Prevalence and Diversity of Avian Haemosporidians May Vary with Anthropogenic Disturbance in Tropical Habitats in Myanmar
Abstract
1. Introduction
2. Materials and Methods
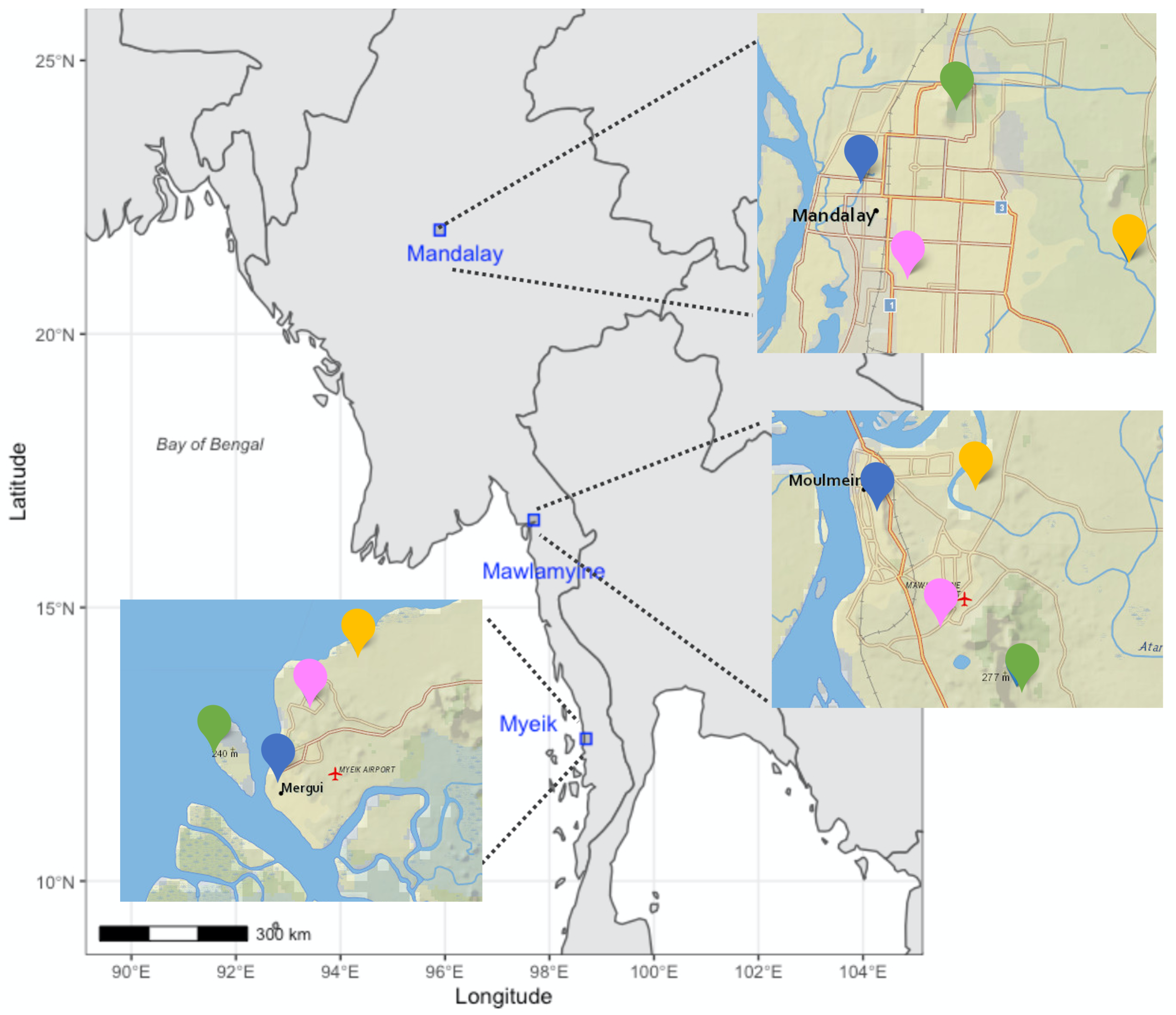
2.1. Study Sites and Field Sampling of Birds
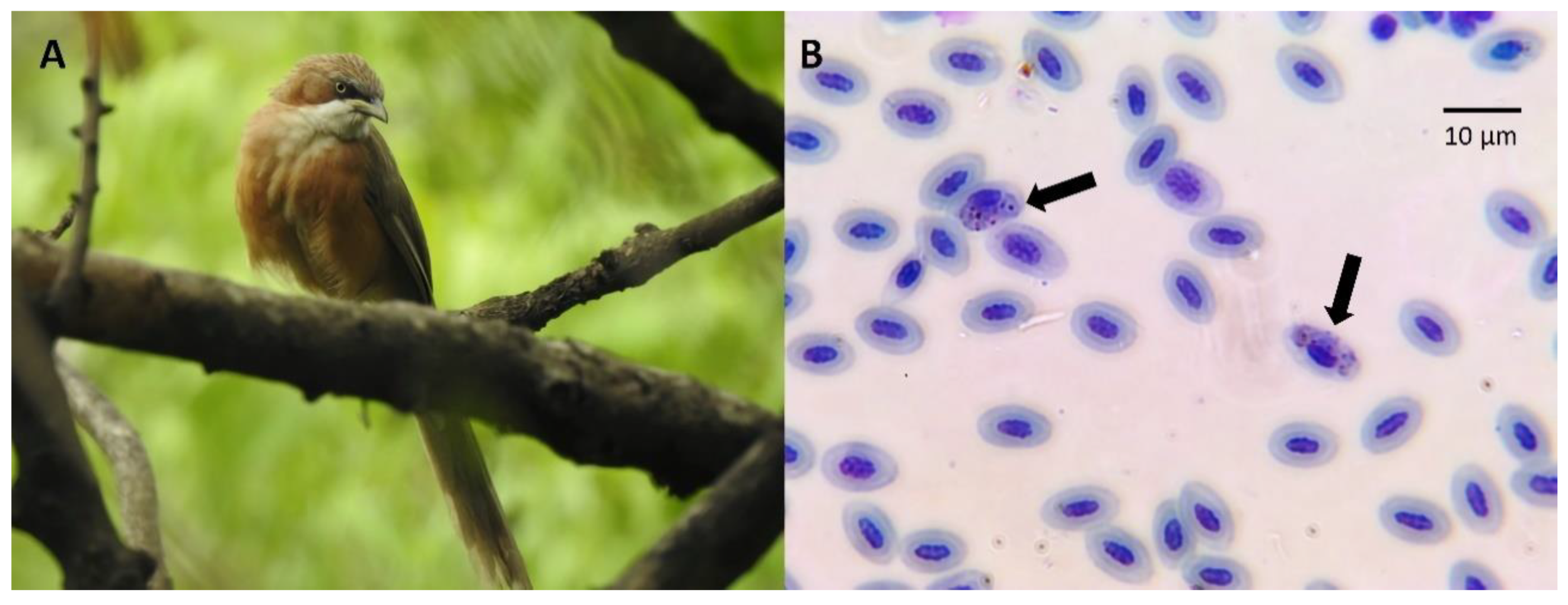
2.2. Blood Sampling
2.3. Molecular Parasite Screening
2.4. Phylogenetic Analyses
2.5. Statistical Procedure
3. Results
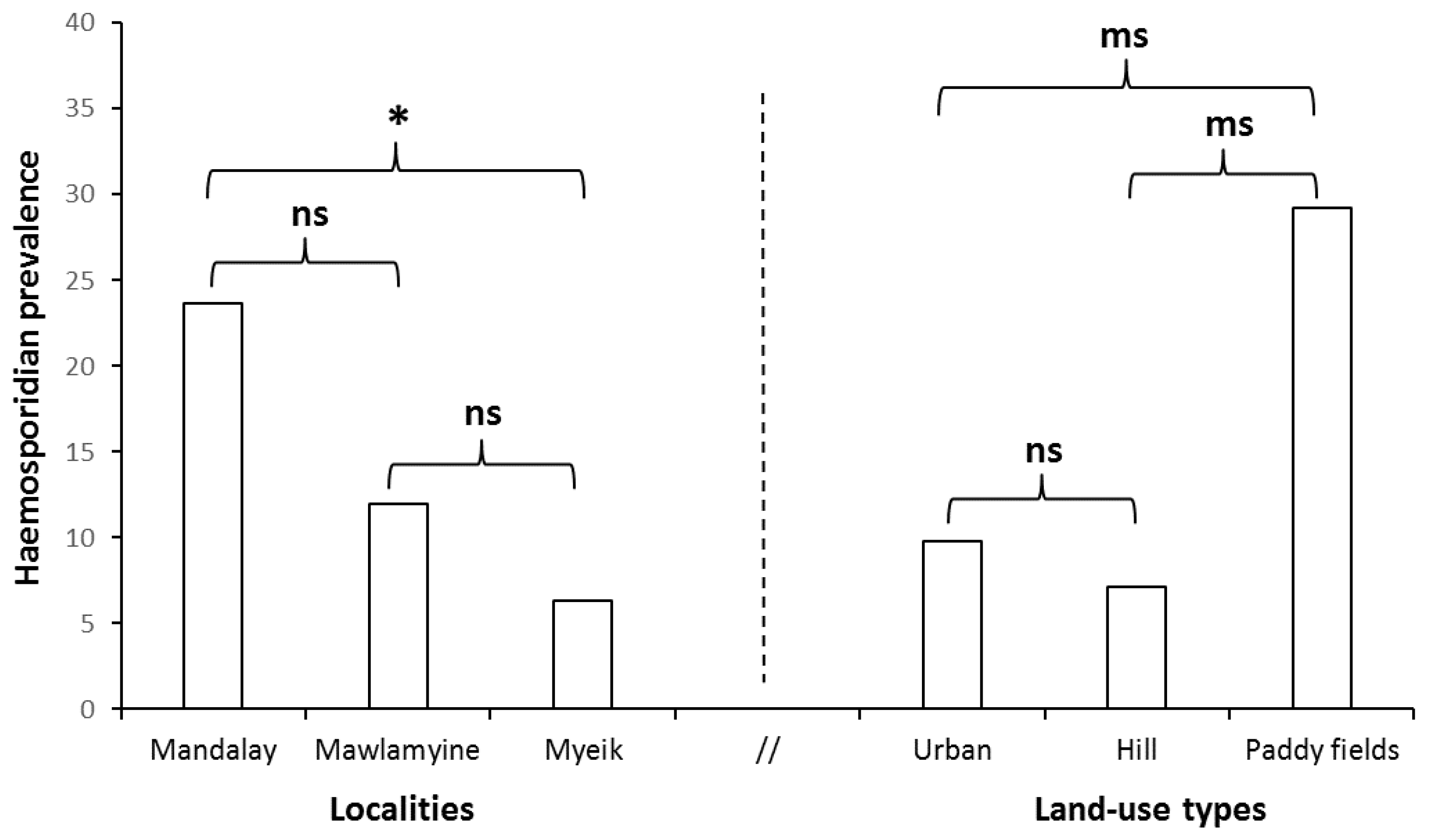
3.1. Prevalence of Haemosporidian Infection
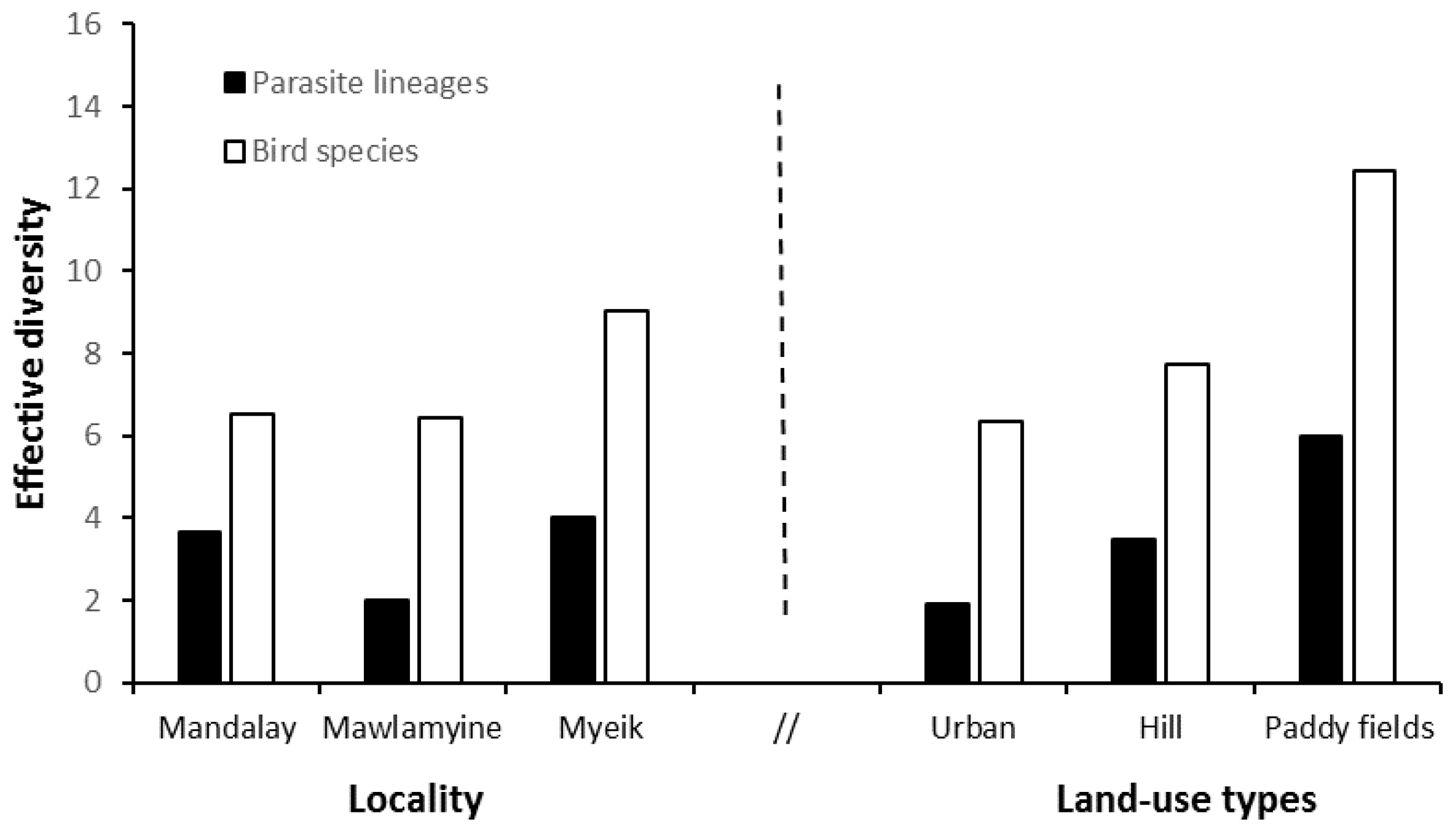
3.2. Estimates of Parasite Lineage and Bird Diversity
4. Discussion
4.1. Genetic Diversity of Bird Haemosporidians in Myanmar
4.2. Differences in Prevalence of Infection among Localities
4.3. Anthropic Disturbance and Avian Haemosporidian Infection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franklin, J. Mapping Species Distributions: Spatial inference and Prediction; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780511810602. [Google Scholar]
- Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B 2018, 285, 20180792. [Google Scholar] [CrossRef]
- Nunez, S.; Arets, E.; Alkemade, R.; Verwer, C.; Leemans, R. Assessing the impacts of climate change on biodiversity: Is below 2 C enough? Clim. Chang. 2019, 154, 351–365. [Google Scholar] [CrossRef]
- Thomas, F.; Renaud, F.; Guégan, J.-F. Parasitism and Ecosystems; Oxford University Press: Oxford, UK, 2005; Volume 1, ISBN 9780198529873. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Antao, T. Evolutionary parasitology applied to control and elimination policies. Trends Parasitol. 2011, 27, 233–234. [Google Scholar] [CrossRef]
- Poulin, R.; Randhawa, H.S. Evolution of parasitism along convergent lines: From ecology to genomics. Parasitology 2013, 142, S6–S15. [Google Scholar] [CrossRef]
- Mouritsen, K.N.; Poulin, R. Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 2002, 124, 101–117. [Google Scholar] [CrossRef]
- Ellis, V.A.; Huang, X.; Westerdahl, H.; Jönsson, J.; Hasselquist, D.; Neto, J.M.; Nilsson, J.; Nilsson, A.; Hegemann, A.; Hellgren, O.; et al. Explaining prevalence, diversity and host specificity in a community of avian haemosporidian parasites. Oikos 2020, 129, 1314–1329. [Google Scholar] [CrossRef]
- Marcogliese, D.J. Parasites: Small Players with Crucial Roles in the Ecological Theater. EcoHealth 2004, 1, 151–164. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Swanson, B.L.; Fallon, S.M.; Martínez-Abraín, A.; Scheuerlein, A.; Gray, J.; Latta, S.C. Community Relationships of Avian Malaria Parasites in Southern Missouri. Ecol. Monogr. 2005, 75, 543–559. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0415300971. [Google Scholar]
- Valkiūnas, G.; Atkinson, C.T. Introduction to Life Cycles, Taxonomy, Distribution, and Basic Research Techniques. In Avian Malaria and Related Parasites in the Tropics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 45–80. [Google Scholar]
