Landscape and Species Traits Co-Drive Roadkills of Bats in a Subtropical Island
Abstract
:1. Introduction
2. Materials and Methods
3. Results
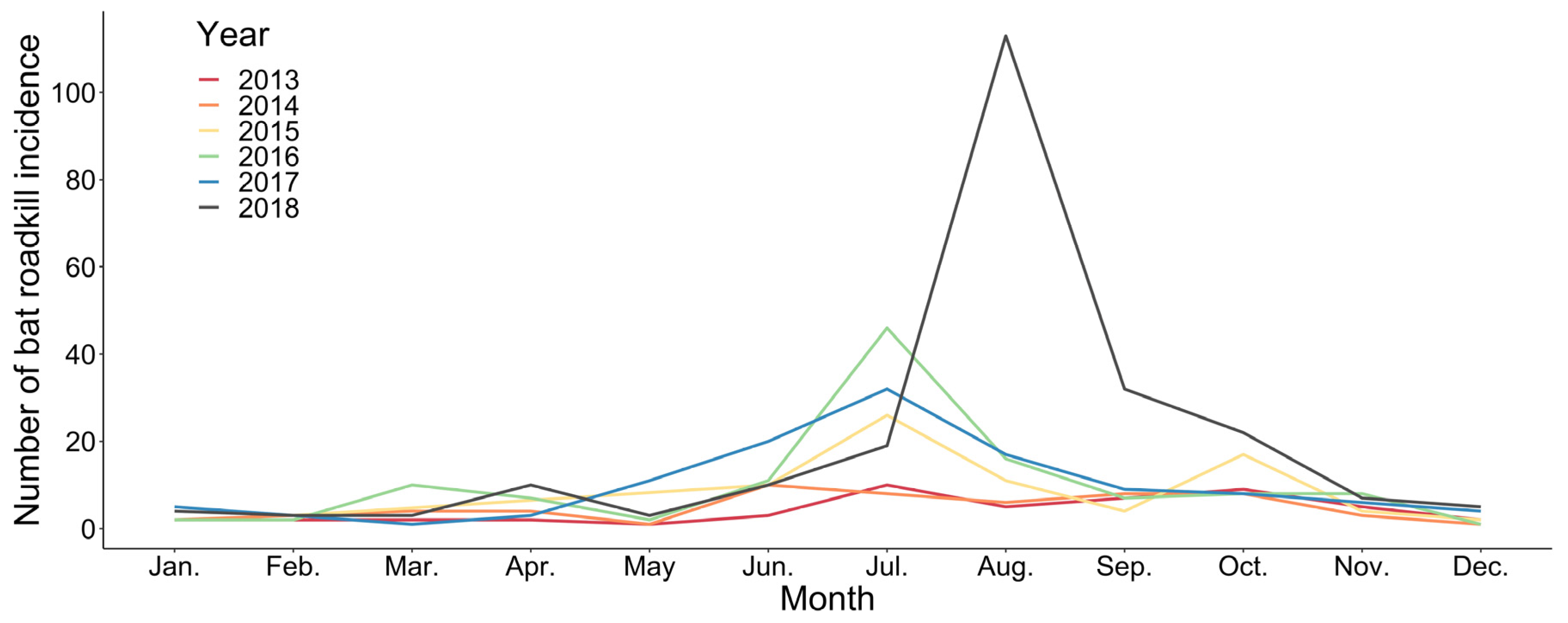
3.1. General Patterns of Bat Roadkill Incidence
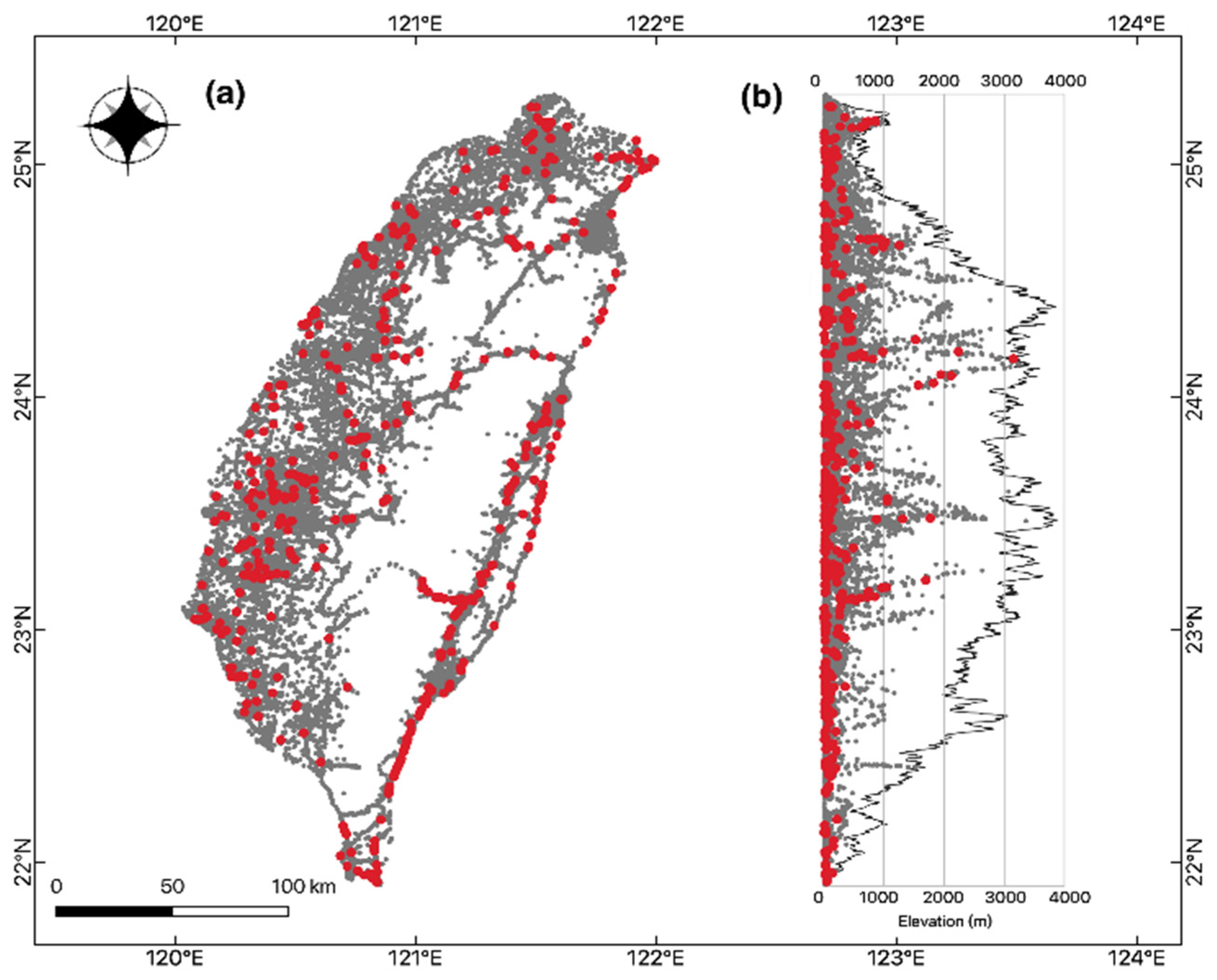
3.2. Elevational Patterns of Occurrences of Bat Roadkill at Species Level
3.3. Elevational Patterns of Bat Roadkill at Assemblage Level
3.4. Associations between the Occurrence of Bat Roadkills and Landscape Features
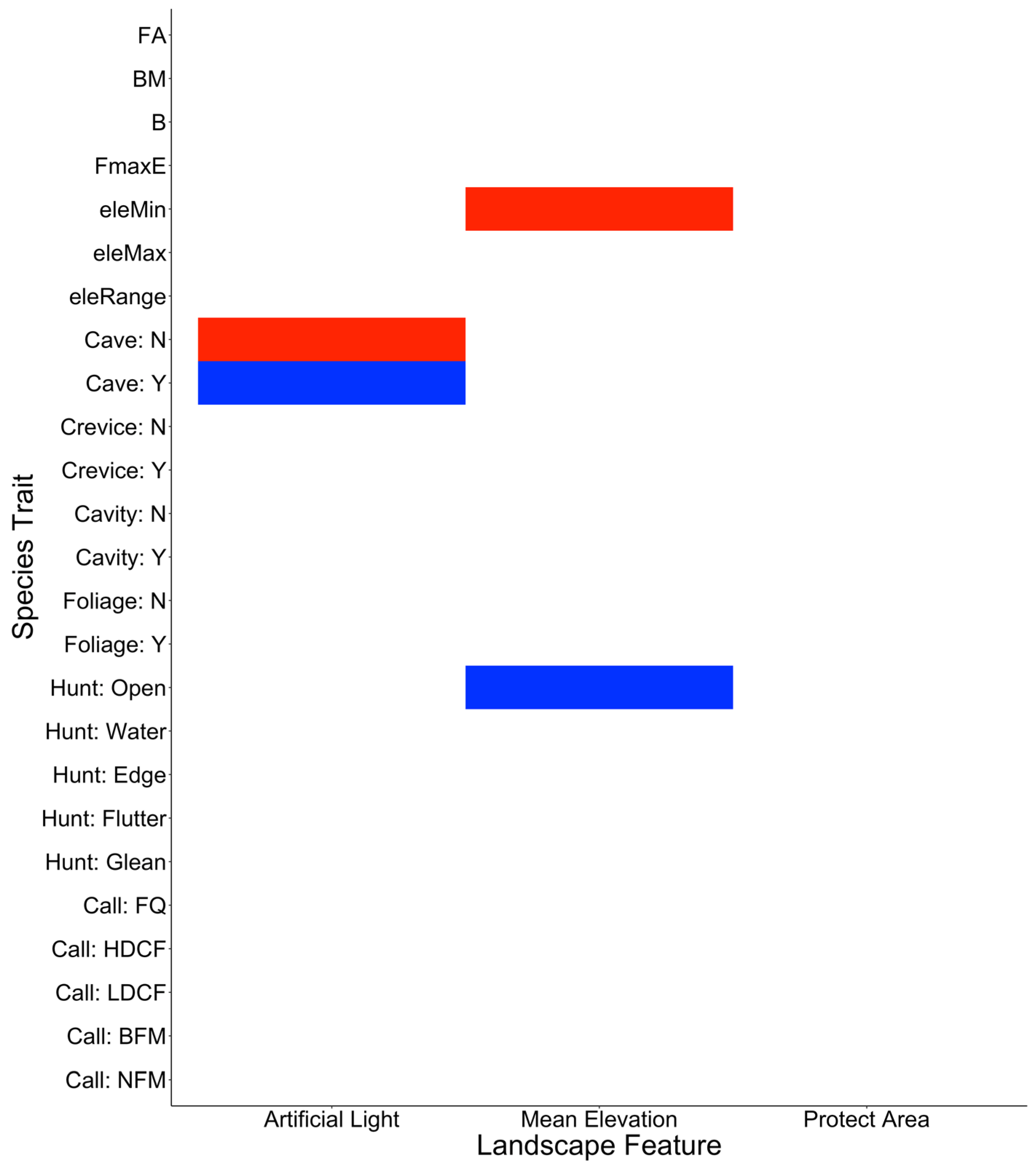
3.5. Trait-Based Responses to Landscape Features in Terms of the Occurrence of Bat Roadkill
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Data Type | Unit | Description | Data Source |
|---|---|---|---|---|
| TOPOGRAPHY | ||||
| Mean of Elevation, ELE | Continuous | meter | Algorithm average of the original elevation measures at a resolution of 30 by 30 m2 | [41] |
| Slope | Continuous | degree | The mean inclination of the terrain surface | [41] |
| Nearest distance to fresh water, DFW | Continuous | meter | Distance to the nearest freshwater body | [41] |
| LAND COVER | [41] | |||
| Farm field, FF | Continuous | m2 | Area of rainfed cropland | [41] |
| Meadow, MD | Continuous | m2 | Area of herbaceous cover | [41] |
| Forest, FO | Continuous | m2 | Area of forested land | [41] |
| Farm wetland, FW | Continuous | m2 | Area of irrigated and post-flooding farmlands | [41] |
| Bushland, BU | Continuous | m2 | Area of shrubland | [41] |
| Wetland, WL | Continuous | m2 | Area of flooded land with fresh, saline, and brackish water | [41] |
| Urban Area, UB | Continuous | m2 | Area of artificial surfaces | [41] |
| Waterbody, WB | Continuous | m2 | Area of freshwater and saltwater bodies | [41] |
| Bare Land, BL | Continuous | m2 | Area of land without any landcover | [41] |
