Cyanobacterial Diversity of the Northern Polar Ural Mountains
Abstract
:1. Introduction
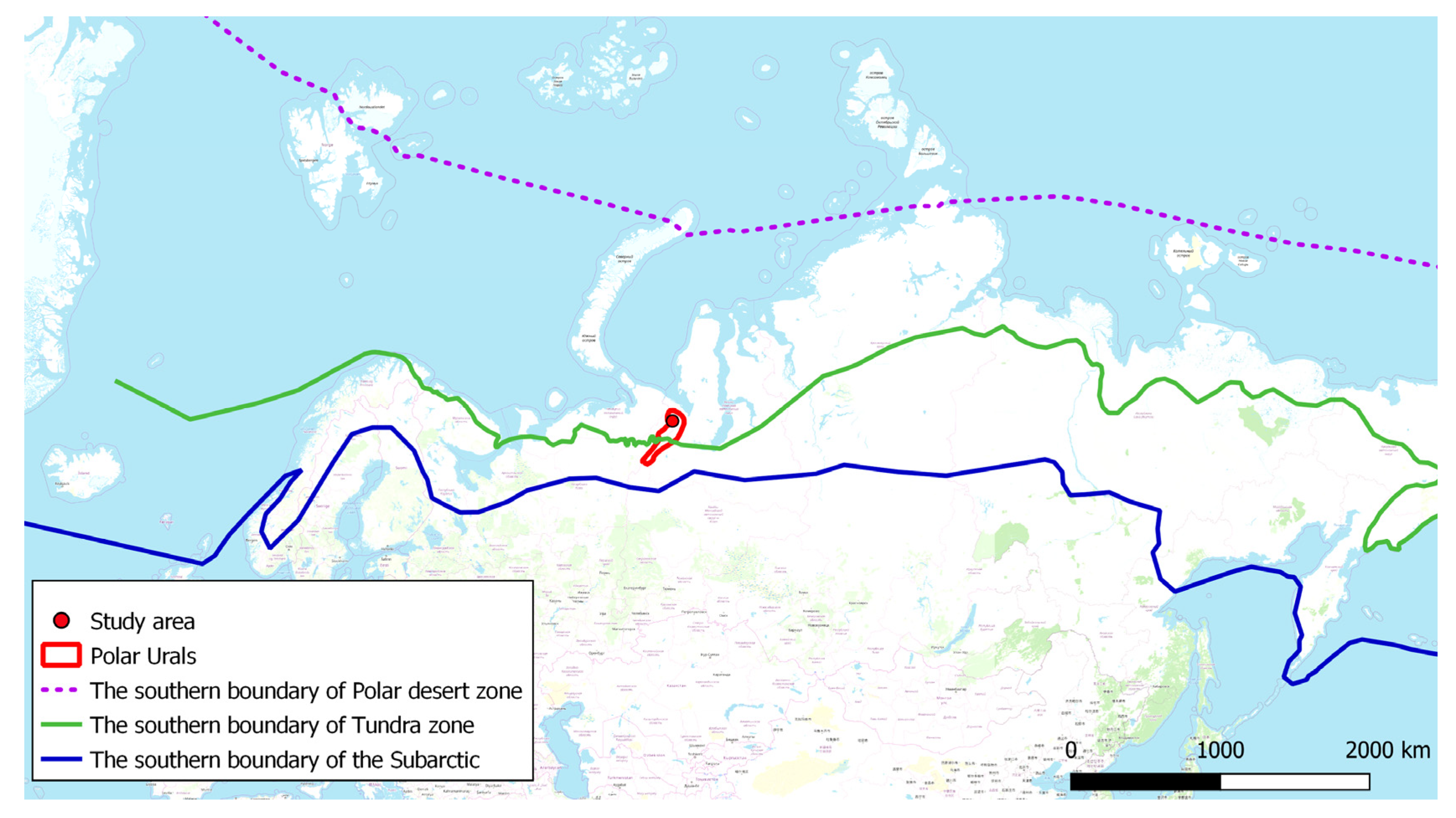
2. Characteristics of the Study Area
3. Materials and Methods
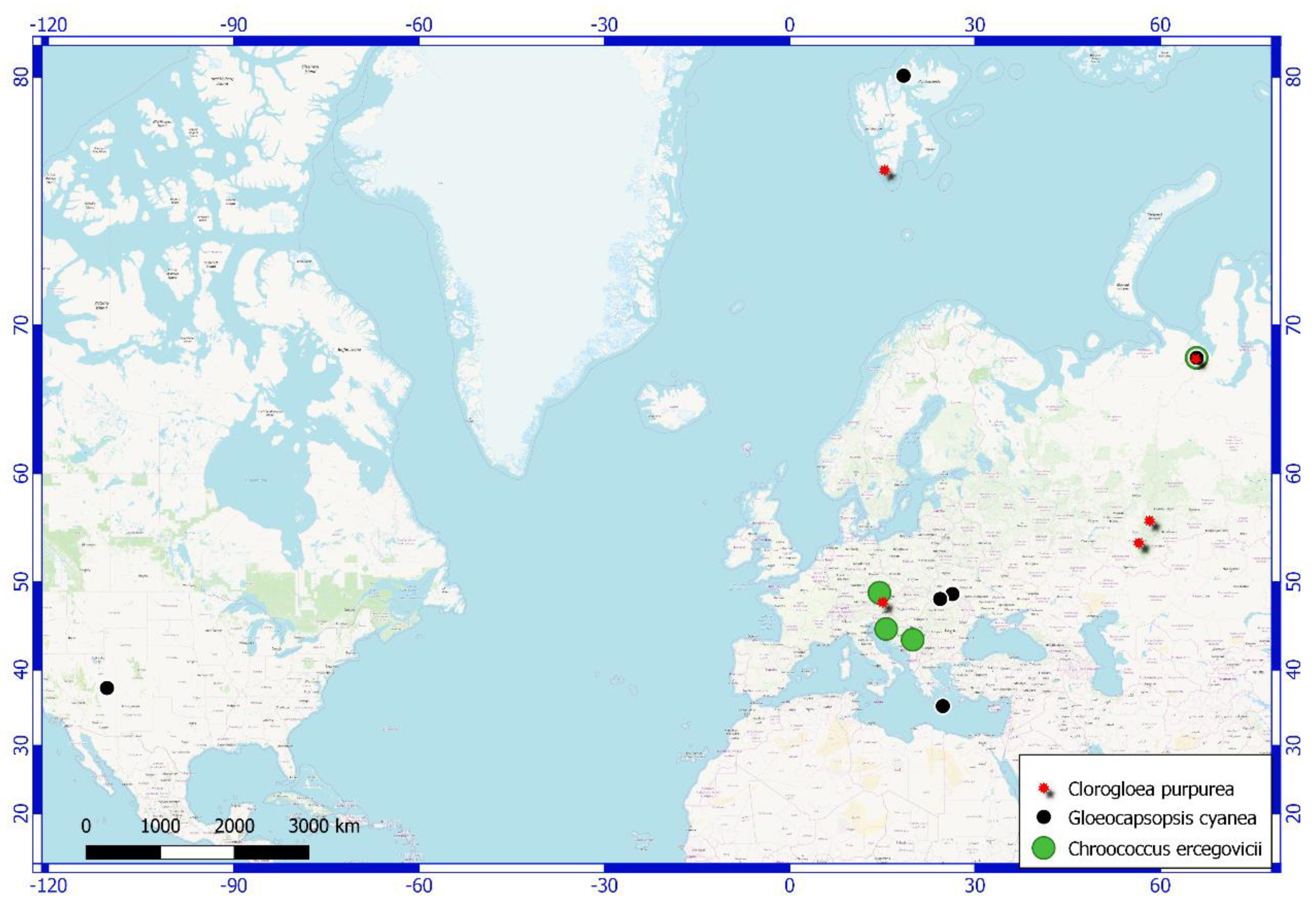
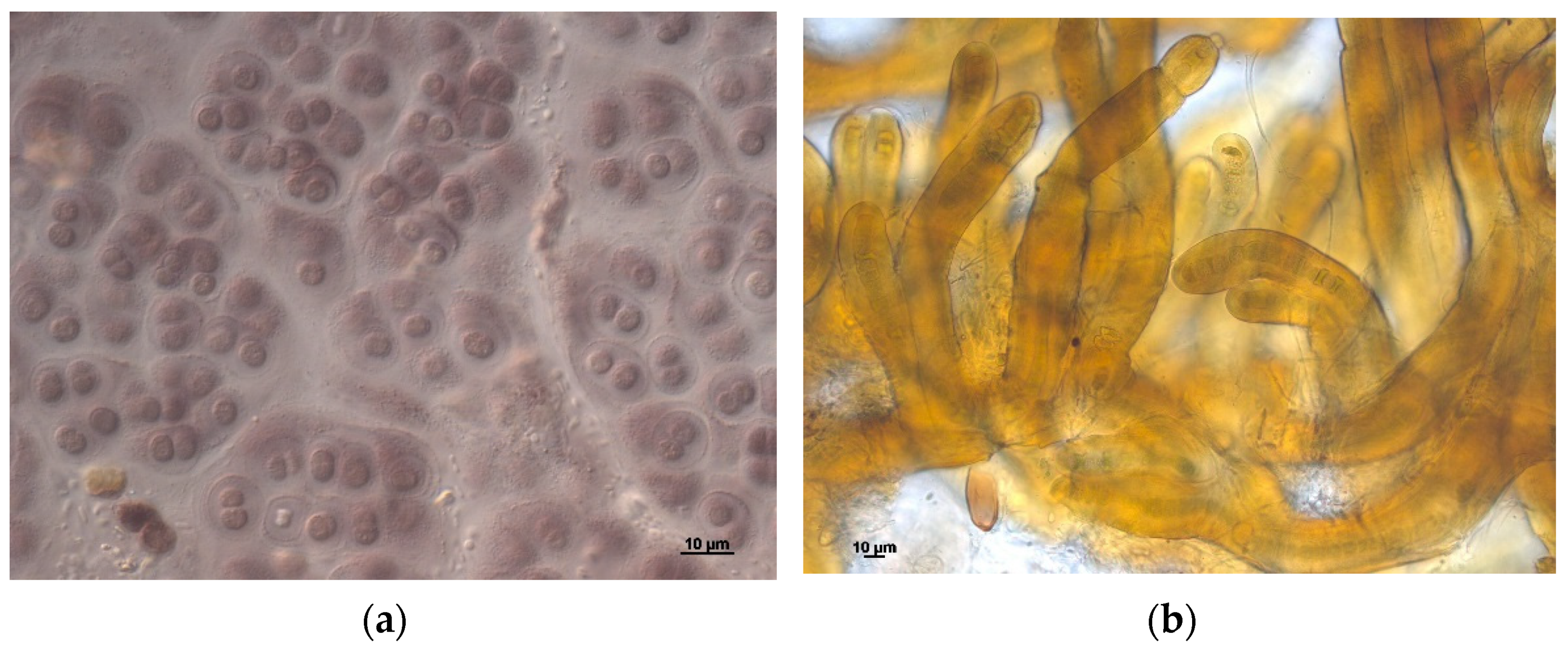
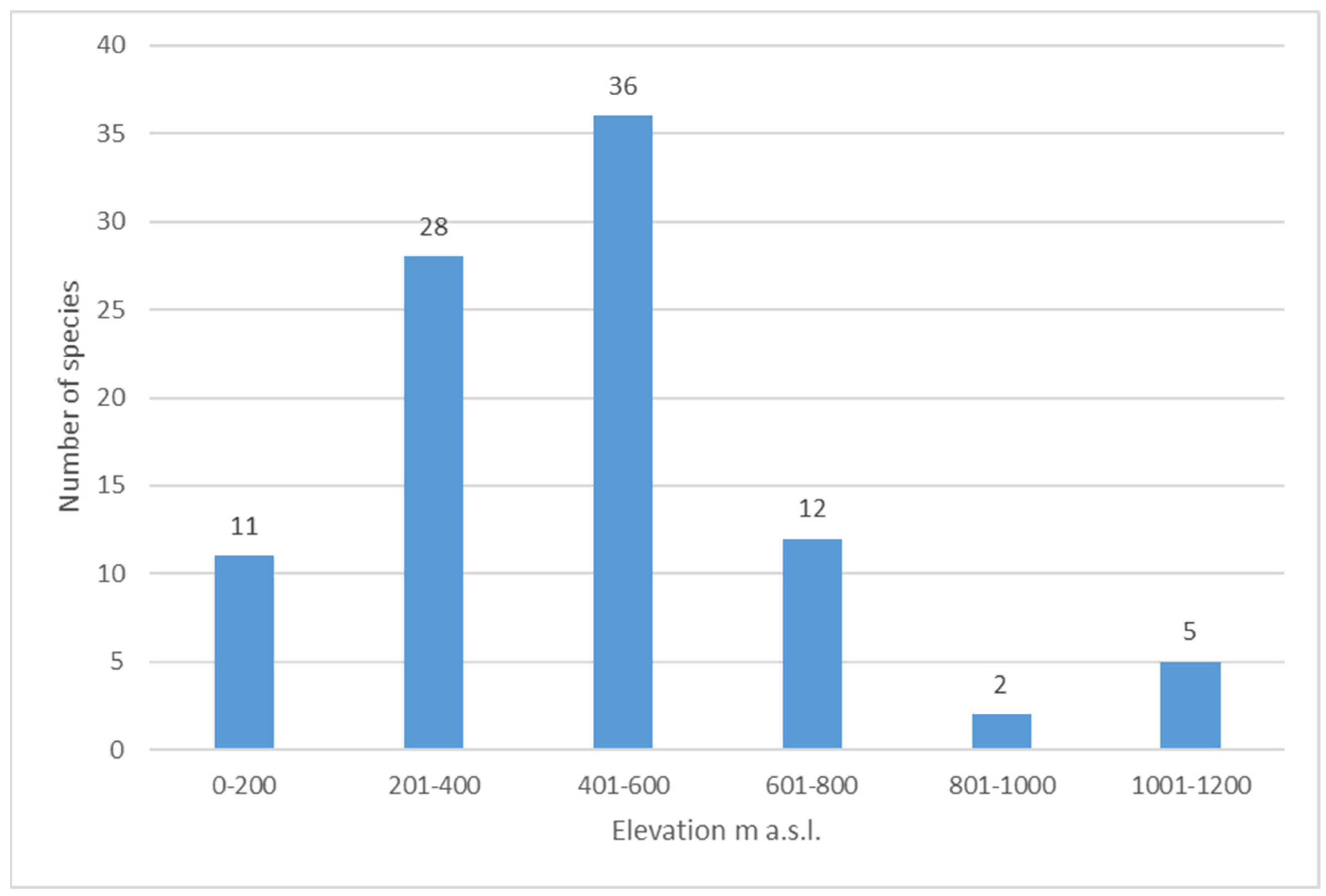
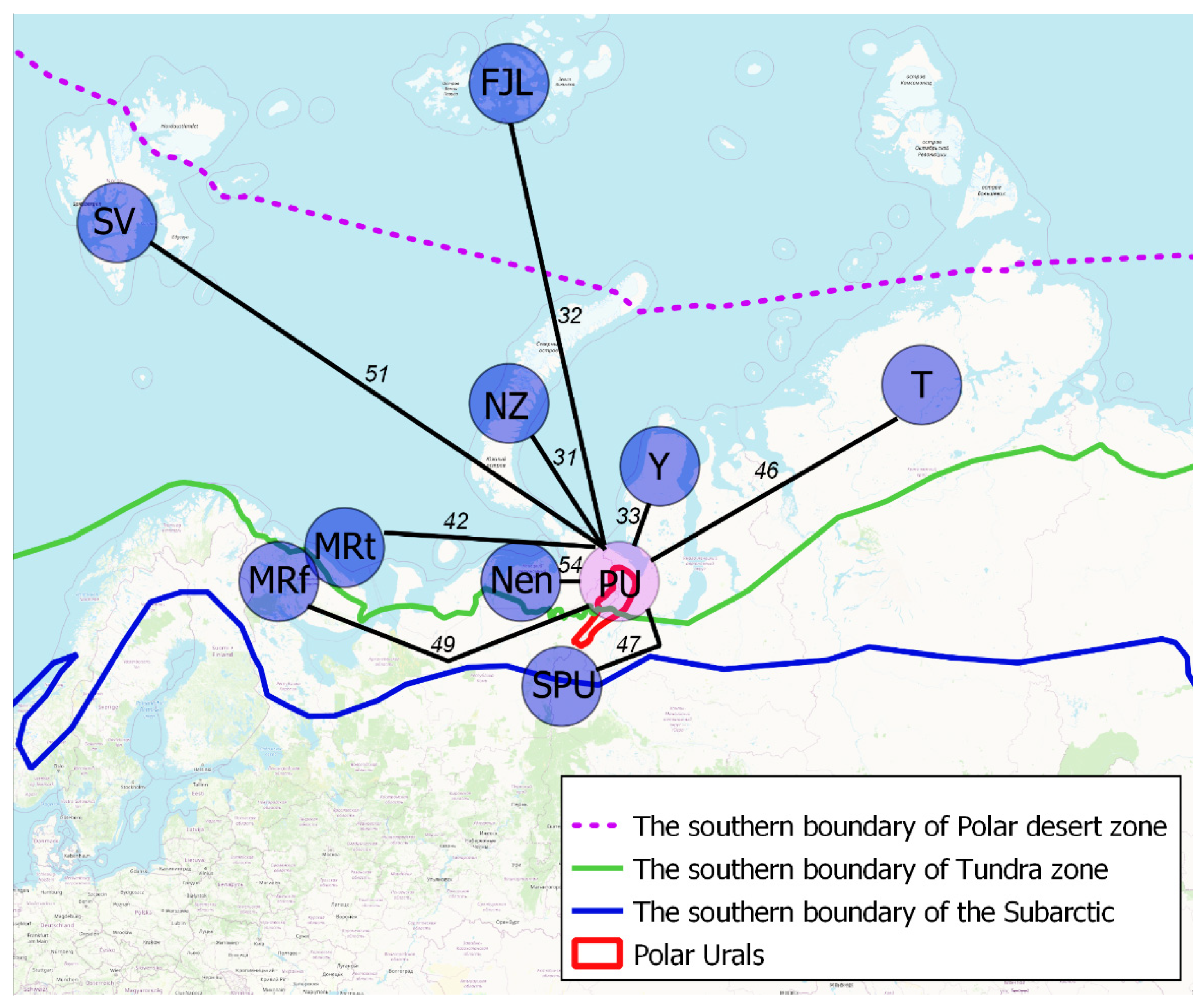
4. Results and Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davydov, D.; Patova, E. The diversity of Cyanoprokaryota from freshwater and terrestrial habitats in the Eurasian Arctic and Hypoarctic. Hydrobiologia 2018, 811, 119–138. [Google Scholar] [CrossRef]
- Ribeiro, K.F.; Duarte, L.; Crossetti, L.O. Everything Is Not Everywhere: A Tale on the Biogeography of Cyanobacteria. Hydrobiologia 2018, 820, 23–48. [Google Scholar] [CrossRef]
- Boyer, S.L.; Johansen, J.R.; Howard, G.L. Phylogeny and genetic variance in terrestrial Microcoleus (Cyanophyceae) species based on sequence analysis of the 16S rRNA gene and associated 16S–23S ITS region. J. Phycol. 2002, 38, 1222–1225. [Google Scholar] [CrossRef]
- Strunecky, O.; Raabova, L.; Bernardova, A.; Ivanova, A.P.; Semanova, A.; Crossley, J.; Kaftan, D. Diversity of Cyanobacteria at the Alaska North Slope with Description of Two New Genera: Gibliniella and Shackletoniella. FEMS Microbiol. Ecol. 2020, 96, fiz189. [Google Scholar] [CrossRef]
- Casamatta, D.A.; Vis, M.L.; Sheath, R.G. Cryptic species in cyanobacterial systematics: A case study of Phormidium retzii (Oscillatoriales) using 16S rDNA and RAPD analyses. Aquat. Bot. 2003, 77, 295–309. [Google Scholar] [CrossRef]
- Mai, T.; Johansen, J.R.; Pietrasiak, N.; Bohunicka, M.; Martin, M.P. Revision of the Synechococcales (Cyanobacteria) through recognition of four families including Oculatellaceae fam. nov. and Trichocoleaceae fam. nov. and six new genera containing 14 species. Phytotaxa 2018, 365, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Davydov, D.; Shalygin, S.; Vilnet, A. New cyanobacterium Nodosilinea svalbardensis sp. nov. (Prochlorotrichaceae, Synechococcales) isolated from alluvium in Mimer river valley of the Svalbard archipelago. Phytotaxa 2020, 442, 61–79. [Google Scholar] [CrossRef]
