Abstract
Sanderia malayensis is a scyphozoan species present in the Indian and Pacific Oceans, ranging from the Suez Canal to Japan. Although this jellyfish is commonly kept in aquariums around the world, there is a knowledge gap regarding its biology and ecology, especially at the polyp stage. In this study, we tested the asexual reproductive activity of S. malayensis at three different temperatures: 10, 15 and 20 °C. Results showed significant increases of polyps at 15 and 20 °C, and a minimum at 10 °C, corresponding with daily budding rates of 6.61% ± 0.92%, 5.85% ± 2.36% and 0.66% ± 0.24%, respectively. Moreover, a second experiment was carried out to report about the ability of S. malayensis to prey on Aurelia solida at the ephyra stage. Unidirectional predation of S. malayensis ephyrae on A. solida and an absence of inverse predation was observed. These results could give new insights on the potential fitness and survival of this species if it will ever invade the Mediterranean Sea.
1. Introduction
In the last decades, jellyfish blooms have increased in various regions of the world [1,2,3,4], including the Mediterranean Sea [4,5,6,7,8]. The Mediterranean Sea hosts about 10% of all known scyphozoan species, six of which (Aurelia spp., Pelagia noctiluca Forskål, 1775, Cotylorhiza tuberculata Macri, 1778, Rhizostoma pulmo Macri, 1778, Marivagia stellata Galil and Gershwin, 2010 and Rhopilema nomadica Galil, Spanier and Ferguson, 1990) may be considered blooming taxa [6]. Jellyfish can assume a dominant role in structuring planktonic communities and large jellyfish aggregations may directly or indirectly interact and interfere with human economic and recreational activities, ecosystem services, public health and local wildlife [6,9,10]. Thus, a thorough knowledge of the ecology and life cycle of these organisms is important.
Up to now, one of the main issues related to the knowledge of scyphozoan jellyfish is the unbalance between the well-studied medusa stage and the poorly studied polyp stage [11,12,13], even if the polyp stage probably represents the key factor for the fitness of many scyphomedusae. The majority of the blooming scyphozoans have a benthic polyp stage that reproduces asexually and in turn produces and releases ephyrae (a process called “strobilation”), which will develop in medusae. The higher the number of polyps, the higher may be the number of ephyrae released and consequently, the number of new medusae, which occasionally may form a bloom. Temperature and food availability have been proposed as the most important environmental factors directly influencing the reproduction rate of the majority of scyphozoan species [14], although their reproductive patterns usually still have to be unveiled.
Sanderia malayensis Goette, 1886 belongs to the family Pelagiidae (Cnidaria, Scyphozoa, Semaeostomeae). At present, the family Pelagiidae contains four genera: Sanderia Goette, 1886 with two species, Pelagia, Péron & Lesueur, 1810 with five species, some of which are doubtful, Chrysaora, Péron & Lesueur, 1810 with sixteen species, some of which are doubtful, and the recently described monospecific Mawia Avian, Ramšak, Tirelli, D’Ambra & Malej, 2016 [15]. Two of them, Sanderia and Chrysaora, have a metagenetic cycle (with polypoid and medusoid stages), Pelagia has an holoplanktonic cycle (with a planula that develops directly into an ephyra) and Mawia still has an unknown life cycle [15]. Sanderia malayensis has been observed for the first time in the Malaysian archipelago [16] and it has later also been recorded in many other areas in the Indo-West Pacific area, such as eastern Africa and Japan [17,18,19,20]. It is also called “Amakusa-Kurage” because it was frequently found in the Amakusa Region on the western coast of Kyūshū island, Japan [18,21]. To the best of our knowledge, this species does not show a clear seasonality as it has been observed during the whole year at low densities [19]. Uchida and Sugiura [20] investigated its life cycle and asexual reproduction for the first time. Subsequent studies described S. malayensis as a species characterized by a large variety of asexual reproductive strategies: its polyps are able to propagate without strobilation, and in some cases, its fragments can regenerate a complete organism [22,23,24,25]. Furthermore, S. malayensis shows unique reproductive traits such as exclusive lateral budding (Modified type 1 budding) [23,25] and a mono-disc strobilation that is typical of the order Rhizostomeae, different from the polydisc strobilation of the order Semaeostomeae, to which S. malayensis belongs [22,26]. Unfortunately, the majority of the experimental studies on S. malayensis have been performed on reared cultures of individuals with no link to their natural environment and no information about their sampling sites [22,23,24,26,27,28]. In fact, very little is known about its natural habitat at the polyp stage. Miyake et al. [12] observed benthic stages of S. malayensis in nature: the authors found S. malayensis polyps attached to vestimentiferan tubeworms (Lamellibrachia satsuma) near submarine smokers in Kagoshima bay. The results of the study of Miyake et al. [12] highlighted that S. malayensis polyps have been found in association with Aurelia aurita (Linnaeus, 1758) polyps on the same substratum. Once transferred to the laboratory on a plate, starved A. aurita polyps preyed on S. malayensis polyps and strongly reduced their number [12].
Submarine smokers may represent a stable environment for S. malayensis polyps to grow in, while S. malayensis medusae have a wide geographical distribution, from Japan to northern Australia (where Sanderia pampinosus Gershwin & Zeidler, 2008 is present) to the north-east African coasts. Furthermore, since S. malayensis is present in the Red Sea and the Suez Canal [15,29], it could become a potential lessepsian invasive species in the Mediterranean Sea.
Based on the current knowledge gap in the ecology of S. malayensis, the purpose of the present work was to verify the effect of temperature on the asexual reproductive capacity of this species. Moreover, we also report on an observational study, carried out in parallel to the first experiment, on the ability of S. malayensis to prey on Aurelia solida Browne, 1905 at the ephyra stage.
2. Materials and Methods
2.1. Cultivation of Sanderia malayensis and Aurelia solida Polyps
Sanderia malayensis polyps used in this study were kindly provided by staff of the Wien Zoo (Schönbrunner Tiergarten, Wien, Austria) in December 2017, and Aurelia solida polyps were provided by the researchers of the NIB (National Institute of Biology, Slovenia), and both were cultured at the laboratory of OGS (National Institute of Oceanography and Applied Geophysics of Trieste, Italy). While A. solida polyps came from the harbor of Koper (Slovenia), unfortunately, we do not know the origin of the S. malayensis polyps. All polyps have been placed in 25 mL borosilicate-glass bowls and were stored in a temperature-controlled chamber at a stable temperature of 20 ± 0.5 °C in filtered seawater (salinity 35). Seawater was collected at a depth of 15 m at the LTER_EU_IT_056 station (45.70083° N, 13.71° E; depth 18 m) in the Gulf of Trieste and filtered through hydrophilic PVDF membrane filters, pore size 0.22 µm, Ø 142 mm, (Durapore® GVWP14250, Millipore, Ireland). Polyps have been fed once a week with cultured Artemia sp. nauplii. The medium of Artemia sp. has been enriched with Isochrysis sp. in order to integrate vitamins [23] and make nauplii a more complete food for jellyfish. Algae were grown in culture medium B [30] at 20 °C under cool white fluorescent light (80 μmoL photons m−2 s−1) with a light–dark cycle of 12/12 h. Polyps have been maintained at the same controlled conditions (20 °C and light–dark cycle of 12/12 h) of algae for four months before the beginning of the experiments (April 2018).