| Building, BD | Continuous | m2 | Area of buildings regardless usage | [42] |
| Habitat heterogeneity, HHabitat | Index | value | Heterogeneity of land cover composition estimated as Shannon’s Diversity Index. BD is not included in the estimation due its alternative source from other land cover variables. | This study |
| ANTHROPOGENIC | ||||
| Artificial Light intensity, ALight | total lighting electricity usage (TLEU) | Estimation of night light pollution, downloaded the Earth of Night image in 2016 from NASA Earth Observatory [87,88] and converted the color model of the image from either the red, green, and blue (RGB) to hue, saturation, and value (HSV) by ArcGIS 10.6 to get the percentage of night light | This study | |
| Road Length, LROAD | Continuous | meter | The total length of all provincial highway, county highway, country road, industrial road, and old logging road systems that currently may not use for public transportation. | [41] |
| Population, Pop | Count | people | Number of residences registered in Department of Household Registration, Ministry of the Interior [89]. The original data are people per neighborhood and rescaled to the spatial scale of 1km by 1km weighted by the proportion of the area of each neighborhood within a grid. | This study |
| Protected Area, PA | Category | - | Whether a grid is overlapped with any existing protected area in Taiwan. The boundary data of protected areas are based on the definitions by the Council of Agriculture, Executive Yuan, of Taiwan [90]. | This study |
| Distance to the nearest protected area, NEAR_DIST | Continuous | meter | The shortest distance from a grid to the boundary of the nearest protected area. | This study |
| Fragmentation, seff | Index | value | Effective mesh density (seff)—a measure of to which movement within a landscape is interrupted by transportation infrastructure and geographic barriers. See more details in [91] | This study |