- Voronikhin, N.N. Algae Polar and Northern Urals. Proc. Leningr. Soc. Nat. 1930, 60, 1–71. (In Russian) [Google Scholar]
- Yarushina, M.I. Algae of reservoirs of the Polar Urals. In Biological Resources of the Polar Urals; Pashalny, S.P., Ed.; Department of Information and Socio-Political Research of the Yamalo-Nenets Autonomous District: Salekhard, Russia, 2002; Volume 3, pp. 71–77. (In Russian) [Google Scholar]
- Yarushina, M.I. Phytoplankton of lakes on the western slope of the Polar Urals. In Biological Resources of the Polar Urals; Pashalny, S.P., Ed.; Department of Information and Socio-Political Research of the Yamalo-Nenets Autonomous District: Salekhard, Russia, 2003; Volume 10, pp. 30–36. (In Russian) [Google Scholar]
- Bogdanov, V.D.; Bogdanova, E.N.; Gavrilov, A.L.; Melnichenko, I.P.; Stepanov, L.N.; Yarushina, M.I. Bioresources Aquatic Ecosystems of the Polar Urals; Urals Dep. RAS: Yekaterinburg, Russia, 2004; pp. 1–167. (In Russian) [Google Scholar]
- Voloshko, L.N.; Demina, I.V.; Estafiev, A.A.; Illarionov, V.V.; Kolesnikova, A.A.; Kochanov, S.K.; Kulakova, O.I.; Kulikova, K.V.; Kulyugina, E.E.; Loskutova, O.A.; et al. Biodiversity the Polar Urals Ecosystems; Institute of Biology: Syktyvkar, Russia, 2007; pp. 1–252. (In Russian) [Google Scholar]
- Patova, E.N.; Demina, I.V. Algae of water bodies of the Polar Urals, not subject to anthropogenic impact. Inland Water Biol. 2008, 1, 58–67. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Patova, E.N. Algae of mountain tundra soils in the North and Polar Urals. Bull. MOIP. Otd. Biol. 2013, 118, 57–66. (In Russian) [Google Scholar]
