; Hoey, A.S.; Hay, M.E. Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 2013, 94, 1347–1358. [Google Scholar] [CrossRef]
- Bonaldo, R.M.; Pires, M.M.; Guimarães, P.R.; Hoey, A.S.; Hay, M.E. Small marine protected areas in Fiji provide refuge for reef fish assemblages, feeding groups, and corals. PLoS ONE 2017, 12, e0170638. [Google Scholar] [CrossRef]
- Loffler, Z.; Hoey, A.S. Canopy-forming macroalgal beds (Sargassum) on coral reefs are resilient to physical disturbance. J. Ecol. 2018, 106, 1156–1164. [Google Scholar] [CrossRef]
- Cvitanovic, C.; Bellwood, D.R. Local variation in herbivore feeding activity on an inshore reef of the Great Barrier Reef. Coral Reefs 2008, 28, 127–133. [Google Scholar] [CrossRef]
- Plass-Johnson, J.G.; Ferse, S.C.A.; Jompa, J.; Wild, C.; Teichberg, M. Fish herbivory as key ecological function in a heavily degraded coral reef system. Limnol. Oceanogr. 2015, 60, 1382–1391. [Google Scholar] [CrossRef]
- Paul, V.; Hay, M. Seaweed susceptibility to herbivory: Chemical and morphological correlates. Mar. Ecol. Prog. Ser. 1986, 33, 255–264. [Google Scholar] [CrossRef]
- Choat, J.H.; Robbins, W.D.; Clements, K.D. The trophic status of herbivorous fishes on coral reefs. Mar. Biol. 2004, 145, 445–454. [Google Scholar] [CrossRef]
- Clements, K.D.; Raubenheimer, D.; Choat, J.H. Nutritional ecology of marine herbivorous fishes: Ten years on. Funct. Ecol. 2009, 23, 79–92. [Google Scholar] [CrossRef]
- Fox, R.J.; Bellwood, D.R. Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 2008, 27, 605–615. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Limited functional redundancy in a high diversity system: Single species dominates key ecological process on coral reefs. Ecosystems 2009, 12, 1316–1328. [Google Scholar] [CrossRef]
- Ford, A.K.; Bejarano, S.; Marshell, A.; Mumby, P.J. Linking the biology and ecology of key herbivorous unicornfish to fisheries management in the Pacific. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 790–805. [Google Scholar] [CrossRef]
- Hoey, A.S.; Brandl, S.J.; Bellwood, D.R. Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: Implications for ecosystem function. Coral Reefs 2013, 32, 973–984. [Google Scholar] [CrossRef]
- Loffler, Z.; Bellwood, D.R.; Hoey, A.S. Among-habitat algal selectivity by browsing herbivores on an inshore coral reef. Coral Reefs 2015, 34, 597–605. [Google Scholar] [CrossRef]
- Streit, R.P.; Hoey, A.S.; Bellwood, D.R. Feeding characteristics reveal functional distinctions among browsing herbivorous fishes on coral reefs. Coral Reefs 2015, 34, 1037–1047. [Google Scholar] [CrossRef]
- Kelly, E.L.A.; Eynaud, Y.; Clements, S.M.; Gleason, M.; Sparks, R.T.; Williams, I.D.; Smith, J.E. Investigating functional redundancy versus complementarity in Hawaiian herbivorous coral reef fishes. Oecologia 2016, 182, 1151–1163. [Google Scholar] [CrossRef]
- Brandl, S.J.; Bellwood, D.R. Individual-based analyses reveal limited functional overlap in a coral reef fish community. J. Anim. Ecol. 2014, 83, 661–670. [Google Scholar] [CrossRef]
- Cheevaporn, V.; Menasveta, P. Water pollution and habitat degradation in the Gulf of Thailand. Mar. Pollut. Bull. 2003, 47, 43–51. [Google Scholar] [CrossRef]