2.2. Sanderia Budding at Different Temperatures
Polyps were split in 12 borosilicate-glass bowls (25 mL volume) filled with filtered seawater and then randomly assigned to one of the 3 experimental temperatures: 10, 15 and 20 °C. The number of polyps for each bowl was variable (mean 20 ± 13 standard deviation (SD)) due to the difficulties in polyps transferring from the bowls of cultivation (where many polyps were living together) to the experimental bowls. Salinity was maintained constant at 35 for each treatment. Cells were exposed to a light–dark cycle of 12/12 h (light intensity 50 μmoL photons m−2 s−1 obtained using cool white fluorescence lamps).
Polyps were overfed three times a week with Artemia sp. nauplii to ensure equal nutrition for each treatment group, as suggested by Purcell et al. [31], thus excluding hunger as a limiting factor for growth and leaving temperature as the only source of variation between treatments. Cells were cleaned one hour after adding food, removing all the surviving Artemia sp. to avoid any contamination and reduce bacteria biofilm formation and microalgae growth. The number of polyps and mouths (new polyps still undivided from the body of the parent organism), types of budding, strobilation and presence of ephyrae (immediately removed when present) were recorded daily. The daily increase in number of polyps was calculated as follows:
where “N final” and “N initial” are the number of polyps/mouths at the end and at the beginning of the experiment respectively, and “Total Days” is the duration in days of the experiment. The daily budding rate was expressed as percentage, considering the formation of one new polyp per day as a budding rate of 100%.
Budding types were identified following the classification introduced by Adler and Jarms [23].
2.3. Predation: S. malayensis vs. A. solida Ephyrae
The experimental design was composed of five cells filled with 20 mL filtered seawater: in three replicates, one starved S. malayensis ephyra was incubated with ten A. solida ephyrae, and the remaining two cells were used to incubate ten A. solida ephyrae and ten S. malayensis ephyrae, respectively. The latter were used to evaluate potential cannibalism.
After the introduction of the ephyrae, cells were monitored every 10 min for 2 h. Predation events consisted in “stinging and ingestion” and “stinging without ingestion” (the poisoned ephyra escaped and died later on).
2.4. Statistical Analysis
In order to assess how different temperature levels affected the reproductive success of S. malayensis, we estimated two different Generalized Linear Mixed Models (GLMMs) [32] using a Penalized Quasi-likelihood (PQL) method by means of the ‘MASS’ R package [33]. Their specifications were as follows: the number of polyps and the number of mouths were selected as the response variables of two distinct GLMMs, while treatment (a factor with three levels: T10, T15 and T20) and the number of days since the experiment started (considered in the models as a quantitative, discrete variable) were both included as explanatory variables in the two models. To determine whether the increase in number of polyps and the increase in the number of mouths changed with the length of the experiment at different temperatures, the interaction term treatment x days was also estimated. In order to account for the different number of polyps in each bowl and for auto-correlated longitudinal data available (repeated measures for the bowls within treatment along the time series), each model estimated a random intercept and a random slope for the group of replicates within each treatment. A Quasi-Poisson distribution (with a logarithmic transformation as the link function) was used to model the error structure since both models showed overdispersion when tested for the Poisson distribution. The models’ coefficients of determination (R2) were estimated using the ‘r2glmm’ package [34]. The above-mentioned statistical models were developed under the R statistical environment v.3.6.3 [35]. In the Supplementary S1, model formulas, model estimated coefficients, effect sizes and diagnostics are described in detail.
To investigate statistical differences in daily budding rate, calculated over the 39 days of the experiment (as calculated applying Equation (1)), at the different temperatures (treatment), we performed a one-way permutational multivariate analysis of variance (PERMANOVA, [36]) on the dataset, where Euclidean distances were calculated among % budding rate values. The test was performed using 9999 permutations and considering a standard α-level = 0.05. When the treatment resulted significant, a posteriori pairwise comparisons were performed among treatments’ levels via PERMANOVA t statistic with 9999 permutations. Non-parametric analyses were performed using PRIMER v6 computer program [37], including the add-on package PERMANOVA+.
