Floral Patches and Their Impact on Pollinator Attraction and Yield Production on Cucurbita maxima Var. Paine in Central Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Study Site
2.3. Experimental Design
2.4. Characterization of Pollinator Assemblages
2.5. Quality and Quantity of Crop Yield
2.6. Data Analysis
3. Results
3.1. Characterization of Pollinator Assemblages
3.2. Quantity and Quality of C. maxima Production
3.3. Pollinators and Their Impact on C. maxima Production
4. Discussion
4.1. Effect of the Addition of Flower Patches on the Diversity and Frequency of Pollinator Visits
4.2. Effect of Pollinators on Quality and Quantity in the Production of C. maxima
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garibaldi, L.A.; Carvalheiro, L.G.; Vaissière, B.E.; Gemmill-Herren, B.; Hipólito, J.; Freitas, B.M.; Ngo, H.T.; Azzu, N.; Sáez, A.; Åström, J.; et al. Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 2016, 351, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Greenleaf, S.S.; Kremen, C. Wild bees enhance honeybees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. USA 2006, 103, 13890–13895. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.D.; Reiners, S.; Nault, B.A. Pollination Services Provided by Bees in Pumpkin Fields Supplemented with Either Apis mellifera or Bombus impatiens or Not Supplemented. PLoS ONE 2013, 8, e69819. [Google Scholar] [CrossRef] [PubMed]
- Bartomeus, I.; Potts, S.G.; Steffan-Dewenter, I.; Vaissière, B.E.; Woyciechowski, M.; Krewenka, K.M.; Tscheulin, T.; Roberts, S.P.M.; Szentgyörgyi, H.; Westphal, C.; et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ 2014, 2, e328. [Google Scholar] [CrossRef]
- Tuell, J.K.; Isaacs, R. Weather During Bloom Affects Pollination and Yield of Highbush Blueberry. J. Econ. Entomol. 2010, 103, 557–562. [Google Scholar] [CrossRef]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132440. [Google Scholar] [CrossRef]
- Button, L.; Elle, E. Wild bumble bees reduce pollination deficits in a crop mostly visited by managed honey bees. Agric. Ecosyst. Environ. 2014, 197, 255–263. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Breeze, T.D.; Boreux, V.; Fountain, M.T.; McKerchar, M.; Webber, S.M.; Coston, D.J.; Jenner, N.; Dean, R.; Westbury, D.B.; et al. Apple Pollination: Demand Depends on Variety and Supply Depends on Pollinator Identity. PLoS ONE 2016, 11, e0153889. [Google Scholar] [CrossRef]
- Miñarro, M.; García, D.; Sastre, R.M. Los insectos polinizadores en la agricultura: Importancia y gestión de su biodiversidad. Ecosistemas 2018, 27, 81–90. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Eilers, E.J.; Kremen, C.; Smith Greenleaf, S.; Garber, A.K.; Klein, A.-M. Contribution of Pollinator-Mediated Crops to Nutrients in the Human Food Supply. PLoS ONE 2011, 6, e21363. [Google Scholar] [CrossRef]
- Bongaarts, J. IPBES, 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Popul. Dev. Rev. 2019, 45, 680–681. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Veldtman, R.; Shenkute, A.G.; Tesfay, G.B.; Pirk, C.W.W.; Donaldson, J.S.; Nicolson, S.W. Natural and within-farmland biodiversity enhances crop productivity. Ecol. Lett. 2011, 14, 251–259. [Google Scholar] [CrossRef]
- Barbir, J.; Badenes-Pérez, F.R.; Fernández-Quintanilla, C.; Dorado, J. Can floral field margins improve pollination and seed production in coriander Coriandrum sativum L. (Apiaceae)? Agric. For. Entomol. 2015, 17, 302–308. [Google Scholar] [CrossRef]
- Feltham, H.; Park, K.; Minderman, J.; Goulson, D. Experimental evidence that wildflower strips increase pollinator visits to crops. Ecol. Evol. 2015, 5, 3523–3530. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Seymour, C.L.; Nicolson, S.W.; Veldtman, R. Creating patches of native flowers facilitates crop pollination in large agricultural fields: Mango as a case study. J. Appl. Ecol. 2012, 49, 1373–1383. [Google Scholar] [CrossRef]
- FAO. Identificación de Sistemas de Producción Agrícola de Importancia Económica Impactados por la Zoopolinización. In Estado del Arte del Servicio Ecosistémico de la Polinización en Chile, Paraguay y Perú. 2017, p. 30. Available online: https://www.fao.org/publications/card/en/c/c2114ea8-bd36-4aad-8c24-e5923bbe80f9/ (accessed on 20 May 2021).