- Adger, W.N.; Hughes, T.P.; Folke, C.; Carpenter, S.R.; Rockström, J. Social-ecological resilience to coastal disasters. Science 2005, 309, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Stuhldreier, I.; Bastian, P.; Schönig, E.; Wild, C.; Schoenig, E. Effects of simulated eutrophication and overfishing on algae and invertebrate settlement in a coral reef of Koh Phangan, Gulf of Thailand. Mar. Pollut. Bull. 2015, 92, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Buranapratheprat, A.; Bunpapong, M. A two dimensional hydrodynamic model for the Gulf of Thailand. In Proceedings of the IOC/WESTPAC Fourth International Scientific Symposium, Okinawa, Japan, 2 February 1998; Volume 469, p. 478. [Google Scholar]
- Kulbicki, M.; Guillemot, N.; Amand, M. A general approach to length-weight relationships for New Caledonian lagoon fishes. Cybium 2005, 29, 235–252. [Google Scholar]
- Hill, J.; Wilkinson, C.R. Methods for ecological monitoring of coral reefs. In A Resource for Managers, Version 1; Australian Institute of Marine Science: Townsville, Australia, 2004; ISBN 9780642322371. [Google Scholar]
- Low, J.K.Y.; Fong, J.; Todd, P.A.; Chou, L.M.; Bauman, A.G. Seasonal variation of Sargassum ilicifolium (phaeophyceae) growth on equatorial coral reefs. J. Phycol. 2018, 55, 289–296. [Google Scholar] [CrossRef]
- Vieira, C.; D’Hondt, S.; De Clerck, O.; Payri, C.E. Toward an inordinate fondness for stars, beetles and Lobophora? Species diversity of the genus Lobophora (Dictyotales, Phaeophyceae) in New Caledonia. J. Phycol. 2014, 50, 1101–1119. [Google Scholar] [CrossRef] [PubMed]
- Mantyka, C.S.; Bellwood, D.R. Direct evaluation of macroalgal removal by herbivorous coral reef fishes. Coral Reefs 2007, 26, 435–442. [Google Scholar] [CrossRef]
- Davis, A.; Manly, B.; McDonald, L.; Thomas, D. Resource selection by animals: Statistical design and analysis for field studies. J. Anim. Ecol. 1994, 63, 745. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 26 July 2020).
- Strauss, R.E. Reliability estimates for Ivlev’s electivity index, the forage ratio, and a proposed linear index of food selection. Trans. Am. Fisheries Soc. 1979, 108, 344–352. [Google Scholar] [CrossRef]
- Lechowicz, M.J. The sampling characteristics of electivity indices. Oecologia 1982, 52, 22–30. [Google Scholar] [CrossRef]
- Roa, R. Design and analysis of multiple-choice feeding-preference experiments. Oecologia 1992, 89, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, J.R., III. On the statistical analysis of multiple-choice feeding preference experiments. Oecologia 1998, 116, 475–481. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 0470387378. [Google Scholar]
- Roff, G.; Mumby, P.J. Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 2012, 27, 404–413. [Google Scholar] [CrossRef]
- Edwards, C.B.; Friedlander, A.M.; Green, A.G.; Hardt, M.J.; Sala, E.; Sweatman, H.P.; Williams, I.D.; Zgliczynski, B.; Sandin, S.A.; Smith, J.E. Global assessment of the status of coral reef herbivorous fishes: Evidence for fishing effects. Proc. R. Soc. B Boil. Sci. 2014, 281, 20131835. [Google Scholar] [CrossRef]
- Chong-Seng, K.M.; Nash, K.L.; Bellwood, D.R.; Graham, N.A.J. Macroalgal herbivory on recovering versus degrading coral reefs. Coral Reefs 2014, 33, 409–419. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Suppression of herbivory by macroalgal density: A critical feedback on coral reefs? Ecol. Lett. 2011, 14, 267–273. [Google Scholar] [CrossRef]
- Madin, E.M.P.; Gaines, S.D.; Warner, R.R. Field evidence for pervasive indirect effects of fishing on prey foraging behavior. Ecology 2010, 91, 3563–3571. [Google Scholar] [CrossRef]