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.; Fragner, K.; Platonova, E.; Weissenböck, H.; Valkiūnas, G. Patterns of Plasmodium homocircumflexum virulence in experimentally infected passerine birds. Malar. J. 2019, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Merino, S.; Tomás, G.; Moreno, J.; Morales, J.; Lobato, E.; García-Fraile, S.; Belda, E.J. The blood parasite Haemoproteus reduces survival in a wild bird: A medication experiment. Biol. Lett. 2010, 6, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; Balbontín, J.; Reviriego, M.; García-Longoria, L.; Relinque, C.; Hermosell, I.G.; Magallanes, S.; López-Calderón, C.; De Lope, F.; Møller, A.P. A longitudinal study of age-related changes in Haemoproteus infection in a passerine bird. Oikos 2016, 125, 1092–1099. [Google Scholar] [CrossRef]
- Marzal, A.; Bensch, S.; Reviriego, M.; Balbontin, J.; De Lope, F. Effects of malaria double infection in birds: One plus one is not two. J. Evol. Biol. 2008, 21, 979–987. [Google Scholar] [CrossRef]
- Palinauskas, V.; Valkiūnas, G.; Bolshakov, C.V.; Bensch, S. Plasmodium relictum (lineage P-SGS1): Effects on experimentally infected passerine birds. Exp. Parasitol. 2008, 120, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; De Lope, F.; Navarro, C.; Møller, A.P. Malarial parasites decrease reproductive success: An experimental study in a passerine bird. Oecologia 2005, 142, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Moreno, J.; Sanz, J.J.; Arriero, E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc. R. Soc. B Biol. Sci. 2000, 267, 2507–2510. [Google Scholar] [CrossRef]
- Lapointe, D.A.; Atkinson, C.T.; Samuel, M.D. Ecology and conservation biology of avian malaria. Ann. N. Y. Acad. Sci. 2012, 1249, 211–226. [Google Scholar] [CrossRef]
- Marzal, A.; Garcia-Longoria, L. The Role of Malaria Parasites in Invasion Biology. In Avian Malaria and Related Parasites in the Tropics; Springer International Publishing: Cham, Switzerland, 2020; pp. 487–512. [Google Scholar]
- Marzal, A.; Albayrak, T. Geographical variation of haemosporidian parasites in Turkish populations of Krüper’s Nuthatch Sitta krueperi. J. Ornithol. 2012, 153, 1225–1231. [Google Scholar] [CrossRef]
- Muriel, J.; Graves, J.A.; Gil, D.; Magallanes, S.; Salaberria, C.; Casal-Lopez, M.; Marzal, A. Molecular characterization of avian malaria in the spotless starling (Sturnus unicolor). Parasitol. Res. 2018, 117, 919–928. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Marzal, A. Research on Avian Haemosporidian Parasites in the Tropics Before the Year 2000. In Avian Malaria and Related Parasites in the Tropics; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–44. [Google Scholar]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Conservation: The Critical Role of Hotspots. In Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Marchese, C. Biodiversity hotspots: A shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef]
- Renner, S.C.; Rappole, J.H.; Kyaw, M.; Milensky, C.M.; Päckert, M. Genetic confirmation of the species status of Jabouilleia naungmungensis. J. Ornithol. 2018, 159, 63–71. [Google Scholar] [CrossRef]
- Oo, S.S.L.; Kyaw, M.; Hlaing, N.M.; Renner, S.C. New to Myanmar: The rosy starling Pastor roseus (Aves: Passeriformes: Sturnidae) in the Hkakabo Razi Landscape. J. Threat. Taxa 2020, 12, 15493–15494. [Google Scholar] [CrossRef]
- Renner, S.C.; Rappole, J.H. Bird diversity, biogeographic patterns, and endemism of the eastern Himalayas and southeastern sub-Himalayan mountains. Ornithol. Monogr. 2011, 70, 153–166. [Google Scholar] [CrossRef]