References
- Coffin, A.W. From roadkill to road ecology: A review of the ecological effects of roads. J. Transp. Geogr. 2007, 15, 396–406. [Google Scholar] [CrossRef]
- Goosem, M. Fragmentation impacts caused by roads through rainforests. Curr. Sci. 2007, 93, 1587–1595. [Google Scholar]
- Gibbs, J.P.; Shriver, W.G. Estimating the effects of road mortality on turtle populations. Conserv. Biol. 2002, 16, 1647–1652. [Google Scholar] [CrossRef]
- Hobday, A.J.; Minstrell, M.L. Distribution and abundance of roadkill on Tasmanian highways: Human management options. Wildl. Res. 2008, 35, 712–726. [Google Scholar] [CrossRef]
- Jackson, N.D.; Fahrig, L. Relative effects of road mortality and decreased connectivity on population genetic diversity. Biol. Conserv. 2011, 144, 3143–3148. [Google Scholar] [CrossRef]
- Silva, I.; Crane, M.; Savini, T. High roadkill rates in the Dong Phayayen-Khao Yai World Heritage Site: Conservation implications of a rising threat to wildlife. Anim. Conserv. 2020, 23, 466–478. [Google Scholar] [CrossRef]
- Jaeger, J.A.G.; Bowman, J.; Brennan, J.; Fahrig, L.; Bert, D.; Bouchard, J.; Charbonneau, N.; Frank, K.; Gruber, B.; Von Toschanowitz, K.T. Predicting when animal populations are at risk from roads: An interactive model of road avoidance behavior. Ecol. Model. 2005, 185, 329–348. [Google Scholar] [CrossRef]
- Dulac, J.; Cuenot, F. Global Travel Growth, Estimated Future Needs for Road Infrastructure and Impacts on Energy Demands and Carbon Emissions: An Analysis; World Road Association (PIARC): Paris, France, 2013; pp. 26–33. [Google Scholar]
- Bats of the World: A Taxonomic and Geographic Database. Available online: https://batnames.org/ (accessed on 23 December 2020).