- Fox, R.J.; Bellwood, D.R. Niche partitioning of feeding microhabitats produces a unique function for herbivorous rabbitfishes (Perciformes, Siganidae) on coral reefs. Coral Reefs 2012, 32, 13–23. [Google Scholar] [CrossRef]
- Brandl, S.J.; Bellwood, D.R. Microtopographic refuges shape consumer-producer dynamics by mediating consumer functional diversity. Oecologia 2016, 182, 203–217. [Google Scholar] [CrossRef]
- Fox, R.J.; Bellwood, D.R. Quantifying herbivory across a coral reef depth gradient. Mar. Ecol. Prog. Ser. 2007, 339, 49–59. [Google Scholar] [CrossRef]
- Russ, G. Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. I. Levels of variability across the entire continental shelf. Mar. Ecol. Prog. Ser. 1984, 20, 23–34. [Google Scholar] [CrossRef]
- Wismer, S.; Hoey, A.; Bellwood, D.R. Cross-shelf benthic community structure on the Great Barrier Reef: Relationships between macroalgal cover and herbivore biomass. Mar. Ecol. Prog. Ser. 2009, 376, 45–54. [Google Scholar] [CrossRef]
- Choat, J.H.; Bellwood, D.R. Interactions amongst herbivorous fishes on a coral reef: Influence of spatial variation. Mar. Biol. 1985, 89, 221–234. [Google Scholar] [CrossRef]
- Klumpp, D.; McKinnon, A. Temporal and spatial patterns in primary production of a coral-reef epilithic algal community. J. Exp. Mar. Biol. Ecol. 1989, 131, 1–22. [Google Scholar] [CrossRef]
- Russ, G.R. Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 2003, 22, 63–67. [Google Scholar] [CrossRef]
- Mumby, P.J.; Harborne, A.R.; Williams, J.; Kappel, C.V.; Brumbaugh, D.R.; Micheli, F.; Holmes, K.E.; Dahlgren, C.P.; Paris, C.B.; Blackwell, P.G. Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl. Acad. Sci. USA 2007, 104, 8362–8367. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.G.; Seah, J.C.L.; Januchowski-Hartley, F.A.; Hoey, A.S.; Fong, J.; Todd, P.A. Fear effects associated with predator presence and habitat structure interact to alter herbivory on coral reefs. Biol. Lett. 2019, 15, 20190409. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.R.; Sura, S.A.; Ford, A.T.; Howard, H.B.; Molina, N.E.; Smith, N.N.; Fong, P. Testing the conceptual and operational underpinnings of field herbivory assays: Does variation in predictability of resources, assay design, and deployment method affect outcomes? J. Exp. Mar. Biol. Ecol. 2020, 533, 151469. [Google Scholar] [CrossRef]
- Steinberg, P.; Paul, V. Fish feeding and chemical defenses of tropical brown algae in Western Australia. Mar. Ecol. Prog. Ser. 1989, 58, 253–259. [Google Scholar] [CrossRef]
- Stiger, V.; Deslandes, E.; Payri, C.E. Phenolic contents of two brown algae, Turbinaria ornata and Sargassum mangarevense on Tahiti (French Polynesia): Interspecific, ontogenic and spatio-temporal variations. Bot. Mar. 2004, 47. [Google Scholar] [CrossRef]
- Bittick, S.J.; Bilotti, N.D.; Peterson, H.A.; Stewart, H.L. Turbinaria ornata as an herbivory refuge for associate algae. Mar. Biol. 2009, 157, 317–323. [Google Scholar] [CrossRef]
- Prathep, A.; Wichachucherd, B.; Thongroy, P. Spatial and temporal variation in density and thallus morphology of Turbinaria ornata in Thailand. Aquat. Bot. 2007, 86, 132–138. [Google Scholar] [CrossRef]
- Seah, J.C.L.; Bauman, A.G.; Todd, P.A. Temporal variation in macroalgal removal: Insights from an impacted equatorial coral reef system. Mar. Biol. 2021, 168, 1–12. [Google Scholar] [CrossRef]