- Päckert, M.; Milensky, C.M.; Martens, J.; Kyaw, M.; Suarez-Rubio, M.; Thaw, W.N.; Oo, S.S.L.; Wolfgramm, H.; Renner, S.C. Pilot biodiversity assessment of the Hkakabo Razi passerine avifauna in northern Myanmar—Implications for conservation from molecular genetics. Bird Conserv. Int. 2019, 30, 267–288. [Google Scholar] [CrossRef]
- Beadell, J.S.; Ishtiaq, F.; Covas, R.; Melo, M.; Warren, B.H.; Atkinson, C.T.; Bensch, S.; Graves, G.R.; Jhala, Y.V.; Peirce, M.A.; et al. Global phylogeographic limits of Hawaii’s avian malaria. Proc. R. Soc. B Biol. Sci. 2006, 273, 2935–2944. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Gering, E.; Rappole, J.H.; Rahmani, A.R.; Jhala, Y.V.; Dove, C.J.; Milensky, C.; Olson, S.L.; Peirce, M.A.; Fleischer, R.C. Prevalence and diversity of avian hematozoan parasites in Asia: A regional survey. J. Wildl. Dis. 2007, 43, 382–398. [Google Scholar] [CrossRef]
- Mya, M.M.; Oo, N.S.E.; Oo, C.C.; Maung, K.G. Prevalence of Plasmodium relictum in residential birds from Hpa-an Township Kayin State, Myanmar. J. Biol. Eng. Res. Rev. 2017, 4, 23–30. [Google Scholar]
- Ferraguti, M.; Martínez-de la Puente, J.; Bensch, S.; Roiz, D.; Ruiz, S.; Viana, D.S.; Soriguer, R.C.; Figuerola, J. Ecological determinants of avian malaria infections: An integrative analysis at landscape, mosquito and vertebrate community levels. J. Anim. Ecol. 2018, 87, 727–740. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Vora, N. Impact of anthropogenic environmental alterations on vector-borne diseases. Medscape J. Med. 2008, 10, 238. [Google Scholar] [PubMed]
- Van Hoesel, W.; Marzal, A.; Magallanes, S.; Santiago-Alarcon, D.; Ibáñez-Bernal, S.; Renner, S.C. Management of ecosystems alters vector dynamics and haemosporidian infections. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Chasar, A.; Loiseau, C.; Valkiūnas, G.; Iezhova, T.; Smith, T.B.; Sehgal, R.N.M. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol. Ecol. 2009, 18, 4121–4133. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.C.; Lüdtke, B.; Kaiser, S.; Kienle, J.; Schaefer, H.M.; Segelbacher, G.; Tschapka, M.; Santiago-Alarcon, D. Forests of opportunities and mischief: Disentangling the interactions between forests, parasites and immune responses. Int. J. Parasitol. 2016, 46, 571–579. [Google Scholar] [CrossRef]
- Hernández-Lara, C.; González-García, F.; Santiago-Alarcon, D. Spatial and seasonal variation of avian malaria infections in five different land use types within a Neotropical montane forest matrix. Landsc. Urban Plan. 2017, 157, 151–160. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Khimoun, A.; Ollivier, A.; Eraud, C.; Faivre, B.; Garnier, S. Habitat fragmentation, not habitat loss, drives the prevalence of blood parasites in a Caribbean passerine. Ecography 2018, 41, 1835–1849. [Google Scholar] [CrossRef]
- Tchoumbou, M.A.; Mayi, M.P.A.; Malange, E.N.F.; Foncha, F.D.; Kowo, C.; Fru-Cho, J.; Tchuinkam, T.; Awah-Ndukum, J.; Dorazio, R.; Anong, D.N.; et al. Effect of deforestation on prevalence of avian haemosporidian parasites and mosquito abundance in a tropical rainforest of Cameroon. Int. J. Parasitol. 2020, 50, 63–73. [Google Scholar] [CrossRef]
- Ferraguti, M.; Hernández-Lara, C.; Sehgal, R.N.M.; Santiago-Alarcon, D. Anthropogenic Effects on Avian Haemosporidians and Their Vectors. In Avian Malaria and Related Parasites in the Tropics; Springer International Publishing: Cham, Switzerland, 2020; pp. 451–485. [Google Scholar]