- Wien, H. (Ed.) The cucurbits: Cucumber, melon, squash and pumpkin. In The Physiology of Vegetable Crops; CAB International: Wallingford, UK, 1997; pp. 345–386. [Google Scholar]
- Eguillor, P. El Mercado del Zapallo: Producción, Precios y Perspectivas. 2011, Volume 11. Available online: https://www.odepa.gob.cl/odepaweb/publicaciones/doc/3424.pdf (accessed on 20 May 2021).
- Krarup, C.K.P. Hortalizas de Estaciones Cálidas. Available online: http://www7.uc.cl/sw_educ/hortalizas/html/zapallo/zapallo.html (accessed on 20 May 2021).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 20 May 2021).
- Brittain, C.; Kremen, C.; Klein, A.-M. Biodiversity buffers pollination from changes in environmental conditions. Glob. Chang. Biol. 2013, 19, 540–547. [Google Scholar] [CrossRef]
- Pisanty, G.; Afik, O.; Wajnberg, E.; Mandelik, Y. Watermelon pollinators exhibit complementarity in both visitation rate and single-visit pollination efficiency. J. Appl. Ecol. 2016, 53, 360–370. [Google Scholar] [CrossRef]
- Muñoz, A.E.; Amouroux, P.; Zaviezo, T. Native flowering shrubs promote beneficial insects in avocado orchards. Agric. For. Entomol. 2021, 23, 463–472. [Google Scholar] [CrossRef]
- Flores-Prado, L. Evolución de la sociabilidad en Hymenoptera: Rasgos conductuales vinculados a niveles sociales y precursores de sociabilidad en especies solitarias. Rev. Chil. Hist. Nat. 2012, 85, 245–266. [Google Scholar] [CrossRef][Green Version]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems: A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl. Ecol. 2014, 15, 701–711. [Google Scholar] [CrossRef]
- Phillips, B.W.; Gardiner, M.M. Use of video surveillance to measure the influences of habitat management and landscape composition on pollinator visitation and pollen deposition in pumpkin (Cucurbita pepo) agroecosystems. PeerJ 2015, 3, e1342. [Google Scholar] [CrossRef]
- Fountain, M.T.; Mateos-Fierro, Z.; Shaw, B.; Brain, P.; Delgado, A. Insect pollinators of conference pear (Pyrus communis L.) and their contribution to fruit quality. J. Pollinat. Ecol. 2019, 25, 103–114. [Google Scholar] [CrossRef]
- Gajc-Wolska, J.; Kowalczyk, K.; Mikas, J.; Drajski, R. Efficiency of cucumber (cucumis sativus L.) pollination by bumblebees (Bombus terrestris). Acta Sci. Pol. Hortorum Cultus 2011, 10, 159–169. [Google Scholar]