3. Results
3.1. Budding Rate at Different Temperatures
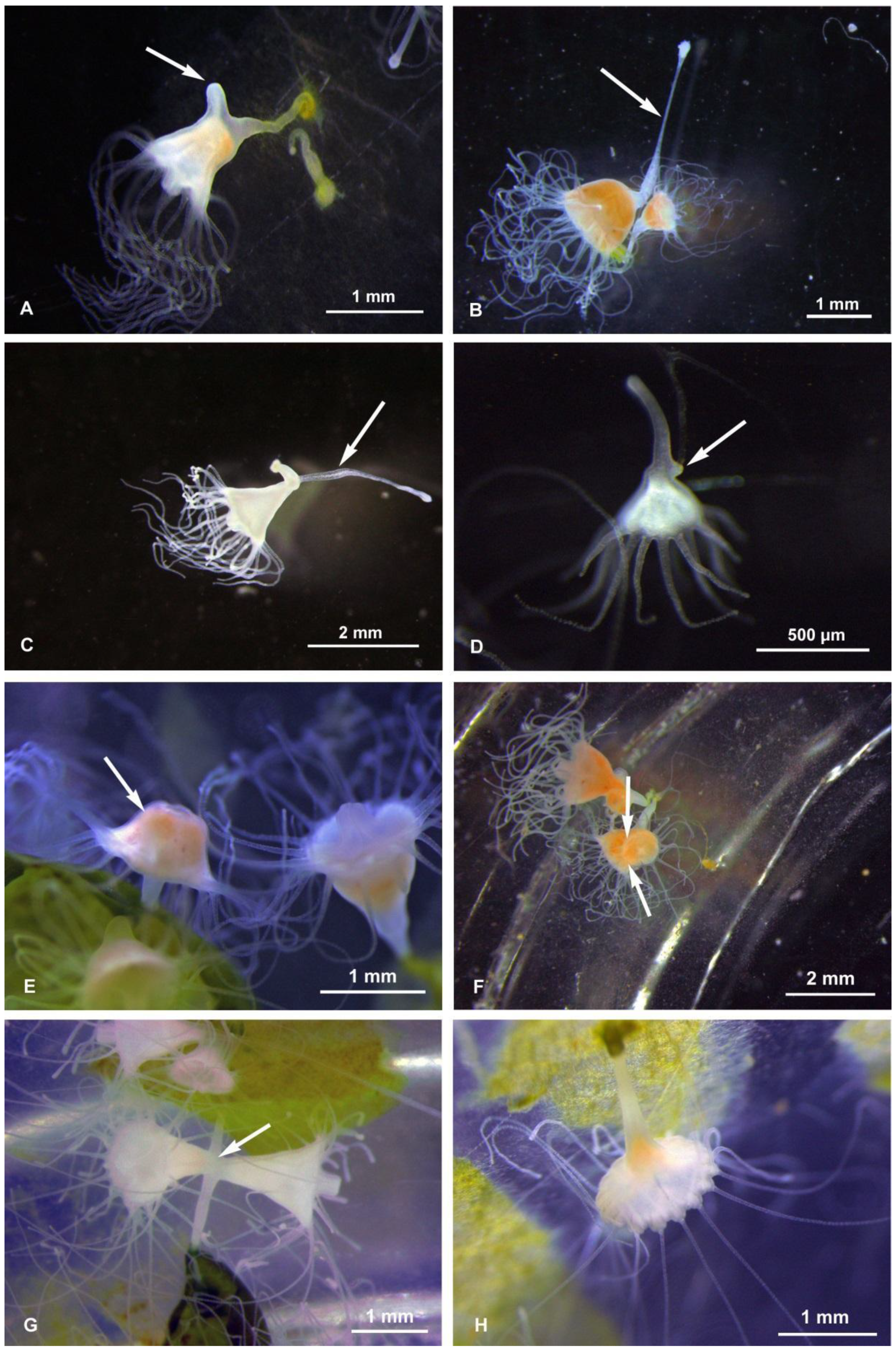
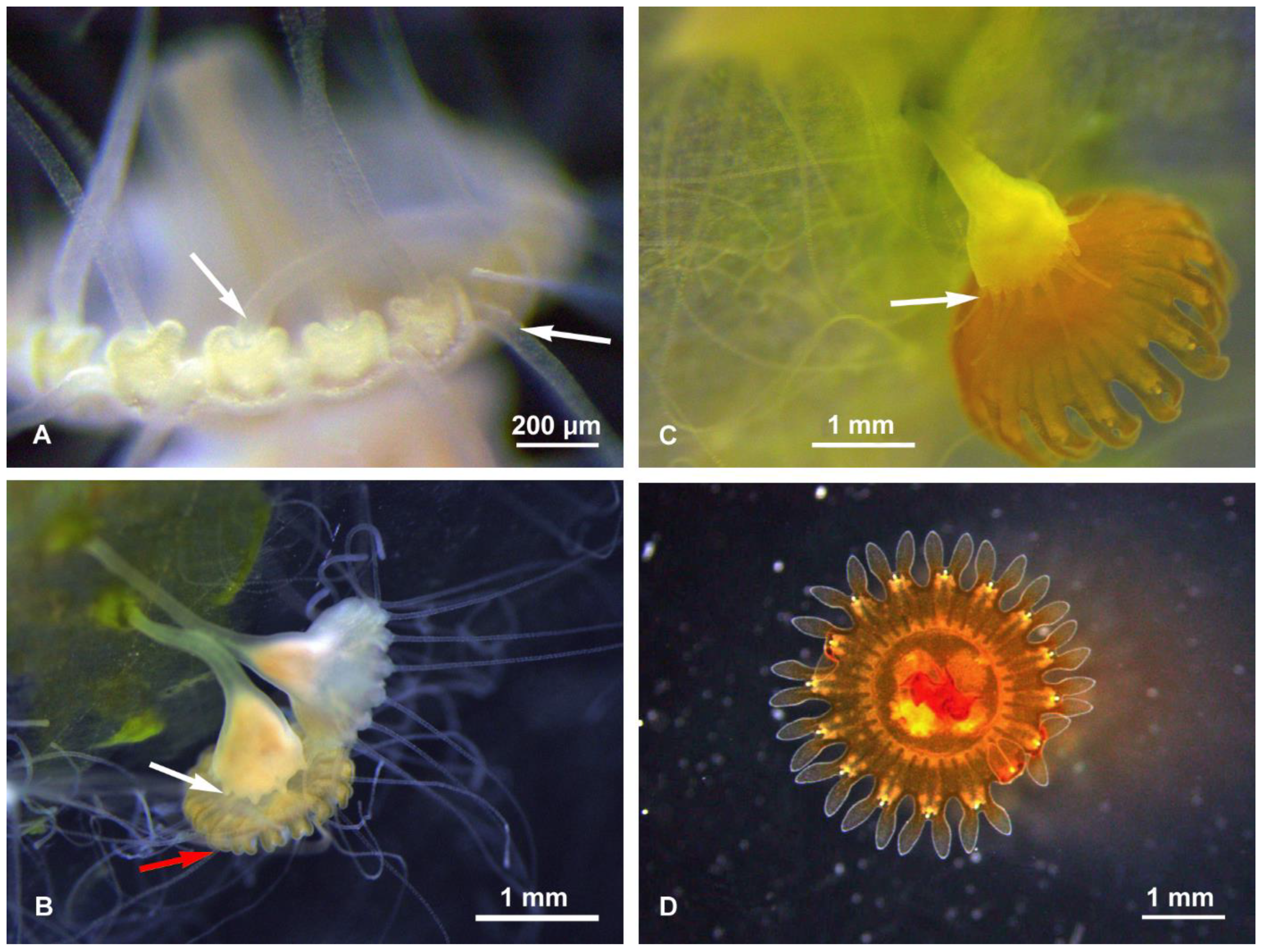
The budding types were observed and photographed both during the maintenance period preceding the experiments (January–April 2018) and during the experiments themselves. At every temperature, we observed the following budding types: Type 1–2, ‘Sanderia-type’ lateral budding (Figure 1A,B), Type 4, reproduction from parts of stolons/stalks, Type 6b, motile bud-like tissue particles (Figure 1C), Type 6c, planuloids originating from tentacles tips/tentacle pieces (Figure 1D), Type 7, regenerations from the gastric cavity (Figure 1E), Type 8, longitudinal fission, either intra-tentacular (starting from the oral disk) and from the bottom (we observed that this consists in the breaking of the polyp; thanks to two stolons attached, the polyp stretches itself between its original calix and the new stolon. During the process, the oral disc is stretched like a band. The tentacles hang from this band and are equally distributed between the two new polyps during the break) (Figure 1F,G), and the Type 9, strobilation (monodisk in Sanderia malayensis) (Figure 2).