- Lim, C.L.; Prescott, G.W.; De Alban, J.D.T.; Ziegler, A.D.; Webb, E.L. Untangling the proximate causes and underlying drivers of deforestation and forest degradation in Myanmar. Conserv. Biol. 2017, 31, 1362–1372. [Google Scholar] [CrossRef]
- Murray, N.J.; Keith, D.A.; Duncan, A.; Tizard, R.; Ferrer-Paris, J.R.; Worthington, T.A.; Armstrong, K.; Hlaing, N.; Htut, W.T.; Oo, A.H.; et al. Myanmar’s terrestrial ecosystems: Status, threats and conservation opportunities. Biol. Conserv. 2020, 252, 108834. [Google Scholar] [CrossRef]
- Zhang, Y.; Prescott, G.W.; Tay, R.E.; Dickens, B.L.; Webb, E.L.; Htun, S.; Tizard, R.J.; Rao, M.; Carrasco, L.R. Dramatic cropland expansion in Myanmar following political reforms threatens biodiversity. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Rubio, M.; Connette, G.; Aung, T.; Kyaw, M.; Renner, S.C. Hkakabo Razi landscape as one of the last exemplar of large contiguous forests. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de La Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016, 6, 29002. [Google Scholar] [CrossRef]
- Renner, S.C.; Bates, P.J.J. Historic changes in species composition for a globally unique bird community. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Bichet, C.; Sorci, G.; Robert, A.; Julliard, R.; Lendvai, Á.Z.; Chastel, O.; Garnier, S.; Loiseau, C. Epidemiology of Plasmodium relictum Infection in the House Sparrow. J. Parasitol. 2014, 100, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- Krams, I.; Cīrule, D.; Krama, T.; Hukkanen, M.; Rytkönen, S.; Orell, M.; Iezhova, T.; Rantala, M.J.; Tummeleht, L. Effects of Forest Management on Haematological Parameters, Blood Parasites, and Reproductive Success of the Siberian Tit (Poecile cinctus) in Northern Finland. Ann. Zoöl. Fenn. 2010, 47, 335–346. [Google Scholar] [CrossRef]
- Wood, M.J.; Cosgrove, C.L.; Wilkin, T.A.; Knowles, S.C.L.; Day, K.P.; Sheldon, B.C. Within-population variation in prevalence and lineage distribution of avian malaria in blue tits, Cyanistes caeruleus. Mol. Ecol. 2007, 16, 3263–3273. [Google Scholar] [CrossRef] [PubMed]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. Bioscience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. Bioscience 2001, 51, 933. [Google Scholar] [CrossRef]
- Chotamonsak, C.; Salathé, E.P.; Kreasuwan, J.; Chantara, S.; Siriwitayakorn, K. Projected climate change over Southeast Asia simulated using a WRF regional climate model. Atmos. Sci. Lett. 2011, 12, 213–219. [Google Scholar] [CrossRef]
- Suarez-Rubio, M.; Aung, T.; Oo, S.S.L.; Shwe, N.M.; Hlaing, N.M.; Naing, K.M.; Oo, T.; Sein, M.M.; Renner, S.C. Nonbreeding Bird Communities Along an Urban–Rural Gradient of a Tropical City in Central Myanmar. Trop. Conserv. Sci. 2016, 9. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New Pcr Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Bensch, S.; Pérez-Tris, J.; Waldenström, J.; Hellgren, O. Linkage Between Nuclear and Mitochondrial Dna Sequences in Avian Malaria Parasites: Multiple Cases of Cryptic Speciation? Evolution 2004, 58, 1617–1621. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Valkiūnas, G.; Iezhova, T.A.; Smith, T.B. Blood Parasites of Chickens in Uganda And Cameroon With Molecular Descriptions of Leucocytozoon schoutedeni and Trypanosoma gallinarum. J. Parasitol. 2006, 92, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 December 2020).