- Vinokurova, G.V. Phytoepiliton of Lake Bolshoye Shchuchye and associated rivers (Polar Urals). Sci. Bull. YNAO 2017, 94, 11–14. (In Russian) [Google Scholar]
- Mitrofanova, E.Y. Phytoplankton of Lake Bolshoye Shchuchye and the rivers of its basin in August 2016. Sci. Bull. Yamalo-Nenets Auton. Okrug. 2017, 1, 55–61. (In Russian) [Google Scholar]
- Patova, E.N.; Novakovskaya, I.V. Diversity of soil algae and cyanoprokaryota in terrestrial communities of the Polar and Subpolar Urals. Theor. Appl. Ecol. 2014, 1, 32–34. [Google Scholar]
- Patova, E.N.; Novakovskaya, I.V. Soil algae of the Northeastern European Russia. Nov. Sist. Nizshikh Rastenii 2018, 52, 311–353. [Google Scholar] [CrossRef]
- Kemmerikh, A.O. Polar Urals; Publishing House “Physical Culture and Sport”: Moscow, Russia, 1966; pp. 1–112. (In Russian) [Google Scholar]
- Solomina, O.; Ivanov, M.; Bradwell, T. Lichenometric studieson moraines in the Polar Urals. Geogr. Ann. Ser. A Phys. Geogr. 2010, 92, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Golovin, A.A.; Gusev, G.S.; Gushchin, A.V.; Zaikov, V.V.; Kilipko, V.A.; Krinochkin, L.A.; Mezhelovsky, N.V.; Morozov, A.F.; Sirotkina, O.N.; Flerova, K.V. Mineragenic Potential of the Subsoil of Russia; Geokart, Geos: Moscow, Russia, 2013; Volume 1, pp. 1–484. (In Russian) [Google Scholar]
- Kulikova, K.V.; Patova, E.N.; Kulyugina, E.E.; Demina, I.V. The study area. In Biodiversity the Polar Urals Ecosystems; Getzen, M.V., Ed.; Institute of Biology: Syktyvkar, Russia, 2007; pp. 10–15. (In Russian) [Google Scholar]
- Shiyatov, S.G. Dynamics of Tree and Shrub Vegetationin the Polar Urals under the Impact of Modern Climate Changes; Urals Department RAS: Yekaterinburg, Russia, 2009; pp. 1–215. (In Russian) [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Unaltered, repr., Ettl, H., Gärtner, G., Heynig, G., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2008; pp. 1–548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schlager, M., Eds.; Unaltered repr., 2. print; Spektrum Akademischer Verlag: Heidelberg, Germany, 2008; pp. 1–759. [Google Scholar]