- Park, M.G.; Raguso, R.A.; Losey, J.E.; Danforth, B.N. Per-visit pollinator performance and regional importance of wild Bombus and Andrena (Melandrena) compared to the managed honey bee in New York apple orchards. Apidologie 2016, 47, 145–160. [Google Scholar] [CrossRef]
- Eeraerts, M.; Vanderhaegen, R.; Smagghe, G.; Meeus, I. Pollination efficiency and foraging behaviour of honey bees and non-Apis bees to sweet cherry. Agric. For. Entomol. 2020, 22, 75–82. [Google Scholar] [CrossRef]
- Sapir, G.; Baras, Z.; Azmon, G.; Goldway, M.; Shafir, S.; Allouche, A.; Stern, E.; Stern, R.A. Synergistic effects between bumblebees and honey bees in apple orchards increase cross pollination, seed number and fruit size. Sci. Hortic. (Amst.) 2017, 219, 107–117. [Google Scholar] [CrossRef]
- Garibaldi, L.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.; Bommarco, R.; Cunningham, S.; Carvalheiro, L.; Harder, L.; Afik, O.; Bartomeus, I.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Hoehn, P.; Tscharntke, T.; Tylianakis, J.M.; Steffan-Dewenter, I. Functional group diversity of bee pollinators increases crop yield. Proc. R. Soc. B Biol. Sci. 2008, 275, 2283–2291. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Aizen, M.A.; Cunningham, S.; Klein, A.M. Communicative & Integrative Biology Pollinator shortage and global crop yield Looking at the whole spectrum of pollinator dependency. Commun. Integr. Biol. 2009, 2, 37–39. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Alvarez-Garreton, C.; Barichivich, J.; Boisier, J.P.; Christie, D.; Galleguillos, M.; LeQuesne, C.; McPhee, J.; Zambrano-Bigiarini, M. The 2010–2015 megadrought in central Chile: Impacts on regional hydroclimate and vegetation. Hydrol. Earth Syst. Sci. 2017, 21, 6307–6327. [Google Scholar] [CrossRef]
- Takkis, K.; Tscheulin, T.; Petanidou, T. Differential Effects of Climate Warming on the Nectar Secretion of Early- and Late-Flowering Mediterranean Plants. Front. Plant Sci. 2018, 9, 874. [Google Scholar] [CrossRef]


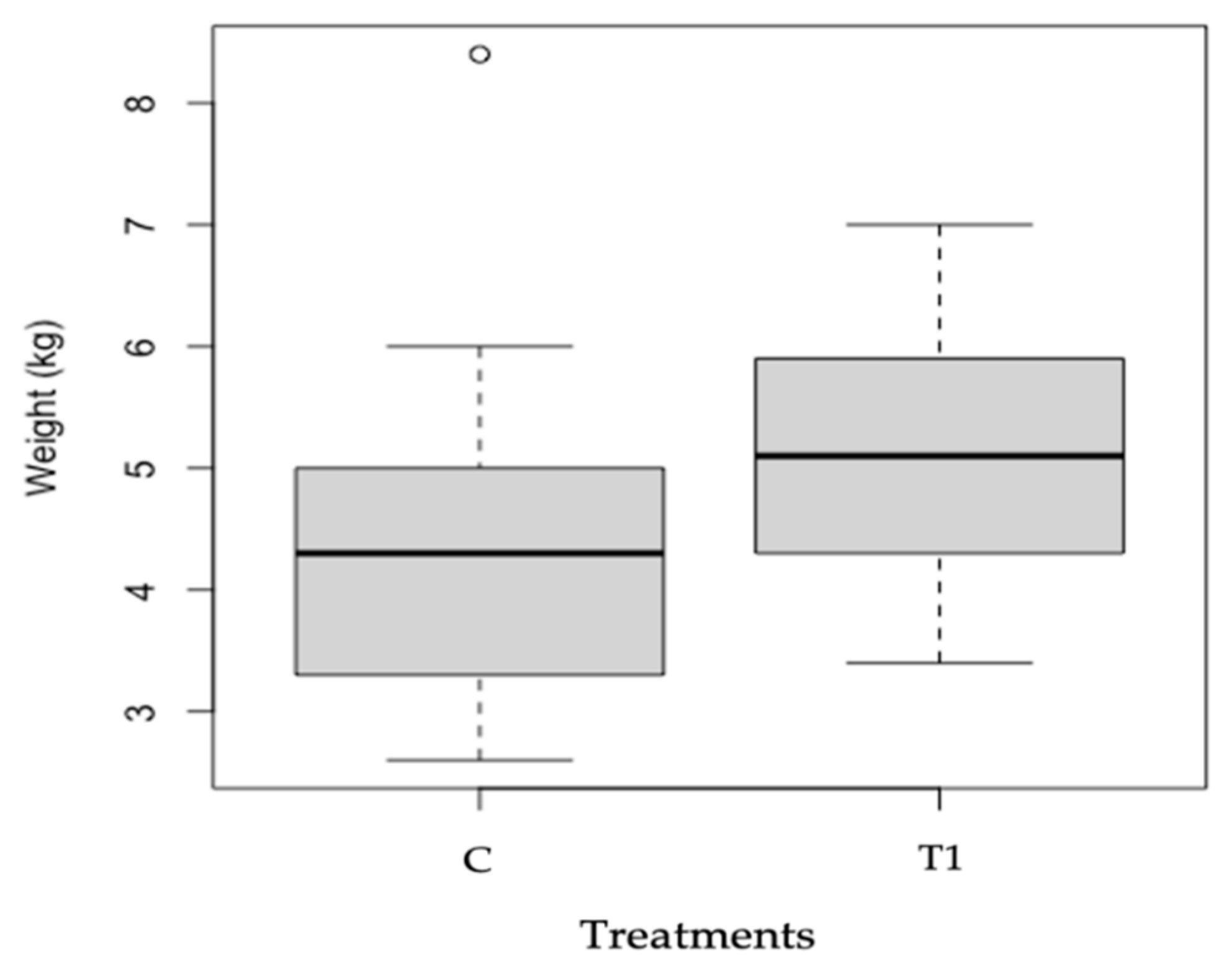

| Plot | Visitation Rate | Fruit Weight | Seed Set | SP |
|---|---|---|---|---|
| (nº Visit × Flower−1 × h−1) | (kg) | (nº Seed per Fruit) | (º Brix) | |
| Control | 0.63 | 2.7 | 209 | 4.2 |
| 1.00 | 2.6 | 267 | 5.0 | |
| 0.75 | 5.0 | 410 | 7.6 | |
| 0.88 | 5.0 | 232 | 9.0 | |
| 0.88 | 4.2 | 246 | 7.0 | |
| 0.25 | 3.2 | 200 | 5.4 | |
| 0.5 | 6.0 | 217 | 8.0 | |
| 0.63 | 4.3 | 178 | 5.9 | |