- Wilkinson, L. ggplot2: Elegant Graphics for Data Analysis by WICKHAM, H. Biometrics 2011, 67, 678–679. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T. ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2016, 8, 28–36. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package “Vegan”: Community Ecology Package. R Packag. Version 2.4-4. 2017. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 December 2020).
- Mittelbach, G.G. Community Ecology, 1st ed.; Sinauer Associates: Sunderland, MA, USA, 2012. [Google Scholar]
- Heck, K.L.; Van Belle, G.; Simberloff, D. Explicit Calculation of the Rarefaction Diversity Measurement and the Determination of Sufficient Sample Size. Ecology 1975, 56, 1459–1461. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. [Google Scholar] [CrossRef]
- Dorman, M.; Dorman, M. Leaflet. In Introduction to Web Mapping; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020; pp. 139–167. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer Publishing Co: New York, NY, USA, 2002. [Google Scholar]
- Ishtiaq, F.; Bowden, C.G.R.; Jhala, Y.V. Seasonal dynamics in mosquito abundance and temperature do not influence avian malaria prevalence in the Himalayan foothills. Ecol. Evol. 2017, 7, 8040–8057. [Google Scholar] [CrossRef]
- Ejiri, H.; Sato, Y.; Kim, K.-S.; Hara, T.; Tsuda, Y.; Imura, T.; Murata, K.; Yukawa, M. Entomological Study on Transmission of Avian Malaria Parasites in a Zoological Garden in Japan: Bloodmeal Identification and Detection of Avian Malaria Parasite DNA From Blood-Fed Mosquitoes. J. Med. Èntomol. 2011, 48, 600–607. [Google Scholar] [CrossRef]
- Martinsen, E.S.; Perkins, S.L.; Schall, J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogenetics Evol. 2008, 47, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Olsson-Pons, S.; Ishtiaq, F.; Clegg, S.M. Specialist enemies, generalist weapons and the potential spread of exotic pathogens: Malaria parasites in a highly invasive bird. Int. J. Parasitol. 2015, 45, 891–899. [Google Scholar] [CrossRef]
- Gupta, P.; Vishnudas, C.K.; Ramakrishnan, U.; Robin, V.V.; Dharmarajan, G. Geographical and host species barriers differentially affect generalist and specialist parasite community structure in a tropical sky-island archipelago. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190439. [Google Scholar] [CrossRef] [PubMed]
- Lwin, K.N.; Thwin, K.M.M. Birds of Myanmar; Silkworm Books: Chiang Mai, Thailand, 2005; ISBN 9789749575680. [Google Scholar]
- Win, S.Y.; Chel, H.M.; Hmoon, M.M.; Htun, L.L.; Bawm, S.; Win, M.M.; Murata, S.; Nonaka, N.; Nakao, R.; Katakura, K. Detection and molecular identification of Haemoproteus and Plasmodium species from village chickens in different areas of Myanmar. Acta Trop. 2020, 212, 105719. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Ostfeld, R.; Daszak, P. New Directions in Conservation Medicine: Applied Cases of Ecological Health; Oxford University Press Inc: New York, NY, USA, 2012; ISBN 978-0-19-973147-3. [Google Scholar]
- BirdLife. Data Zone. Available online: http://datazone.birdlife.org/home (accessed on 20 December 2020).