- Komárek, J. Cyanoprokaryota 3. Teil: Heterocytous genera. In Süsswasserflora von Mitteleuropa 19/3; Büdel, B., Gärtner, G., Krienitz, L., Schlager, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–1133. [Google Scholar] [CrossRef]
- Melechin, A.V.; Davydov, D.; Shalygin, S.; Borovichev, E.A. Open information system on biodiversity cyanoprokaryotes and lichens CRIS (Cryptogamic Russian Information System). Bull. MOIP. Otd. Biol. 2013, 118, 51–56. (In Russian) [Google Scholar]
- Melekhin, A.V.; Davydov, D.; Borovichev, E.A.; Shalygin, S.S.; Konstantinova, N.A. CRIS—Service for input, storage and analysis of the biodiversity data of the cryptogams. Folia Cryptogam. Est. 2019, 56, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Kondratyeva, N.V.; Kovalenko, O.V. A Short Guide to the Types of Toxic Blue-Green Algae; Naukova dumka: Kiev, Ukraine, 1975; pp. 1–80. [Google Scholar]
- Nowakowskiy, A.B. Interaction between Excel and statistical package R for ecological data analysis. Vestn. Insituta Biol. Komi Sci. Ural. Branch 2016, 3, 26–33. [Google Scholar]
- Geitler, L. Über die Tiefenflora an Lelsen im Lunzer Untersee. Arch. Für Protistenkd. 1928, 62, 96–104. [Google Scholar]
- Gaysina, L.A.; Bohunická, M.; Hazuková, V.; Johansen, J.R. Biodiversity of terrestrial cyanobacteria of the South Ural region. Cryptogam. Algol. 2018, 39, 167–198. [Google Scholar] [CrossRef] [Green Version]
- Getsen, M.V.; Stenina, A.S.; Patova, E.N. Algoflora of Bolshezemelskaya Tundra in the Conditions of Anthropogenic Pollution; Komi Science Centre Publishers: Syktyvkar, Russia, 1994; pp. 1–148. (In Russian) [Google Scholar]
- Davydov, D. Cyanoprokaryota and Their Role in the Process of Nitrogen Fixation in Terrestrial Ecosystems of the Murmansk Region; GEOS: Moscow, Russia, 2010; pp. 1–184. (In Russian) [Google Scholar]