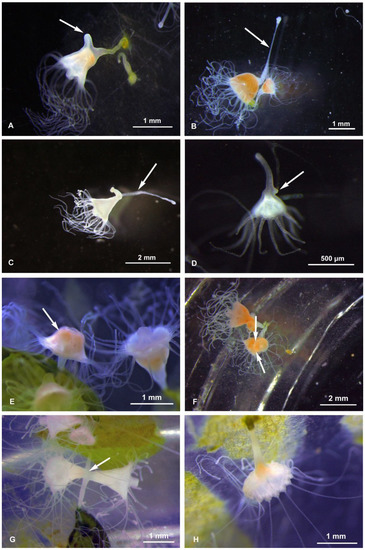
Figure 1.
Sanderia malayensis polyps. (A) Budding type 2, with a developing stolon (arrow), (B) budding type 2, almost complete detachment of the “parental” polyp from the “child” polyp (on the right), by means of the tension exerted by the stolon (arrow), (C) budding type 4, the old stolon takes on a clavate shape while creating the choke for separation from the new stolon (arrow), (D) budding Type 6b, a freely floating bud that has already developed the tentacles, and a developing new stolon (arrow), (E) budding type 7, inverted polyp (arrow) exposing the gastroderm, (F) budding type 8, advanced tentacular cleavage with the polyp possessing two heads (arrows indicate the area of constriction between them), (G) budding type 8, two polyps that are completing the tentacular budding—in the center is the still common portion (arrow), (H) budding type 9 (strobilation), polyp (scyphistoma) starting strobilation.
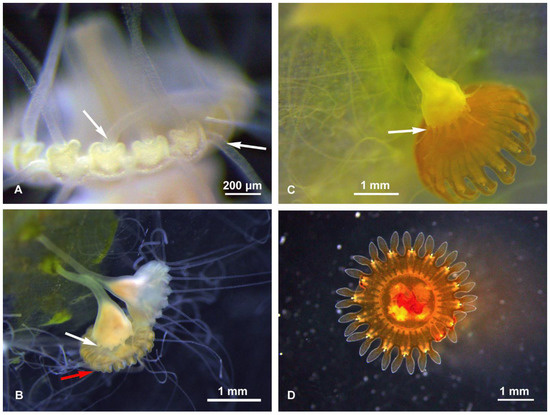
Figure 2.
Sanderia malayensis, strobilation and ephyra. (A) Detail of the oral disc margin, with the marginal lappets of the future ephyra in formation, and polyp tentacles still present (arrows). (B) Two scyphistoma in different strobilation stages, early (top) and more advanced (bottom), where the polyp is regenerating its tentacles (white arrow) even before those present on the edge of the ephyra are totally reabsorbed (red arrow). (C) Strobilation in final phase, polyp with new tentacles (arrow), and ephyra close to detachment and already colored. (D) Just released ephyra, sub-umbrellar view.
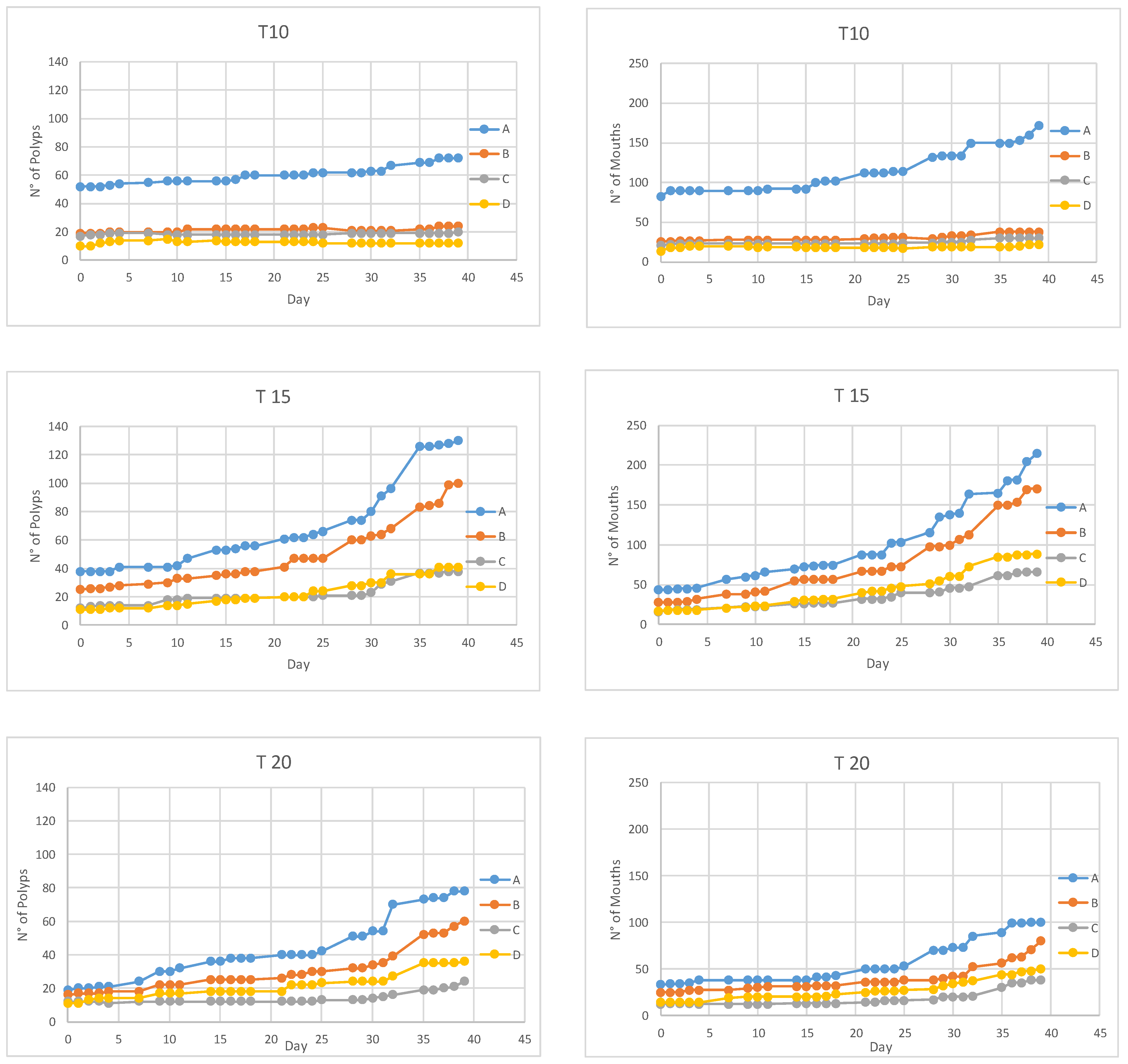
Polyps maintained at 10 °C, over 39 days, reproduced by budding at a rate of 0.66% ± 0.24% per day (mean ± SD) (Figure 3). The total number of mouths at the beginning of the experiment was 144, while, at the end of the experiment, we counted 262 mouths, representing a mean increase of 1.6% ± 0.8% per day (Figure 3). Overall, seven polyps died (Table 1) and 12 ephyrae were released.