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nat. Cell Biol. 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Dobson, A.; Lafferty, K.D.; Kuris, A.M.; Hechinger, R.F.; Jetz, W. Homage to Linnaeus: How many parasites? How many hosts? Proc. Natl. Acad. Sci. USA 2008, 105, 11482–11489. [Google Scholar] [CrossRef]
- Møller, A.P.; Czirjak, G.A.; Heeb, P. Feather micro-organisms and uropygial antimicrobial defences in a colonial passerine bird. Funct. Ecol. 2009, 23, 1097–1102. [Google Scholar] [CrossRef]
- Cibois, A.; Gelang, M.; Alström, P.; Pasquet, E.; Fjeldså, J.; Ericson, P.G.P.; Olsson, U. Comprehensive phylogeny of the laughingthrushes and allies (Aves, Leiothrichidae) and a proposal for a revised taxonomy. Zoöl. Scr. 2018, 47, 428–440. [Google Scholar] [CrossRef]
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the world’s worst invasive alien species. Biol. Invasions 2013, 16, 981–985. [Google Scholar] [CrossRef]
- Platonova, E.; Aželytė, J.; Iezhova, T.; Ilgūnas, M.; Mukhin, A.; Palinauskas, V. Experimental study of newly described avian malaria parasite Plasmodium (Novyella) collidatum n. sp., genetic lineage pFANTAIL01 obtained from South Asian migrant bird. Malar. J. 2021, 20, 82. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Renner, S.C. Bird Migration and Vector-Borne Parasite Transmission. In Avian Malaria and Related Parasites in the Tropics; Springer International Publishing: Cham, Switzerland, 2020; pp. 513–526. [Google Scholar]
- Garcia-Longoria, L.; Marzal, A.; De Lope, F.; Garamszegi, L. Host-parasite interaction explains variation in the prevalence of avian haemosporidians at the community level. PLoS ONE 2019, 14, e0205624. [Google Scholar] [CrossRef]
- Clark, N.J. Phylogenetic uniqueness, not latitude, explains the diversity of avian blood parasite communities worldwide. Glob. Ecol. Biogeogr. 2018, 27, 744–755. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Fernández-González, S.; De La Hera, I.; Pérez-Tris, J. Finding the appropriate variables to model the distribution of vector-borne parasites with different environmental preferences: Climate is not enough. Glob. Chang. Biol. 2013, 19, 3245–3253. [Google Scholar] [CrossRef]
- Lachish, S.; Knowles, S.C.L.; Alves, R.; Wood, M.J.; Sheldon, B.C. Infection dynamics of endemic malaria in a wild bird population: Parasite species-dependent drivers of spatial and temporal variation in transmission rates. J. Anim. Ecol. 2011, 80, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S.; Beaty, B.J. Natural cycles of vector-borne pahogens. In Biology of Disease Vectors; Marquardt, M.C., Ed.; Elsevier Academic Press: New York, NY, USA, 2005; pp. 167–185. [Google Scholar]
- Knowles, S.C.L.; Wood, M.J.; Alves, R.; Wilkin, T.A.; Bensch, S.; Sheldon, B.C. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol. Ecol. 2011, 20, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Balls, M.J.; Bødker, R.; Thomas, C.J.; Kisinza, W.; Msangeni, H.A.; Lindsay, S.W. Effect of topography on the risk of malaria infection in the Usambara Mountains, Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 400–408. [Google Scholar] [CrossRef]
- Basto, N.; Rodríguez, O.; Marinkelle, C.; Caldasia, R.G.U. Haematozoa in birds from La Macarena National Natural Park (Colombia). Caldasia 2006, 28, 371–377. [Google Scholar]
- Imura, T.; Suzuki, Y.; Ejiri, H.; Sato, Y.; Ishida, K.; Sumiyama, D.; Murata, K.; Yukawa, M. Prevalence of avian haematozoa in wild birds in a high-altitude forest in Japan. Veter Parasitol. 2012, 183, 244–248. [Google Scholar] [CrossRef]
- Cosgrove, C.L.; Wood, M.J.; Day, K.P.; Sheldon, B.C. Seasonal variation in Plasmodium prevalence in a population of blue tits Cyanistes caeruleus. J. Anim. Ecol. 2008, 77, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Aung, S.T.; Wai, S.S.; Htun, L.L.; Bawm, S. Investigation of seasonal distribution of mosquito species in Nay Pyi Taw Area, Myanmar. S. Asian J. Life Sci. 2018, 6, 7–13. [Google Scholar] [CrossRef]
- Figuerola, J. Effects of salinity on rates of infestation of waterbirds by haematozoa. Ecography 1999, 22, 681–685. [Google Scholar] [CrossRef]
- Mendes, L.; Piersma, T.; Lecoq, M.; Spaans, B.; Ricklefs, R.E. Disease-limited distributions? Contrasts in the prevalence of avian malaria in shorebird species using marine and freshwater habitats. Oikos 2005, 109, 396–404. [Google Scholar] [CrossRef]
- Martínez-de La Puente, J.; Eberhart-Phillips, L.J.; Carmona-Isunza, M.C.; Zefania, S.; Navarro, M.J.; Kruger, O.; Hoffman, J.I.; Székely, T.; Figuerola, J. Extremely low Plasmodium prevalence in wild plovers and coursers from Cape Verde and Madagascar. Malar. J. 2017, 16, 243. [Google Scholar] [CrossRef]