- Davydov, D. Terrestrial Cyanoprokaryota of the western part of Khibiny Mountains. Bull. MOIP. Otd. Biol. 2012, 117, 72–77. (In Russian) [Google Scholar]
- Davydov, D. Diversity of the Cyanoprokaryota in polar deserts of Innvika cove North-East Land (Nordaustlandet) Island, Spitsbergen. Czech Polar Rep. 2016, 6, 66–79. [Google Scholar] [CrossRef]
- Davydov, D. Cyanoprokaryotes of the west part of Oscar II Land, West Spitsbergen Island, Spitsbergen archipelago. Czech Polar Rep. 2017, 7, 94–108. [Google Scholar] [CrossRef]
- Davydov, D. Diversity of the Cyanoprokaryota in polar deserts of Rijpfjorden east coast, North-East Land (Nordaustlandet) Island, Spitsbergen. Algol. Stud. 2013, 142, 29–43. [Google Scholar] [CrossRef]
- Davydov, D. Diversity of the Cyanoprokaryota of the area of settlement Pyramiden, West Spitsbergen Island, Spitsbergen archipelago. Folia Cryptogam. Est. 2014, 51, 13–23. [Google Scholar] [CrossRef]
- Davydov, D. Checklist of cyanobacteria from the European polar desert zone. Botanica 2018, 24, 185–201. [Google Scholar] [CrossRef] [Green Version]
- Gorchakovskiy, P.L. The Flora of the High Mountains of the Urals; Nauka: Moscow, Russia, 1975; pp. 1–283. (In Russian) [Google Scholar]
- Gorchakovskiy, P.L.; Kuvaev, V.B. Ecological aspects of vertical differentiation of vegetation cover in the boreal highlands. Russ. J. Ecol. 1985, 3, 12–20. [Google Scholar]
- Morozova, L.M. The current state of the vegetation cover on the eastern slope of the Polar Urals. Biol. Resour. Polar Ural. 2002, 10, 78–89. [Google Scholar]
- Davydov, D. Cyanobacterial diversity of Svalbard Archipelago. Polar Biol. 2021, 44, 1967–1978. [Google Scholar] [CrossRef]





| No 1 | Latitude N | Longitude E | Elevation (m a.s.l.) | Description of Study Site |
|---|---|---|---|---|