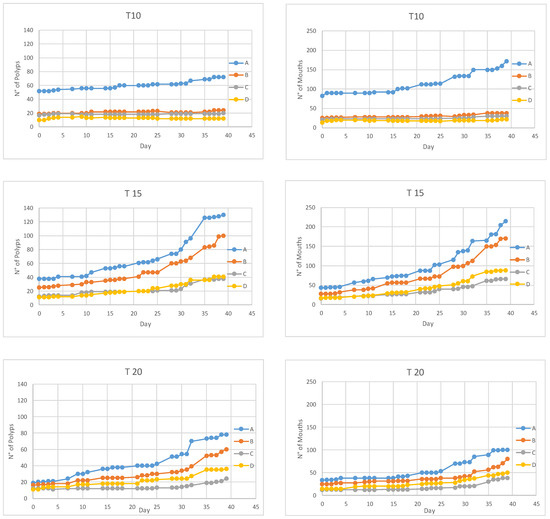
Figure 3.
Total number of polyps (left) and mouths (right) observed during the 39 days of incubations at 10 °C (top), 15 °C (middle) and 20 °C (bottom). (A–D) The four replicates for each temperature.

Table 1.
Number of polyps, mouths, dead polyps and released ephyrae counted at the beginning and at the end of incubation at 10, 15 and 20 °C.
At 15 °C, the budding rate was 6.61% ± 0.92% per day (Figure 3, Table 1). The number of mouths was 105 and 541 at the beginning and at the end of the experiment respectively (Figure 3), having an increase of 10.5% ± 2.1% per day. Only one polyp died and eight ephyrae were released.
At 20 °C, the budding rate was 5.85% ± 2.36% per day (mean ± SD) (Figure 3, Table 1). Overall, over 39 days, the number of mouths increased at a rate of 5.7% ± 0.6% per day (Table 1). Only one polyp died and four ephyrae were released.
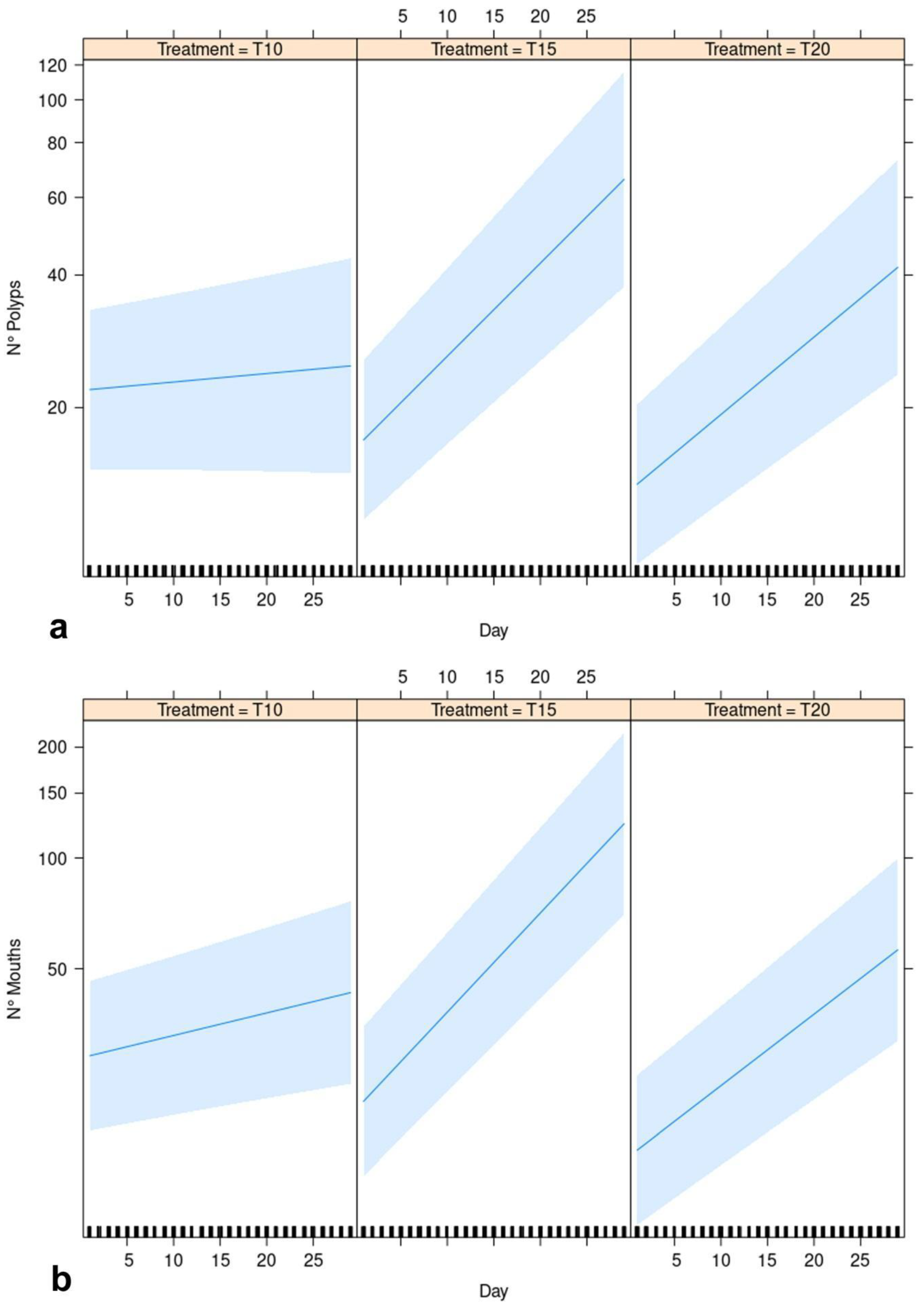
Results of the GLMM provided positive and significant relationships for the three levels of the treatment and the number of polyps as a function of the observational time, with significantly different slopes of the regression lines in the three levels of the treatment factor (Table 2, Figure 4a, Supplementary S1). The estimated regression slope for T15 (betaT15xDay = 0.044) resulted higher than for T20 (betaT20xDay = 0.036). The estimated model goodness of fit is higher than 90% (R2 = 0.91). Similar results were observed for the GLMM considering the number of mouths as the response variable (Table 2, Figure 4b, Supplementary S1): the estimated regression slope for T15 (betaT15xDay = 0.047) resulted higher than for T20 (betaT20xDay = 0.030) and the R2 resulted equal to 0.94.