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Wilig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef] [Green Version]
- Altringham, J.; Kerth, G. Bats and roads. In Bats of the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Berlin, Germany, 2015; pp. 35–62. [Google Scholar]
- Collinson, W.J. A Standardised Protocol for Roadkill Detection and the Determinants of Roadkill in the Greater Mapungubwe Transfrontier Conservation Area, Limpopo Province, South Africa. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2013. [Google Scholar]
- Fensome, A.G.; Mathews, F. Roads and bats: A meta-analysis and review of the evidence on vehicle collisions and barrier effects. Mamm. Rev. 2016, 46, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Medinas, D.; Marques, J.T.; Mira, A. Assessing road effects on bats: The role of landscape, road features, and bat activity on road-kills. Ecol. Res. 2013, 28, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Novaes, R.L.M.; Laurindo, R.S.; Dornas, R.A.P.; Esbérard, C.E.L.; Bueno, C. On a collision course: The vulnerability of bats to roadkills in Brazil. Mastozool. Neotrop. 2018, 25, 115–128. [Google Scholar] [CrossRef]
- Jones, C.; Borkin, K.; Smith, D. Roads and wildlife: The need for evidence-based decisions; New Zealand bats as a case study. N. Z. J. Ecol. 2019, 43, 1–18. [Google Scholar] [CrossRef]
- Berthinussen, A.; Altringham, J. Do bat gantries and underpasses help bats cross roads safely? PLoS ONE 2012, 7, e38775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesiński, G. Bat road casualties and factors determining their number. Mammalia 2007, 71, 138–142. [Google Scholar] [CrossRef]
- Secco, H.; Gomes, L.A.; Lemos, H.; Mayer, F.; Machado, T.; Guerreiro, M.; Gregorin, R. Road and landscape features that affect bat roadkills in southeastern Brazil. Oecologia Aust. 2017, 21, 323–336. [Google Scholar] [CrossRef]
- Medinas, D.; Ribeiro, V.; Marques, J.T.; Silva, B.; Barbosa, A.M.; Rebelo, H.; Mira, A. Road effects on bat activity depend on surrounding habitat type. Sci. Total Environ. 2019, 660, 340–347. [Google Scholar] [CrossRef]
- Schaub, A.; Ostwald, J.; Siemers, B.M. Foraging bats avoid noise. J. Exp. Biol. 2008, 211, 3174–3180. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, M.; Soanes, K.; Lahoz-Monfort, J.J.; Lumsden, L.F.; van der Ree, R. Artificial lighting reduces the effectiveness of wildlife-crossing structures for insectivorous bats. J. Environ. Manag. 2020, 262, 11013. [Google Scholar] [CrossRef] [PubMed]
- Azam, C.; Le Viol, I.; Yves, B.; Zissis, G.; Vernet, A.; Julien, J.-F.; Kerbiriou, C. Evidence for distance and illuminance thresholds in the effects of artificial lighting on bat activity. Landsc. Urban Plan. 2018, 175, 123–135. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Street Lighting Disturbs Commuting Bats. Curr. Biol. 2009, 19, 1123–1127. [Google Scholar] [CrossRef] [Green Version]
- Lesiński, G.; Sikora, A.; Olszewski, A. Bat casualties on a road crossing a mosaic landscape. Eur. J. Wildl. Res. 2011, 57, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.C.C.; Ho, Y.Y.; Kuo, H.C. Illustrated field keys to the bats (Mammalia: Chiroptera) of Taiwan. J. Threat. Taxa 2020, 12, 15675–15710. [Google Scholar] [CrossRef]
- Ding, T.S.; Yuan, H.W.; Geng, S.; Lin, Y.S.; Lee, P.F. Energy flux, body size and density in relation to bird species richness along an elevational gradient in Taiwan. Glob. Ecol. Biogeogr. 2005, 14, 299–306. [Google Scholar] [CrossRef]
- Li, C.F.; Chytrý, M.; Zelený, D.; Chen, M.Y.; Chen, T.Y.; Chiou, C.R.; Hsia, Y.J.; Liu, H.Y.; Yang, S.Z.; Yeh, C.L.; et al. Classification of Taiwan forest vegetation. Appl. Veg. Sci. 2013, 16, 698–719. [Google Scholar] [CrossRef]
- Koh, C.N.; Lee, P.F.; Lin, R.S. Bird species richness patterns of northern Taiwan: Primary productivity, human population density, and habitat heterogeneity. Divers. Distrib. 2006, 12, 546–554. [Google Scholar] [CrossRef]
- Chang, C.W. Bat Species and Fauna in Relation to Habitat Types and Environment Factors in Central-Southern Taiwan. Master’s Thesis, National Chiayi University, Chiayi City, Taiwan, 2015. [Google Scholar]
- Cheng, H.C.; Fang, Y.P.; Chou, C.H. A Photographic Guide to the Bats of Taiwan, 3rd ed.; Endemic Species Research Institute: Jiji Township, Taiwan, 2017; p. 151. [Google Scholar]
- Macro ECOnomy Meter. Available online: http://mecometer.com (accessed on 31 July 2019).