- Clements, K.D.; German, D.P.; Piché, J.; Tribollet, A.; Choat, J.H. Integrating ecological roles and trophic diversification on coral reefs: Multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linn. Soc. 2016. [Google Scholar] [CrossRef]
- Perpetua, M.D.; Gorospe, J.G.; Torres, M.A.J.; Demayo, C.G. Diet composition based on stomach content of the Streaked spinefoot (Siganus javus) from three coastal bays in Mindanao, Philippines. Adv. Environ. Sci. 2013, 5, 49–61. [Google Scholar]
- Miyake, S.; Ngugi, D.K.; Stingl, U. Diet strongly influences the gut microbiota of surgeonfishes. Mol. Ecol. 2015, 24, 656–672. [Google Scholar] [CrossRef]
- Ebrahim, A.; Martin, T.S.H.; Mumby, P.J.; Olds, A.D.; Tibbetts, I.R. Differences in diet and foraging behaviour of commercially important rabbitfish species on coral reefs in the Indian Ocean. Coral Reefs 2020, 39, 977–988. [Google Scholar] [CrossRef]
- Nanami, A. Spatial distributions, feeding ecologies, and behavioral interactions of four rabbitfish species (Siganus unimaculatus, S. virgatus, S. corallinus, and S. puellus). PeerJ 2018, 6, e6145. [Google Scholar] [CrossRef]
- Bauman, A.G.; Hoey, A.S.; Dunshea, G.; Feary, D.A.; Low, J.; Todd, P.A. Macroalgal browsing on a heavily degraded, urbanized equatorial reef system. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuriiwa, K.; Hanzawa, N.; Yoshino, T.; Kimura, S.; Nishida, M. Phylogenetic relationships and natural hybridization in rabbitfishes (Teleostei: Siganidae) inferred from mitochondrial and nuclear DNA analyses. Mol. Phylogenet. Evol. 2007, 45, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Sunderland, T.L.; Hoey, A.; Bellwood, D.R. Estimating ecosystem function: Contrasting roles of closely related herbivorous rabbitfishes (Siganidae) on coral reefs. Mar. Ecol. Prog. Ser. 2009, 385, 261–269. [Google Scholar] [CrossRef]
- Cheal, A.; Emslie, M.; Miller, I.; Sweatman, H. The distribution of herbivorous fishes on the Great Barrier Reef. Mar. Biol. 2012, 159, 1143–1154. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Choat, J.H. A functional analysis of grazing in parrotfishes (family Scaridae): The ecological implications. Environ. Boil. Fishes 1990, 28, 189–214. [Google Scholar] [CrossRef]
- Brandl, S.J.; Rasher, D.B.; Côté, I.M.; Casey, J.M.; Darling, E.S.; Lefcheck, J.S.; Duffy, J.E. Coral reef ecosystem functioning: Eight core processes and the role of biodiversity. Front. Ecol. Environ. 2019, 17, 445–454. [Google Scholar] [CrossRef]
- Fong, C.R.; Chancellor, K.S.; Renzi, J.J.; Robinson, D.R.; Barber, P.H.; Habtes, S.Y.; Fong, P. Epibionts on Turbinaria ornata, a secondary foundational macroalga on coral reefs, provide diverse trophic support to fishes. Mar. Environ. Res. 2018, 141, 39–43. [Google Scholar] [CrossRef]
- Clements, K.D.; Choat, J.H. Comparison of herbivory in the closely-related marine fish genera Girella and Kyphosus. Mar. Biol. 1997, 127, 579–586. [Google Scholar] [CrossRef]
- Puk, L.; Cernohorsky, N.; Marshell, A.; Dwyer, J.; Wolfe, K.; Mumby, P. Species-specific effects of herbivorous fishes on the establishment of the macroalga Lobophora on coral reefs. Mar. Ecol. Prog. Ser. 2020, 637, 1–14. [Google Scholar] [CrossRef]
- Fulton, C.J.; Abesamis, R.A.; Berkström, C.; Depczynski, M.; Graham, N.A.; Holmes, T.H.; Tinkler, P. Form and function of tropical macroalgal reefs in the Anthropocene. Funct. Ecol. 2019, 33, 989–999. [Google Scholar] [CrossRef]