Table 2.
Quasi-Poisson Generalized Linear Mixed Models (GLMM) and statistical significance of the fixed effects in explaining the variation of the number of polyps and the number of mouths. Both the main explanatory terms and their interaction resulted highly significant in the models. R2 (N° of Polyps) = 0.91. *** p < 0.001, R2 (N° of Mouths) = 0.94. Chisq = Χ2, Df = degrees of freedom. *** p < 0.001.
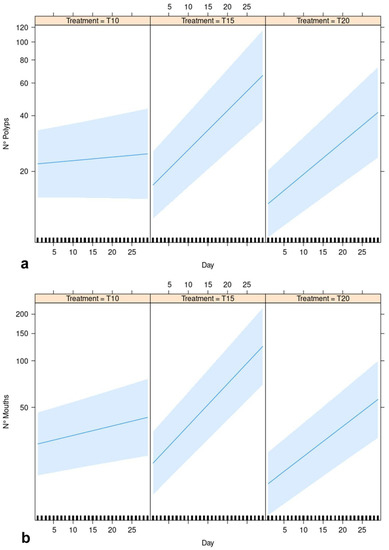
Figure 4.
GLMM effect plot of estimated linear responses of (a) No. of polyps and (b) No. of mouths (both as response variables) for each level of the treatment (T10, T15 and T20).
Temperature also significantly affected budding rate in S. malayensis (Pseudo F(2) = 19.43, p < 0.005, Table 3), and, after post-hoc tests, significant differences between T10 and T15 (t = 12.44, p < 0.05) and T10 and T20 (t = 4.378, p < 0.05) were observed. No significant differences were found between T15 and T20 (t = 0.60, p = 0.687).

Table 3.
One-way permutation analysis of variance (PERMANOVA) results on % budding rates. Statistical significance for the treatment was tested under a null model (9999 permutations). MS: mean squares. In bold, p-value < 0.005.
3.2. Predation: S. malayensis vs. A. solida Ephyrae
Predation of S. malayensis ephyrae on Aurelia solida ephyrae has been observed in all replicates with a maximum of four events of predation (Table 4, please see the clip in Video S2). Conversely, no predation of A. solida ephyrae on S. malayensis ephyrae was observed. In cells where only one species of jellyfish was incubated, no events of intraspecific predation were observed.

Table 4.
Interaction between S. malayensis and A. solida ephyrae. T0–T120 (min). * Indicates predation without ingestion.
4. Discussion
Sanderia malayensis was discovered over a century ago and it is now commonly held in aquaria around the world [38]. Observations in nature of this species are rare and spatially far from each other. Its distribution area extends from the Red Sea to the Western Pacific Ocean (Japan). Recently, a second species was added to the genus Sanderia as a few specimens from Western Australia have been described as a new species, namely S. pampinosus [39]. This discovery was based on morphological differences but Morandini and Gul [28] assumed that those differences were just due to the age of the organisms, rejecting the new species hypothesis. Previous studies on cultured polyps of S. malayensis focused on various budding types [22,23,25], ephyra morphology [26,27] and the development of the gastrovascular system in juvenile medusae [19]. Aside from laboratory observations, data in the natural environment are quite uncommon, at present there is only one observation of S. malayensis bloom [40] (even if this observation is doubtful as the photo presented in the paper is not a Sanderia specimen). So, very little is known about its biology and ecology, especially at the polyp stage. For the considered levels of the treatment, our results show that cultured polyps of Sanderia malayensis have the highest rate of asexual reproduction and the lowest mortality rate (0.3%) at 15 °C. Similarly, the number of mouths follows the same trend, with the highest presence at 15 °C, followed by 20 and 10 °C, respectively. These results suggest a close resemblance with those obtained by Miyake et al. [12] at 16 °C and 34 salinity, supporting the idea that the temperature optimum for the polyp stage reproduction of this species lies in the 15–20 °C range, which requires further experiments. However, even if the restrictiveness of our study allows us only to hypothesize, the suitable habitat for S. malayensis polyps could consist of shore bottoms at intermediate depths, where water temperature is more stable than in coastal surface waters and warmer than in the deeper ones. At the same time, S. malayensis could be found deeper near heat sources such as fumaroles. Furthermore, the ability to reproduce also at higher temperatures (20 °C) could justify the propagation of this species all the way from Africa to Japan. Moreover, since the only observation of S. malayensis polyps has been made near fumaroles, an ecosystem where abiotic parameters do not show significant variations, it could suggest a preference of this species to live in stable conditions. In fact, the environmental parameters of the deeper area of Kagoshima Bay have been reported to be stable throughout the year (Oki 2000, in Miyake et al. [12]). If this preference is confirmed, coastal waters may present conditions that are too variable for this species.
Its apparent rarity may also be caused by the fact that S. malayensis is the only species of the family Pelagiidae (order Semaeostomeae) that presents a mono-disc strobilation (a common characteristic of part of the order Rhizostomeae) [15] and shows higher asexual reproduction and a dominant polypoid phase with scarce production of medusae [41], making the adult stage difficult to observe in nature.
Strobilation is another fundamental factor in the jellyfish lifecycle, and S. malayensis ephyrae have been observed just once in the Misaki area in Japan in 1973, at a recorded temperature between 18.8 and 21.7 °C [26]. This study showed the highest strobilation at 10 °C, followed by 15 and 20 °C. These results could be linked to the stress of S. malayensis in consequence to the decrease of temperature during the experiment, compared to the acclimation temperature (20 °C). In fact, the shift from the acclimation rearing tanks’ temperature (20 °C) to 10 and 15 °C may have triggered early strobilation as occurs in other scyphozoans [42]. Miyake et al. [12] observed S. malayensis strobilation when temperature increased, and they concluded that strobilation could have been a response to environmental temperature changes.