| 1 | 67.9715 | 65.45875 | 153 | Gnettyvis river, a pebble on the bottom. |
| 2 | 67.967 | 65.54814 | 196 | Gnetty lake area. On the dirt road. The biological soil crust. |
| 3 | 67.99013 | 65.67176 | 218 | Ochenyrd ridge. Bolshaya Kara river valley. A fast stream near a waterfall. |
| 4 | 68.00411 | 65.66261 | 365 | Ochenyrd ridge. Bolshaya Kara river valley. On the SW exposure wall of an outlier. |
| 5 | 68.005 | 65.66225 | 351 | Ibid. On the W exposure wall of an outlier. |
| 6 | 68.01703 | 65.70358 | 365 | Ochenyrd ridge. On the bottom of a small lake at the upper part of a pass. |
| 7 | 68.03303 | 65.71507 | 451 | Ochenyrd ridge. On the shore of a small lake. |
| 8 | 68.03416 | 65.71313 | 463 | Ochenyrd ridge. The lake is near a glacier. The fast stream, a pebble on the bottom. |
| 9 | 68.03989 | 65.72366 | 510 | Ochenyrd ridge. The upper part of a pass, a small slow stream, on a boulder. |
| 10 | 68.04331 | 65.71349 | 654 | Ochenyrd ridge. An unnamed mountain, N exposure slope. Near snow patch, a bare permafrost ground polygon. |
| 11 | 68.04346 | 65.71719 | 638 | Ochenyrd ridge. An unnamed mountain, N exposure slope. On the jasper outlier. |
| 12 | 68.04728 | 65.70953 | 574 | Ibid. On a wet rock. |
| 13 | 68.04742 | 65.70971 | 558 | Ibid. On a small slow stream, on a pebble. |
| 14 | 68.04803 | 65.72273 | 477 | Ochenyrd ridge. A soil crust on the shore of a lake near a snow patch. |
| 15 | 68.04932 | 65.71127 | 498 | Ochenyrd ridge. On a wet wall of an outlier stone. |
| 16 | 68.05112 | 65.73135 | 479 | Ochenyrd ridge. Morennyy (Moraine) stream valley. The bouldering plateau near the lake. On an upper side of a boulder in a puddle. |
| 17 | 68.05721 | 65.76206 | 485 | Ochenyrd ridge. An unnamed mountain (994.8 m a.s.l.). Rocks of S exposure, on a wet wall. |
| 18 | 68.05724 | 65.69468 | 958 | Ochenyrd ridge. An unnamed mountain (1375 m a.s.l.). Rocks of E exposure, on a wet wall. |