- NationMaster: Global Industry Market Sizing. Available online: https://www.nationmaster.com/ (accessed on 31 July 2019).
- Chyn, K.; Lin, T.E.; Chen, Y.K.; Chen, C.Y.; Fitzgerald, L.A. The magnitude of roadkill in Taiwan: Patterns and consequences revealed by citizen science. Biol. Conserv. 2019, 237, 317–326. [Google Scholar] [CrossRef]
- Central Weather Bureau. Climate of Taiwan. Available online: https://www.cwb.gov.tw/V8/C/C/Taiwan/index.html (accessed on 23 December 2020).
- Lin, Y.C.; Huang, S.L.; Budd, W.W. Assessing the environmental impacts of high-altitude agriculture in Taiwan: A Driver-Pressure-State-Impact-Response (DPSIR) framework and spatial emergy synthesis. Ecol. Indic. 2013, 32, 42–50. [Google Scholar] [CrossRef]
- Lee, P.F.; Ding, T.S.; Hsu, F.H.; Geng, S. Breeding bird species richness in Taiwan: Distribution on gradients of elevation, primary productivity and urbanization. J. Biogeogr. 2004, 31, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Taiwan Roadkill Observation Network. Available online: https://roadkill.tw/en (accessed on 12 April 2019).
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Leprieur, F. Comparing methods to separate components of beta diversity. Methods Ecol. Evol. 2015, 6, 1069–1079. [Google Scholar] [CrossRef]
- Chen, W.J.; Lo, C.C.; Tsai, F.A.; Chang, A.Y. Using open data to establish a multi-temporal and terrestrial environmental dataset of Taiwan. Taiwan J. Biodivers 2020, 22, 13–44. [Google Scholar]
- Shiu, H.J. A GIS-Based Environmental Database of Taiwan and Penghu Islands. Available online: http://mountain-ecology.blogspot.tw/2016/06/gis.html (accessed on 7 April 2016).
- Jones, G.; Teeling, E.C. The evolution of echolocation in bats. Trends Ecol. Evol. 2006, 21, 148–156. [Google Scholar] [CrossRef]
- Ho, Y.Y.; Fang, Y.P.; Chou, C.H.; Cheng, H.C.; Chang, H.W. High duty cycle to low duty cycle: Echolocation behaviour of the hipposiderid bat Coelops frithii. PLoS ONE 2013, 8, e62938. [Google Scholar] [CrossRef] [PubMed]
- Denzinger, A.; Schnitzler, H.U. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 2013, 4, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lie, C.C. Wing Morphology of Insectivorous Bats in Taiwan. Master’s Thesis, Tunghai University, Taichung, Taiwan, 2000. [Google Scholar]
- Masuda, R.; Ohdachi, S.D.; Ishibashi, Y.; Iwasa, M.A.; Saitoh, T. The Wild Mammals of Japan, 2nd ed.; Shoukadoh Book Sellers: Kyoto, Japan, 2015; p. 506. [Google Scholar]
- Yoon, K.B.; Rahman, M.M.; Park, Y.C. Acoustic Species identification of Korean Myotis bats (Chiroptera: Vespertilionidae). J. For. Environ. Sci. 2016, 32, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Min, C.; Jiang, F.; Zhenxin, L.; Huihua, Z.; Jiang, Z.; Shuyi, Z. Relationship between the echolocation frequency and body size in six species (Chiroptera: Vespertilionidae). J. Nor. Nor. Univ. 2002, 34, 64–69. [Google Scholar]
- Bats in China. Available online: http://www.bio.bris.ac.uk/research/bats/China%20bats/index.htm (accessed on 3 January 2021).