The second experiment of this study showed the predation of S. malayensis ephyrae on Aurelia solida ephyrae but we did not observe the opposite (in Supplementary Video S2). A possible explanation relies in the fact that newly released S. malayensis ephyrae were much larger (3.67 mm diameter) than Aurelia (2–2.5 mm diameter) and S. malayensis could behave like many species of Chrysaora (genus of the same family of Sanderia) which are voracious consumers of gelatinous zooplankton [43]. It is interesting to remember that at the polyp stage, Miyake et al. [12] observed a unidirectional predation of Aurelia aurita on S. malayensis. Predation was so intense that Sanderia malayensis polyps disappeared where Aurelia was present. S. malayensis was otherwise more able to survive on vertical surfaces or other substrates avoided by Aurelia, like the vestimentiferan worm tubes, where its own characteristics (the stolon anatomy) made this species a better competitor against Aurelia.
Predation at polyp and ephyra stages could be an important factor regulating the propagation of this species. If S. malayensis possesses a polypoid-dominating life cycle and this stage may be heavily predated by polyps of other scyphozoans, as seen by Miyake et al. [12], this low competitiveness could justify the scarcity of presence of this species, which may be confined in particular areas (i.e., vestimentiferan tubeworms of fumaroles). On the other hand, its greater ability to settle on substrates commonly avoided by other polyp species can result in a greater ability to colonize different environments. At the same time, even if S. malayensis is not reported among invasive jellyfish species [44], as it is widespread from the Red Sea to Japan [15], it could become a potential lessepsian invasive species in the Mediterranean Sea up to the Adriatic Sea, where it could compete with the species of the genus Aurelia (A. solida, the most frequent, A. relicta Scorrano, Aglieri, Boero, Dawson & Piraino, 2017 and A. coerulea von Lendenfeld, 1884 [45]). This hypothesis is supported by our results that showed how S. malayensis polyps can survive even at 10 °C, a temperature often recorded in winter in the shallower areas of the Adriatic Sea.
Supplementary Materials
The following supporting information are available online at https://www.mdpi.com/1424-2818/13/2/37/s1, S1: GLMM description, formula, models’ estimated coefficients and effect sizes. Video S2: predation of Sanderia malayensis ephyrae on Aurelia solida ephyrae.
Author Contributions
Conceptualization, M.A., V.T., A.B., and M.P.; Investigation, M.P., A.B., and A.G.; supervision, M.A. and V.T.; formal analysis, V.T., G.B., and E.T.; writing—original draft preparation, G.M., V.M., M.A., G.B., and V.T.; writing—review and editing, M.A., V.T., and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in (OGS-NODC: Italian National Oceanographic Data Center) at (http://nodc.inogs.it/doi/documents/Sanderia_asexualreproduction_dataset.xlsx), reference number (doi: 10.6092/8ebc5377-a516-4bf2-a4ae-4834b1503478).
Acknowledgments
We wish to thank A. Weissenbacher, R. Hallbauer, V. Bartsch (Schönbrunner Tiergarten, Wien, Austria) and A. Malej (Marine Biology Station, National Institute of Biology, Piran, Slovenia) for providing the polyps of Sanderia malayensis and Aurelia solida, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Licandro, P.; Conway, D.V.P.; Daly Yahia, M.N.; Fernandez de Puelles, M.L.; Gasparini, S.; Hecq, J.H.; Tranter, P.; Kirby, R.R. A blooming jellyfish in the northeast Atlantic and Mediterranean. Biol. Lett. 2010, 6, 688–691. [Google Scholar] [CrossRef]
- Lynam, C.P.; Lilley, M.K.S.; Bastian, T.; Doyle, T.K.; Beggs, S.E.; Hays, G.C. Have jellyfish in the benefited from climate change and overfishing? Glob. Chang. Biol. 2011, 17, 767–782. [Google Scholar] [CrossRef]
- Brotz, L.; Cheung, W.W.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: Trends in large marine ecosystems. Hydrobiologia 2012, 690, 3–30. [Google Scholar] [CrossRef]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Nat. Acad. Sci. USA 2013, 110, 1000–1005. [Google Scholar] [CrossRef]
- Kogovšek, T.; Bogunović, B.; Malej, A. Recurrence of bloom-forming scyphomedusae: Wavelet analysis of a 200-year time series. Hydrobiologia 2010, 645, 81–96. [Google Scholar] [CrossRef]
- D’Ambra, I.; Malej, A. Scyphomedusae of the Mediterranean: State of the art and future perspectives. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 81–94. [Google Scholar] [CrossRef]
- Gravili, C. Jelly surge in the Mediterranean Sea: Threat or opportunity? Med. Mar. Sci. 2020, 21, 11–21. [Google Scholar] [CrossRef]
- Pierson, J.; Camatti, E.; Hood, R.; Kogovšek, T.; Lučić, D.; Tirelli, V.; Malej, A. Mesozooplankton and gelatinous zooplankton in the face of environmental stressors. In Coastal Ecosystems in Transition: A Comparative Analysis of the Northern Adriatic and Chesapeake Bay; Malone, T., Malej, A., Faganeli, J., Eds.; American Geophysical Union: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Graham, W.M.; Gelcich, S.; Robinson, K.L.; Duarte, C.M.; Brotz, L.; Purcell, J.E.; Pitt, K.A. Linking human well-being and jellyfish: Ecosystem services, impacts, and societal responses. Front. Ecol. Environ. 2014, 12, 515–523. [Google Scholar] [CrossRef]
- Palmieri, M.G.; Barausse, A.; Luisetti, T.; Turner, K. Jellyfish blooms in the Northern Adriatic Sea: Fishermen’s perceptions and economic impacts on fisheries. Fish. Res. 2014, 155, 51–58. [Google Scholar] [CrossRef]
- Mills, C.E. Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobiologia 2001, 451, 55–68. [Google Scholar] [CrossRef]
- Miyake, H.; Hashimoto, J.; Chikuchishin, M.; Miura, T. Scyphopolyps of Sanderia malayensis and Aurelia aurita attached to tubes of vestimentiferan tubeworm (Lamellibrachia satsuma) at submarine fumaroles in Kagoshima Bay. Mar. Biotechnol. 2004, 6, S174–S178. [Google Scholar] [CrossRef]
- Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Purcell, J.E.; Uye, S.I.; Robinson, K.; Madin, L.P.; Mianzan, H.; Sutherland, K.R.; Uye, S.; et al. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 2013, 11, 91–97. [Google Scholar] [CrossRef]
- Lucas, C.H.; Graham, W.M.; Widmer, C. Jellyfish life histories: Role of polyps in forming and maintaining scyphomedusa populations. Adv. Mar. Biol. 2012, 63, 133–196. [Google Scholar] [CrossRef] [PubMed]
- Jarms, G.; Morandini, A.C.; Schmidt-Rhaesa, A.; Giere, O.; Straehler-Pohl, I. World Atlas of Jellyfish: Scyphozoa except Stauromedusae; Jarms, G., Morandini, A.C., Eds.; Dölling und Galitz Verlag: Hamburg, Germany, 2019; pp. 324–377. [Google Scholar]
- Goette, A.; der Medusen welche, V. Stabsarzt Auf S.M.S. “Prinz Adalbert” Gesammelt Wurden. Sitzungsberichte der Preussischen Stabsarzt auf S.M.S. “Prinz Adalbert” Gesammelt Wurden; Sitzungsberichte der Preussischen Königlichen Akademie der Wissenschaften: Berlin, Germany, 1886; Volume 2, pp. 831–837. [Google Scholar]
- Mayer, A.G. Medusae of the World: The Scyphomedusae; Carnegie Institution of Washington: Washington, DC, USA, 1910; Volume III, p. 735. [Google Scholar]
- Uchida, T. Distribution of Scyphomedusae in Japanese and its adjacent waters. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1954, 12, 209–219. [Google Scholar]
- Kramp, P.L. Synopsis of the medusae of the world. J. Mar. Biol. Assoc. UK 1961, 40, 7–382. [Google Scholar] [CrossRef]
- Fenner, P.J.; Williamson, J.A. Worldwide deaths and severe envenomation from jellyfish stings. Med. J. Australia 1996, 165, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T. Medusae in the vicinity of the Amakusa Marine Biological Station. Bull. Biogeogr. Soc. Japan 1938, 8, 143–149. [Google Scholar]
- Uchida, T.; Sugiura, Y. On the polyp of the Scyphomedusa, Sanderia malayensis and its reproduction. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1978, 21, 279–286. [Google Scholar]
- Adler, L.; Jarms, G. New insights into reproductive traits of scyphozoans: Special methods of propagation in Sanderia malayensis Goette, 1886 (Pelagiidae, Semaeostomeae) enable establishing a new classification of asexual reproduction in the class Scyphozoa. Mar. Biol. 2009, 156, 1411–1420. [Google Scholar] [CrossRef]
- Straehler-Pohl, I.; Widmer, C.L.; Morandini, A.C. Characterizations of juvenile stages of some semaeostome Scyphozoa (Cnidaria), with recognition of a new family (Phacellophoridae). Zootaxa 2011, 2741, 1–37. [Google Scholar] [CrossRef]
- Schiariti, A.; Morandini, A.C.; Jarms, G.; von Glehn Paes, R.; Franke, S.; Mianzan, S. Asexual reproduction strategies and blooming potential in Scyphozoa. Mar. Ecol. Prog. Ser. 2014, 510, 241–253. [Google Scholar] [CrossRef]
- Uchida, T.; Sugiura, Y. On the ephyra and postephyra of Semaeostome medusa, Sanderia malayensis Goette, 1886. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1975, 19, 879–881. [Google Scholar]
- Straehler-Pohl, I.; Jarms, G. Identification key for young ephyrae: A first step for early detection of jellyfish blooms. In Jellyfish Blooms: New Problems and Solutions; Purcell, J.E., Dror, A., Eds.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Morandini, A.C.; Gul, S. Rediscovery of Sanderia malayensis and remarks on Rhopilema nomadica record in Pakistan (Cnidaria: Scyphozoa). Pap. Avulsos Zool. 2016, 56, 171–175. [Google Scholar] [CrossRef]
- Browne, E.T. Zoological results of the Cambridge Expedition to the Suez Canal, 1924. Trans. Zool. Soc. Lond. 1926, 22, 105–115. [Google Scholar] [CrossRef]
- Agatha, S.; Strüder-Kypke, M.C.; Beran, A. Morphologic and genetic variability in the marine planktonic ciliate Laboea strobila Lohmann, 1908 (Ciliophora, Oligotrichia), with notes on its ontogenesis. J. Eukaryot. Microbiol. 2004, 51, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J.E.; Atienza, D.; Fuentes, V.; Olariaga, A.; Tilves, U.; Colahan, C.; Gili, J.M. Temperature effects on asexual reproduction rates of scyphozoan species from the northwest Mediterranean Sea. Hydrobiologia 2012, 690, 169–180. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S., 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Jaeger, B. R2glmm: Computes R Squared for Mixed (Multilevel) Models. R Package Version 0.1, 2. Available online: https://github.com/bcjaeger/r2glmm (accessed on 12 December 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E: Plymouth, UK, 2006; p. 192. [Google Scholar]
- Hall, H.; Warmolts, D. The role of public aquariums in the conservation and sustainability of the marine ornamentals trade. In Marine Ornamental Species: Collection, Culture & Conservation; Cato, J.C., Brown, C.L., Eds.; Iowa State Press: Ames, IA, USA, 2003; ISBN 978-0813829876. [Google Scholar]
- Gershwin, L.A.; Zeidler, W. Two new jellyfishes (Cnidaria: Scyphozoa) from tropical Australian waters. Zootaxa 2008, 1764, 41–52. [Google Scholar] [CrossRef]
- Xian, W.; Kang, B.; Liu, R. Jellyfish blooms in the Yangtze Estuary. Science 2005, 307, 41. [Google Scholar] [CrossRef]
- Gershwin, L.A. Jellyfish: A Natural History; Ivy Press: East Sussex, UK, 2016; p. 224. [Google Scholar]
- Pascual, M.; Fuentes, V.; Canepa, A.; Atienza, D.; Gili, J.M.; Purcell, J.E. Temperature effects on asexual reproduction of the scyphozoan Aurelia aurita sl: Differences between exotic (Baltic and Red seas) and native (Mediterranean Sea) populations. Mar. Ecol. 2014, 36, 994–1002. [Google Scholar] [CrossRef]
- Morandini, A.C.; Silveira, F.L.D.; Jarms, G. The life cycle of Chrysaora lactea Eschscholtz, 1829 (Cnidaria, Scyphozoa) with notes on the scyphistoma stage of three other species. Hydrobiologia 2004, 530, 347–354. [Google Scholar] [CrossRef]
- Bayha, K.M.; Graham, W.M. Nonindigenous marine jellyfish: Invasiveness, invasibility, and impacts. In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2014; pp. 45–77. ISBN 978-94-007-7015-7. [Google Scholar]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2016, 180, 243–267. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).