| 2015 Reef Crest | Single-Choice | |||
|---|---|---|---|---|
| Loc D | Loc E | |||
| Benthos | % Cover | % SE | % Cover | % SE |
| LCC | 42.75 | 3.21 | 48.96 | 3.85 |
| Turf Algae | 32.44 | 1.95 | 7.76 | 2.09 |
| Sand | 1.34 | 0.53 | 20.66 | 5.08 |
| Rubble | 10.65 | 3.18 | 6.34 | 1.42 |
| Other Life | 0.67 | 0.3 | 16.28 | 2.76 |
| Macroalgae | 12.15 | 3.51 | - | - |
| of which | ||||
| Turbinaria | - | - | - | - |
| Lobophora | 12.15 | 3.51 | - | - |
| Padina | - | - | - | - |
| Sargassum | - | - | - | - |
| 2018 Reef Flat | (a) Single-Choice | (b) Multiple-Choice | ||||||
|---|---|---|---|---|---|---|---|---|
| Loc A | Loc B | Loc C | Loc D | |||||
| Benthos | % Cover | % SE | % Cover | % SE | % Cover | % SE | % Cover | % SE |
| LCC | 51.8 | 13.75 | 7.57 | 3.27 | 22.11 | 7.83 | 4.95 | 1.98 |
| Turf Algae | 33.44 | 6.91 | 37.5 | 4.97 | 56.77 | 3.81 | 27.06 | 1.32 |
| Sand | 0.98 | 0.57 | 44.41 | 3.01 | 5.61 | 1.19 | 54.13 | 3.68 |
| Other Life | 2.62 | 1.31 | 0.66 | 0.66 | 0.99 | 0.57 | - | - |
| Macroalgae | 11.15 | 3.88 | 9.87 | 3.89 | 14.52 | 2.35 | 13.86 | 2.51 |
| of which | ||||||||
| Turbinaria | 3.61 | 0.87 | 4.28 | 1.32 | 4.95 | 1.14 | 5.61 | 0.66 |
| Lobophora | 6.23 | 2.15 | 4.61 | 2 | 8.25 | 0.33 | 6.6 | 1.19 |
| Padina | 1.31 | 0.87 | 0.99 | 0.57 | 1.32 | 0.87 | - | - |
| Sargassum | - | - | - | - | - | - | 1.65 | 0.66 |
| Macroalga | Ri | Pi | L |
|---|---|---|---|
| Sargassum sp. | 0.548 | 0.260 | 0.003 |
| Padina sp. | 0.413 | 0.105 | 0.003 |
| Turbinaria sp. | 0.039 | 0.622 | −0.006 |
| Lobophora sp. | 0.000 | 0.014 | 0.000 |
| 2015 Reef Crest | Biomass Loss | ||||
|---|---|---|---|---|---|
| Macroalga | Abs. g h−1 | ±SE | % h−1 | ±SE | |
| (a) loc D | Turbinaria sp. | 0.066 | 0.010 | 0.060 | 0.001 |
| (b) loc E * | Turbinaria sp. | 0.146 | 0.053 | 0.201 | 0.009 |
| 2018 Reef Flat | Biomass Loss | ||||
|---|---|---|---|---|---|
| Macroalga | Abs. g h−1 | ±SE | % h−1 | ±SE | |
| (a) single-choice loc C | Turbinaria sp. | 0.003 | 0.000 | −0.018 | 0.000 |
| (b) multiple-choice loc D * | Turbinaria sp. | 0.013 | 0.002 | 0.026 | 0.007 |
| Sargassum sp. | 0.009 | 0.001 | 0.147 | 0.035 | |
| Padina sp. | 0.020 | 0.003 | 0.209 | 0.031 | |
| Lobophora sp. | 0.000 | 0.000 | 0.000 | 0.000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.; Staab, C.F.K.; Puk, L.D.; Schoenig, E.M.; Ferse, S.C.A.; Wild, C. The Rabbitfish Siganus virgatus as Key Macroalgae Browser in Coral Reefs of the Gulf of Thailand. Diversity 2021, 13, 123. https://doi.org/10.3390/d13030123
Müller M, Staab CFK, Puk LD, Schoenig EM, Ferse SCA, Wild C. The Rabbitfish Siganus virgatus as Key Macroalgae Browser in Coral Reefs of the Gulf of Thailand. Diversity. 2021; 13(3):123. https://doi.org/10.3390/d13030123
Chicago/Turabian StyleMüller, Malika, Constanze F. K. Staab, Laura D. Puk, Eike M. Schoenig, Sebastian C. A. Ferse, and Christian Wild. 2021. "The Rabbitfish Siganus virgatus as Key Macroalgae Browser in Coral Reefs of the Gulf of Thailand" Diversity 13, no. 3: 123. https://doi.org/10.3390/d13030123
APA StyleMüller, M., Staab, C. F. K., Puk, L. D., Schoenig, E. M., Ferse, S. C. A., & Wild, C. (2021). The Rabbitfish Siganus virgatus as Key Macroalgae Browser in Coral Reefs of the Gulf of Thailand. Diversity, 13(3), 123. https://doi.org/10.3390/d13030123