- Kuo, H.C.; Tuanmu, M.-N.; Huang, C.C.; Tsi, W.Y.; Chang, P.S.; Chen, K.Y. Monitoring of Bat Population Dynamics in the Taroko National Park(2/2); Taroko National Park: Xiulin Township, Taiwan, 2017; p. 72. [Google Scholar]
- Hsieh, B.J.; Chen, H.C. Monitoring of Bat Population Dynamics in the Taroko National Park; Taroko National Park: Xiulin Township, Taiwan, 2016; p. 80. [Google Scholar]
- Cheng, H.C. Handbook of the Biological Resources in Hushan-Mammals, 3rd ed.; Endemic Species Research Institute: Jiji Township, Taiwan, 2014; p. 111. [Google Scholar]
- Lin, L.K.; Lee, L.L.; Cheng, C.C. Bats of Taiwan; National Museum of Natural Science: Taichung, Taiwan, 1997; p. 177. [Google Scholar]
- Chen, C.H. A Study of Roost Selection of Plecotus Taivanus and Monitoring of Bat Boxes in Syuejian Area; Shei-Pa National Park: Dahu Township, Taiwan, 2011; p. 61. [Google Scholar]
- Bat Study and Conservation Group of Japan. A Field Guide to Bats of Japan, Rev. ed.; Sano, A., Fukui, D., Eds.; Bun-ich Co.: Tokyo, Japan, 2011; p. 68. [Google Scholar]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. 2020. Available online: https://cran.r-project.org/web/packages/betapart/index.html (accessed on 19 January 2021).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bolker, B.; Team, R.D.C. Bbmle: Tools for General Maximum Likelihood Estimation; 2017. Available online: https://cran.r-project.org/web/packages/bbmle/index.html (accessed on 19 January 2021).
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; Ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Dray, S.; Dufour, A.; Chessel, D. The ade4 Package-II: Two-Table and K-Table Methods. R News 2007, 7, 47–52. [Google Scholar]
- Ter Braak, C.J.; Cormont, A.; Dray, S. Improved testing of species traits–environment relationships in the fourth-corner problem. Ecology 2012, 93, 1525–1526. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cheng, H.C.; Changchien, L.W.; Lin, R.S.; Yang, C.H.; Chang, S.W. The Red List of Terrestrial Mammals of Taiwan, 2017; Endemic Species Research Institute: Jiji Township, Taiwan, 2017; p. 35. [Google Scholar]
- Huang, C.C.; Chang, H.C. Bat Fauna of Shuilin Township, Yunlin County; Formosan Golden Bat’s Home: Sheuilin Township, Taiwan, 2020; p. 31. [Google Scholar]
- Burgin, C.J.; Wilson, D.E.; Mittermeier, R.A.; Rylands, A.B.; Lacher, T.E.; Sechrest, W. (Eds.) Illustrated Checklist of the Mammals of the World; Lynx Edicions: Barcelona, Spain, 2020; p. 1166. [Google Scholar]
- Lee, Y.F.; Kuo, Y.M.; Chu, W.C.; Lin, Y.H. Chiropteran Diversity in Different Settings of the Uplifted Coral Reef Tropical Forest of Taiwan. J. Mammal. 2007, 88, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Senawi, J.; Kingston, T. Clutter negotiating ability in an ensemble of forest interior bats is driven by body mass. J. Exp. Biol. 2019, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C. Bat Surveys in Neiweipi Cultural Park; Kaohsiung Museum of Fine Arts: Taichung City, Taiwan, 2020; p. 37. [Google Scholar]
- Wu, J.T. Taxonomic Study of the Genus Pipistrellus (Chiroptera: Vespertilionidae) in Taiwan. Mater’s Thesis, National Chiayi University, Chiayi City, Taiwan, 2007; p. 59. [Google Scholar]
- Roeleke, M.; Bumrungsri, S.; Voigt, C.C. Bats probe the aerosphere during landscape-guided altitudinal flights. Mammal Rev. 2018, 48, 7–11. [Google Scholar] [CrossRef]
- McCracken, G.F.; Gillam, E.H.; Westbrook, J.K.; Lee, Y.F.; Jensen, M.L.; Balsley, B.B. Brazilian free-tailed bats (Tadarida brasiliensis: Molossidae, Chiroptera) at high altitude: Links to migratory insect populations. Integr. Comp. Biol. 2008, 48, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Ruangwiset, A.; Bumrungsri, S. Vertical stratification in foraging activity of Chaerephon plicatus (Molossidae, Chiroptera) in Central Thailand. Mamm. Biol. 2019, 96, 1–6. [Google Scholar] [CrossRef]
- National Parks of Taiwan. Available online: https://np.cpami.gov.tw/home-en.html (accessed on 14 January 2021).
- Stone, E.L.; Harris, S.; Jones, G. Impacts of artificial lighting on bats: A review of challenges and solutions. Mamm. Biol. 2015, 80, 213–219. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Law, B.; Banks, P.B. The urban matrix and artificial light restricts the nightly ranging behaviour of Gould’s long-eared bat (Nyctophilus gouldi). Austral Ecol. 2013, 38, 921–930. [Google Scholar] [CrossRef]
- Lewanzik, D.; Voigt, C.C. Artificial light puts ecosystem services of frugivorous bats at risk. J. Appl. Ecol. 2014, 51, 388–394. [Google Scholar] [CrossRef]
- Russo, D.; Cistrone, L.; Libralato, N.; Korine, C.; Jones, G.; Ancillotto, L. Adverse effects of artificial illumination on bat drinking activity. Anim. Conserv. 2017, 20, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Straka, T.M.; Greif, S.; Schultz, S.; Goerlitz, H.R.; Voigt, C.C. The effect of cave illumination on bats. Glob. Ecol. Conserv. 2020, 21, e00808. [Google Scholar] [CrossRef]
- Coelops Frithii. The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/5074/22030377 (accessed on 3 January 2021).