- Van Hoesel, W.; Santiago-Alarcon, D.; Marzal, A.; Renner, S.C. Effects of forest structure on the interaction between avian hosts, dipteran vectors and haemosporidian parasites. BMC Ecol. 2020, 20, 47. [Google Scholar] [CrossRef]
- Naing, M.M. Paddy field irrigation systems in Myanmar. In Proceedings of the Regional Workshop on the Future of Large Rice-based Irrigation Systems in Southeast Asia, Ho Chi Minh City, Vietnam, 26–28 October 2005; pp. 120–130. [Google Scholar]
- Thongsripong, P.; Green, A.; Kittayapong, P.; Kapan, D.; Wilcox, B.; Bennett, S. Mosquito Vector Diversity across Habitats in Central Thailand Endemic for Dengue and Other Arthropod-Borne Diseases. PLoS Negl. Trop. Dis. 2013, 7, e2507. [Google Scholar] [CrossRef]
- Wirth, W.W.; Ratanaworabhan, N.C. New species and records of predaceous midges (Diptera: Ceratopogonidae) from rice paddies in Thailand. Pac. Insects 1981, 23, 396–431. [Google Scholar]
- Rosà, R.; Marini, G.; Bolzoni, L.; Neteler, M.; Metz, M.; Delucchi, L.; Chadwick, E.A.; Balbo, L.; Mosca, A.; Giacobini, M.; et al. Early warning of West Nile virus mosquito vector: Climate and land use models successfully explain phenology and abundance of Culex pipiens mosquitoes in north-western Italy. Parasites Vectors 2014, 7, 269. [Google Scholar] [CrossRef]
- Knowles, S.C.; Palinauskas, V.; Sheldon, B.C. Chronic malaria infections increase family inequalities and reduce parental fitness: Experimental evidence from a wild bird population. J. Evol. Biol. 2010, 23, 557–569. [Google Scholar] [CrossRef]
- Loiseau, C.; Sorci, G.; Dano, S.; Chastel, O. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen. Comp. Endocrinol. 2008, 155, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Zichinelli, C.A.; MacGregor-Fors, I.; Quesada, J.; Rohana, P.T.; Romano, M.C.; Valdéz, R.; Schondube, J.E. How Stressed are Birds in an Urbanizing Landscape? Relationships between the Physiology of Birds and Three Levels of Habitat Alteration. Condor 2013, 115, 84–92. [Google Scholar] [CrossRef]
- Lee, H.; Masuda, T.; Yasuda, H.; Hosoi, Y. The pollutant loads from a paddy field watershed due to agricultural activity. Paddy Water Environ. 2014, 12, 439–448. [Google Scholar] [CrossRef]
- Sehgal, R.N. Manifold habitat effects on the prevalence and diversity of avian blood parasites. Int. J. Parasitol. Parasites Wildl. 2015, 4, 421–430. [Google Scholar] [CrossRef]
- Chapa-Vargas, L.; Matta, N.E.; Merino, S. Effects of Ecological Gradients on Tropical Avian Hemoparasites. In Avian Malaria and Related Parasites in the Tropics; Springer International Publishing: Cham, Switzerland, 2020; pp. 349–377. [Google Scholar]
- Chakraborty, D.; Reddy, M.; Tiwari, S.; Umapathy, G. Land Use Change Increases Wildlife Parasite Diversity in Anamalai Hills, Western Ghats, India. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Bonneaud, C.; Sepil, I.; Milá, B.; Buermann, W.; Pollinger, J.; Sehgal, R.N.M.; Valkiūnas, G.; Iezhova, T.A.; Saatchi, S.; Smith, T.B. The prevalence of avian Plasmodium is higher in undisturbed tropical forests of Cameroon. J. Trop. Ecol. 2009, 25, 439–447. [Google Scholar] [CrossRef]
- Poulin, R.; Morand, S. The Diversity of Parasites. Q. Rev. Biol. 2000, 75, 277–293. [Google Scholar] [CrossRef]
- Illera, J.C.; López, G.; García-Padilla, L.; Moreno, Á. Factors governing the prevalence and richness of avian haemosporidian communities within and between temperate mountains. PLoS ONE 2017, 12, e0184587. [Google Scholar] [CrossRef]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef]
- Lacorte, G.A.; Félix, G.M.; Pinheiro, R.R.B.; Chaves, A.V.; Almeida-Neto, G.; Neves, F.S.; Leite, L.O.; Santos, F.R.; Braga, E.M. Exploring the Diversity and Distribution of Neotropical Avian Malaria Parasites – A Molecular Survey from Southeast Brazil. PLoS ONE 2013, 8, e57770. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Cumming, G.S.; Peters, J.L. Host community heterogeneity and the expression of host specificity in avian haemosporidia in the Western Cape, South Africa. Parasitology 2018, 145, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Bulla, L. An Index of Evenness and Its Associated Diversity Measure. Oikos 1994, 70, 167. [Google Scholar] [CrossRef]

| L | A | Bird Species | N (Total/Infected) | Genus | Lineage | GenBank Acc. N | Alt. Host | Alt. Locat. | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MANDALAY | H | Acridotheres burmannicus * | 2/1 | H | AFR084 $ | KM056470 | Gracupica contra | India | [75] |
| Argya gularis | 2/2 | H | TURSTR02 $ | MF565817 | Argya striata | India | [75] | ||
| PF | Aegithina tiphia | 2/0 | - | - | |||||