| 19 | 68.05827 | 65.69228 | 1051 | Ibid. On a wet wall. |
| 20 | 68.05968 | 65.69263 | 1161 | Ochenyrd ridge. A wet wall of rock near a waterfall. |
| 21 | 68.06146 | 65.73355 | 567 | Ochenyrd ridge. S exposure slope of the unnamed mountain (994 m a.s.l.). On a wet rock. |
| 22 | 68.06151 | 65.73597 | 557 | Ochenyrd ridge. S exposure slope of the unnamed mountain (994 m a.s.l.). A wet rock. On the soil, mosses or rock. |
| 23 | 68.06225 | 65.79045 | 424 | Ochenyrd ridge. The slow stream. |
| 24 | 68.06613 | 65.8465 | 361 | The lake between Limbyatayaha river valley and Ochety Lake valley. On the shore of the lake. A puddle. |
| 25 | 68.07383 | 65.88036 | 367 | Unnamed mountains 1375 m a.s.l. A wet rock W exposure. |
| 26 | 68.08432 | 66.04273 | 381 | Tisnenzato lake valley, west shore of the lake. Unnamed mountain 830 m a.s.l. Kan’onnyy stream gorge. A wet rock on the E exposure slope. |
| 27 | 68.08879 | 66.03936 | 377 | Tisnenzato lake valley, south shore of the lake. Unnamed mountain 830 m a.s.l. Kan’onnyy stream gorge. A wet rock on the S exposure slope. |
| 28 | 68.08981 | 66.04167 | 381 | Ibid. On the rock. |
| 29 | 68.09029 | 66.03429 | 381 | Ibid. On the rock. |
| 30 | 68.09108 | 66.03974 | 381 | Ibid. On the rock. |
| 31 | 68.09153 | 65.90435 | 700 | Unnamed mountain, 832,5 m alt. North exposure slope, a wet wall of rock. |
| 32 | 68.0916 | 65.91236 | 669 | Unnamed mountain, 832,5 m alt. North exposure slope, a wet wall of rock. |
| 33 | 68.09182 | 65.90287 | 704 | Ibid. On a wet wall of rock. |
| 34 | 68.09187 | 65.91724 | 669 | Ibid. A slow stream, on a wet rock. |
| 35 | 68.09208 | 65.91968 | 650 | Ibid. |
| 36 | 68.09241 | 65.89986 | 732 | Ibid. A wet wall of rock. |
| 37 | 68.09413 | 66.04542 | 401 | The gorge on the west shore of Tisnenzato lake. On the NE exposure rock of the outlier. |
| 38 | 68.09522 | 66.04769 | 404 | Tisnenzato Lake valley, west shore of the lake. Unnamed mountain 830 m a.s.l. The E exposure slope. The S exposure rock. On the soil. |