- Myotis Formosus. The IUCN Red List of Threatened Species. 2020. Available online: https://www.iucnredlist.org/species/85736120/95642290 (accessed on 3 January 2021).
- Russo, D.; Cosentino, F.; Festa, F.; De Benedetta, F.; Pejic, B.; Cerretti, P.; Ancillotto, L. Artificial illumination near rivers may alter bat-insect trophic interactions. Environ. Pollut. 2019, 252, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Rydell, J. Bats and their insect prey at streetlights. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Washington, DC, USA, 2006; pp. 43–60. [Google Scholar]
- Brown, C.R.; Brown, M.B. Where has all the road kill gone? Curr. Biol. 2013, 23, R233–R234. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.E.; Bielby, J.; Cardillo, M.; Fritz, S.A.; O’Dell, J.; Orme, C.D.L.; Safi, K.; Sechrest, W.; Boakes, E.H.; Carbone, C.; et al. PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 2009, 90, 2648. [Google Scholar] [CrossRef] [Green Version]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef] [Green Version]
- NASA Earth Observatory. Available online: https://earthobservatory.nasa.gov/features/NightLights (accessed on 12 February 2019).
- Román, M.O.; Stokes, E.C. Holidays in lights: Tracking cultural patterns in demand for energy services. Earth’s Future 2015, 3, 182–205. [Google Scholar] [CrossRef]
- Department of Household Registration, Ministry of the Interior. Available online: https://www.ris.gov.tw/app/portal/346 (accessed on 9 September 2019).
- Council of Agriculture, Executive Yuan, of Taiwan. Available online: https://data.coa.gov.tw/ (accessed on 9 September 2019).
- European Environmental Agency. Landscape Fragmentation Indicator Effective Mesh Density (seff)—Major and Medium Anthrophogenic Fragmentation (FGA2_S_2016). Available online: https://www.eea.europa.eu/data-and-maps/data/landscape-fragmentation-indicator-effective-mesh/ (accessed on 18 November 2016).


| Trait | Type | Definition | Data Source |
|---|---|---|---|
| Forearm length, FA | Quantitative: continuous | The length of forearm, in mm | [31] |
| Body mass, BM | Quantitative: continuous | The weight of alive volant individual, in grams | [31] |
| Wingspan, B | Quantitative: continuous | The distance between two wing tips when wings fully expand, in mm | [31,46] |
| Frequency of maximum energy, FmaxE | Quantitative: continuous | The dominant frequency of orientating echolocation calls | [31,44,47,48,49,50], |
| Echolocation call type, CALL | Qualitative: category | Four categories: high duty-cycle constant frequency (HDCF), low duty-cycle constant frequency (LDCF), broadband frequency-modulated (BFM), narrow-tailed frequency modulated (NFM) | [31,44,51] |
| Minimum elevation, eleMin | Qualitative: continuous | Known lowest elevation in records, in meters | [30,52,53] |
| Maximum elevation, eleMax | Qualitative: continuous | Known lowest elevation in records. In meters | [30,54,55], this study |
| Elevation range, eleRange | Qualitative: continuous | Elevation range based on the minimum and maximum elevation, in meters | This study |
| Roost type use | Qualitative: binary | Use of cave, crevice, cavity, and foliage. Data are presented separately for individual type of roost. The definition of each roost type is as: Cave: large chambers in nature cave, abandon and in-used car tunnels, mining tunnel, bunker Crevice: narrow space in rock, cement, and gaps inside buildings Cavity: small cambers inside living tree trunk, log, space under tree bark, woody bat box and woody part of building Foliage: live and dead leaves | [31,47,55,56], empirical data |
| Hunting mode, HUNT | Qualitative: category | How and where a bat species detects and captures prey. Five categories: Open space aerial hawking, edge and gap aerial hawking, water trawling, active gleaning, and fluttering detection | [31,44] |