| Botaurus stellaris | 1/1 | P | IXOMIN02 | KU752579 | Japan | [76] | |||
| Cisticola juncidis | 1/0 | - | - | ||||||
| Merops orientalis | 2/1 | H | MERORI02 $ | MW351709 | |||||
| Ploceus hypoxanthus * | 1/1 | H | PLOHYP01 $ | MW351710 | |||||
| Pycnonotus blanfordi | 2/0 | - | - | ||||||
| Pycnonotus cafer | 2/0 | - | - | ||||||
| U | Cinnyris asiaticus | 1/0 | - | - | |||||
| Passer domesticus | 17/0 | - | - | ||||||
| Pycnonotus blanfordi | 1/0 | - | - | ||||||
| Pycnonotus cafer | 1/0 | - | - | ||||||
| Argya gularis | 3/3 | H | TURSTR02 $ | MF565817 | Argya striata | India | [75] | ||
| MAWLAMYINE | H | Copysychus malabaricus | 1/0 | - | - | ||||
| Phylloscopus fuscatus | 1/0 | - | - | ||||||
| Pycnonotus jocosus | 1/0 | - | - | ||||||
| PF | Acrocephalus aedon | 1/1 | H | ACAED03 $ | MW351708 | ||||
| Aegithina tiphia | 1/1 | P | AEGTIP01 | DQ659581 | Dicrurus leucophaeus | Myanmar | [34,35] | ||
| Orthotomus sutorius | 1/0 | - | - | ||||||
| Phylloscopus fuscatus | 1/0 | - | - | ||||||
| Pycnonotus blanfordi * | 2/1 | Unidentified | - | ||||||
| U | Copsychus saularis | 1/0 | - | - | |||||
| Dicrurus macrocercus | 1/0 | - | - | ||||||
| Lanius cristatus | 2/0 | - | - | ||||||
| Passer montanus | 12/0 | - | - | ||||||
| MYEIK | H | Alcedo atthis | 1/0 | - | - | ||||
| Hirundo tahitica | 1/0 | - | - | ||||||
| Lanius cristatus | 1/0 | - | - | ||||||
| Lonchura punctulata | 8/0 | - | - | ||||||
| Lonchura striata | 14/0 | - | - | ||||||
| Passer montanus | 10/0 | - | - | ||||||
| PF | Acrocephalus sp. | 2/0 | - | - | |||||
| Centropus sinensis | 1/0 | - | - | ||||||
| Cisticola juncidis | 1/0 | - | - | ||||||
| Ficedula albicilla * | 1/1 | H | FIPAR02 $ | EF380197 | Ficedula parva | Myanmar | [35] | ||
| Passer montanus | 2/0 | - | - | ||||||
| U | Acridotheres javanicus | 1/0 | - | - | |||||
| Acridotheres tristis | 12/1 | P | FANTAIL01 | AY714196 | Singapore, Australia | [77,78] | |||
| Columba livia | 1/1 | H | HAECOL1 | AF495554 | India | [79] | |||
| Dicrurus macrocercus | 6/0 | - | - | ||||||
| Halcyon smyrnensis | 1/0 | - | - | ||||||
| Pycnonotus finlaysoni * | 1/1 | P | ORW1 $ | AF254963 | Ficeluda parva, Jynx torquilla, Tephrodornis pondicerianus | Myanmar | [35] |
| Richness | Abundance | Rarefaction ± SE | Shannon–Wiener Index | Evenness | Effective Diversity | |||
|---|---|---|---|---|---|---|---|---|
| (A) Parasite Lineages | Locality | Mandalay | 5 | 9 | 2.28 ± 0.66 | 1.303 | 0.398 | 3.680 |
| Mawlamyine | 2 | 2 | 2.00 ± 0.00 | 0.693 | 0.721 | 2.000 | ||
| Myeik | 4 | 4 | 3.00 ± 0.00 | 1.386 | 0.541 | 4.000 | ||
| Land-use | Urban | 2 | 3 | 2.00 ± 0.00 | 0.637 | 0.641 | 1.889 | |
| Paddy fields | 6 | 6 | 3.00 ± 0.00 | 1.792 | 0.465 | 6.000 | ||
| Hill | 4 | 6 | 2.40 ± 0.58 | 1.242 | 0.481 | 3.464 | ||
| (B) Bird Species | Locality | Mandalay | 11 | 38 | 9.22 ± 1.01 | 1.875 | 0.316 | 6.523 |
| Mawlamyine | 11 | 25 | 11.0 ± 0.00 | 1.859 | 0.308 | 6.422 | ||
| Myeik | 16 | 64 | 9.47 ± 1.47 | 2.200 | 0.308 | 9.026 | ||
| Land-use | Urban | 11 | 42 | 8.07 ± 1.20 | 1.847 | 0.328 | 6.344 | |
| Paddy fields | 14 | 24 | 14.0 ± 0.00 | 2.520 | 0.344 | 12.43 | ||
| Hill | 14 | 61 | 8.54 ± 1.38 | 2.043 | 0.313 | 7.718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muriel, J.; Marzal, A.; Magallanes, S.; García-Longoria, L.; Suarez-Rubio, M.; Bates, P.J.J.; Lin, H.H.; Soe, A.N.; Oo, K.S.; Aye, A.A.; et al. Prevalence and Diversity of Avian Haemosporidians May Vary with Anthropogenic Disturbance in Tropical Habitats in Myanmar. Diversity 2021, 13, 111. https://doi.org/10.3390/d13030111
Muriel J, Marzal A, Magallanes S, García-Longoria L, Suarez-Rubio M, Bates PJJ, Lin HH, Soe AN, Oo KS, Aye AA, et al. Prevalence and Diversity of Avian Haemosporidians May Vary with Anthropogenic Disturbance in Tropical Habitats in Myanmar. Diversity. 2021; 13(3):111. https://doi.org/10.3390/d13030111
Chicago/Turabian StyleMuriel, Jaime, Alfonso Marzal, Sergio Magallanes, Luz García-Longoria, Marcela Suarez-Rubio, Paul J. J. Bates, Htet Htet Lin, Aye Nyein Soe, Khin Swe Oo, Aung Aung Aye, and et al. 2021. "Prevalence and Diversity of Avian Haemosporidians May Vary with Anthropogenic Disturbance in Tropical Habitats in Myanmar" Diversity 13, no. 3: 111. https://doi.org/10.3390/d13030111
APA StyleMuriel, J., Marzal, A., Magallanes, S., García-Longoria, L., Suarez-Rubio, M., Bates, P. J. J., Lin, H. H., Soe, A. N., Oo, K. S., Aye, A. A., Wilbur, N. D., Win, N. N., Soe, Y. T., Linn, K. K., & Renner, S. C. (2021). Prevalence and Diversity of Avian Haemosporidians May Vary with Anthropogenic Disturbance in Tropical Habitats in Myanmar. Diversity, 13(3), 111. https://doi.org/10.3390/d13030111