| 0.33 | 5.0 | 286 | 7.0 | |
| 0.50 | 4.9 | 202 | 8.1 | |
| 0.31 | 3.1 | 114 | 8.0 | |
| 1.25 | 6.0 | 257 | 9.0 | |
| 1.00 | 4.2 | 124 | 6.0 | |
| 0.25 | 4.3 | 189 | 8.1 | |
| 0.38 | 3.3 | 270 | 5.0 | |
| 0.38 | 5.1 | 266 | 7.6 | |
| 0.63 | 8.4 | 291 | 8.6 | |
| 0.42 | 3.3 | 289 | 6.2 | |
| Mean ± SD | 0.61 ± 0.29 | 4.5 ± 1.43 | 236 ± 68.05 | 6.98 ± 1.47 |
| Treatment 1 | 1.75 | 5.0 | 204 | 8.1 |
| 1.25 | 5.3 | 240 | 9.7 | |
| 1.38 | 4.3 | 190 | 8.0 | |
| 1.58 | 4.2 | 246 | 8.0 | |
| 2.75 | 4.9 | 262 | 9.6 | |
| 1.50 | 6.0 | 228 | 8.0 | |
| 0.88 | 5.9 | 198 | 11.0 | |
| 0.38 | 5.1 | 128 | 7.9 | |
| 1.50 | 4.2 | 248 | 7.6 | |
| 0.33 | 5.0 | 191 | 8.7 | |
| 0.50 | 5.1 | 235 | 9.0 | |
| 0.70 | 5.1 | 214 | 7.0 | |
| 1.92 | 7.0 | 304 | 7.1 | |
| 1.25 | 5.0 | 232 | 8.5 | |
| 1.33 | 6.8 | 264 | 9.1 | |
| 1.50 | 3.4 | 216 | 7.0 | |
| 0.40 | 5.9 | 308 | 9.0 | |
| 0.88 | 4.0 | 331 | 7.3 | |
| Mean ± SD | 1.21 ± 0.63 | 5.1 ± 9.41 | 236 ± 48.38 | 8.37 ± 1.07 |
| Seed Set (N = 18) | Estimate | Z Value | p Value |
|---|---|---|---|
| Treatment (T) | −0.04 | −0.22 | 0.82 |
| Visit rate (VR) | 0.09 | 0.56 | 0.58 |
| Weight (W) | 0.001 | 2.48 | 0.01 ** |
| Soluble Solids in Pulp (N = 18) | |||
| Treatment (T) | 0.19 | 8.84 | <0.001 *** |
| Visit rate (VR) | −0.34 | −3.86 | <0.001 *** |
| Weight (W) | 0.001 | 4.22 | <0.001 *** |
| T × VR | 0.17 | 0.48 | 0.63 |
| T × W | 0.02 | −9.42 | <0.001 *** |
| VR × W | 0.001 | 4.02 | <0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoso, S.; Murúa, M. Floral Patches and Their Impact on Pollinator Attraction and Yield Production on Cucurbita maxima Var. Paine in Central Chile. Diversity 2021, 13, 608. https://doi.org/10.3390/d13120608
Donoso S, Murúa M. Floral Patches and Their Impact on Pollinator Attraction and Yield Production on Cucurbita maxima Var. Paine in Central Chile. Diversity. 2021; 13(12):608. https://doi.org/10.3390/d13120608
Chicago/Turabian StyleDonoso, Santiago, and Maureen Murúa. 2021. "Floral Patches and Their Impact on Pollinator Attraction and Yield Production on Cucurbita maxima Var. Paine in Central Chile" Diversity 13, no. 12: 608. https://doi.org/10.3390/d13120608
APA StyleDonoso, S., & Murúa, M. (2021). Floral Patches and Their Impact on Pollinator Attraction and Yield Production on Cucurbita maxima Var. Paine in Central Chile. Diversity, 13(12), 608. https://doi.org/10.3390/d13120608