| Taxa 1 | Roadkill Records | Endemism | National Status 2 | IUCN Status | IUCN Population Trend |
|---|---|---|---|---|---|
| Hipposideridae | |||||
| Hipposideros armiger terasensis, Hiar | 68 | ESS | LC | Unknown | |
| Coelops frithii formosanus, Cofr | 4 | ESS | NVU | NT | Decreasing |
| Hipposideridae gen. sp. | 1 | ||||
| Rhinolophidae | |||||
| Rhinolophus formosae, Rhfo | 1 | ES | LC | Decreasing | |
| Rhinolophus monoceros, Rhmo | 30 | ES | n.a. | n.a. | |
| Miniopteridae | |||||
| Miniopterus fuliginosus, Mifu | 75 | n.a. | n.a. | ||
| Vespertilionidae | |||||
| KERIVOLINAE | |||||
| Kerivoula furva, Kefu | 8 | n.a. | n.a. | ||
| MURININAE | |||||
| Harpiocephalus harpia, Haha | 1 | LC | Decreasing | ||
| Murina bicolor, Mubi | 1 | ES | LC | Unknown | |
| Murina puta, Mupu | 36 | ES | LC | Stable | |
| Murina recondita, Mure | 5 | ES | LC | Unknown | |
| Murina spp. | 9 | ||||
| MYOTINAE | |||||
| Myotis fimbriatus taiwanensis, Myfi | 2 | ESS | LC | Unknown | |
| Myotis formosus flavus, Myfo | 3 | ESS | NVU | NT | Decreasing |
| Myotis frater, Myfr | 1 | DD # | Unknown | ||
| Myotis secundus, Myse | 4 | ES | LC | Stable | |
| Submyotodon latirostris, Sula | 1 | ES | LC | Unknown | |
| Myotinae gen. sp. | 1 | ||||
| VESPERTILIONINAE | |||||
| Eptesicus pachyomus horikawai, Eppa | 25 | ESS | LC | Unknown | |
| Nyctalus plancyi velutinus, Nypl | 6 | LC | Unknown | ||
| Pipistrellus abramus, Piab | 98 | LC | Stable | ||
| Pipistrellus sp. group2, Pisp | 2 | ||||
| Pipistrellus spp. | 20 | ||||
| Scotophilus kuhlii, Scku | 43 | LC | Stable | ||
| Vespertilionidae gen. sp. | 102 | ||||
| Unidentified bat | 114 |
| Model 1 | Significant Variables 2 | Estimate ± SE | p-Value | AIC Score | BIC Score |
|---|---|---|---|---|---|
| All variables (no interaction) | Intercept | 89,850.0 ± 5.454 | <0.001 | 3120.1 | 3357.4 |
| ELE | −2.720 ± 0.805 | <0.001 | |||
| ALight | −1.185 ± 0.242 | <0.001 | |||
| PA | 0.642 ± 0.175 | <0.001 | |||
| BU | 89,850.0 ± 5.454 | <0.001 | |||
| BL | 89,850.0 ± 5.454 | <0.001 | |||
| Each of the other 7 landcovers 3 | 35,650.0 ± 2.536 | <0.001 | |||
| ELE + ALight + PA+ 9 landcover types | ELE | −2.433 ± 0.789 | <0.001 | 3107.9 | 3287.7 |
| ALight | −1.263 ± 0.228 | <0.001 | |||
| PA | 0.563 ± 0.158 | <0.001 | |||
| ELE + ALight + PA | Intercept | −3.415 ± 0.411 | <0.001 | 3103.5 | 3218.6 |
| ELE | −3.321 ± 0.686 | <0.001 | |||
| ALight | −0.864 ± 0.163 | <0.001 | |||
| PA | 0.641 ± 0.149 | <0.001 | |||
| ELE × ALight + PA | Intercept | −3.421 ± 0.409 | <0.001 | 3104.7 | 3226.9 |
| ELE | −3.043 ± 0.731 | <0.001 | |||
| ALight | −0.803 ± 0.175 | <0.001 | |||
| PA | 0.623 ± 0.150 | <0.001 | |||
| ELE × PA + ALight | Intercept | −3.274 ± 0.415 | <0.001 | 3099.6 | 3221.8 |
| ELE | −4.828 ± 0.975 | <0.001 | |||
| ALight | −0.957 ± 0.167 | 0.023 | |||
| PA | 0.406 ± 0.178 | <0.001 | |||
| ELE × PA | 2.857 ± 1.158 | 0.014 | |||
| ELE + ALight × PA | Intercept | −3.460 ± 0.413 | <0.001 | 3100.0 | 3222.3 |
| ELE | −3.564 ± 0.687 | <0.001 | |||
| ALight | −0.758 ± 0.169 | <0.001 | |||
| PA | 1.072 ± 0.230 | <0.001 | |||
| PA × ALight | −0.921 ± 0.399 | 0.0209 | |||
| ELE × ALight × PA | Intercept | −3.357 ± 0.420 | <0.001 | 3103.6 | 3247.4 |
| ELE | −4.255 ± 1.117 | <0.001 | |||
| ALight | −0.819 ± 0.202 | <0.001 | |||
| PA | 0.768 ± 0.318 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.C.-C.; Chen, W.-J.; Lin, T.-E. Landscape and Species Traits Co-Drive Roadkills of Bats in a Subtropical Island. Diversity 2021, 13, 117. https://doi.org/10.3390/d13030117
Huang JC-C, Chen W-J, Lin T-E. Landscape and Species Traits Co-Drive Roadkills of Bats in a Subtropical Island. Diversity. 2021; 13(3):117. https://doi.org/10.3390/d13030117
Chicago/Turabian StyleHuang, Joe Chun-Chia, Wan-Jyun Chen, and Te-En Lin. 2021. "Landscape and Species Traits Co-Drive Roadkills of Bats in a Subtropical Island" Diversity 13, no. 3: 117. https://doi.org/10.3390/d13030117