| 39 | 68.09551 | 66.04517 | 415 | The gorge on the west shore of Tisnenzato lake. On the E exposure rock of the outlier. |
| 40 | 68.09811 | 66.04853 | 446 | Tisnenzato lake valley, west shore of the lake. Unnamed mountain 830 m a.s.l. The E exposure slope. The N exposure rock. On the soil. |
| 41 | 68.10442 | 66.04979 | 512 | Ibid. Dryas octopetala assemblage tundra. A small slow stream. On the soil. |
| 42 | 68.1069 | 66.05212 | 573 | Ibid. Grass tundra assemblage, biological sols crust. |
| 43 | 68.12484 | 65.851 | 502 | The unnamed mountain (1070 m), the slope of the south exposure. On a wet wall of rock. Mosses. |
| 44 | 68.12592 | 65.88839 | 522 | The southern shore of Sidyayambto lake, the unnamed mountain, slope of the north exposure. On a wet wall of rock. |
| 45 | 68.13106 | 65.86433 | 395 | Sidyayambtoso river valley. The waterfall on the river. On a wet wall of the bank’s rock. |
| 46 | 68.13265 | 65.84778 | 338 | Sidyayambtoso river valley. On a dry wall of north exposure rock. Mosses. |
| 47 | 68.16459 | 65.75465 | 224 | The Ochetyvis valley. The left shore of the Ochetyvis river. Dry rocks od N exposure. Soil. |
| 48 | 68.18832 | 65.68006 | 173 | Ibid. A stream inflow to the Ochetyvis river the left shore, on a soil. |
| 49 | 68.1895 | 65.68204 | 173 | The Ochetyvis valley. The right shore of the Ochetyvis river. Epilithic on the pebble in the puddle on the shore. |
| 50 | 68.18995 | 65.67754 | 167 | The Ochetyvis valley. The left shore of the Ochetyvis river. On rocks and boulders underwater. Calcareous rock. |
| 51 | 68.19059 | 65.67815 | 154 | Ibid. On a wet wall of rock, near water. Calcareous rock. |
| N | Species | Number of Loaclities 1 | The Frequency of Species Occurrence (%) |
|---|---|---|---|
| 1 | Aphanocapsa fusco-lutea Hansg. | 14, 21, 22 | 1.3 |
| 2 | * A. fonticola Hansg.2 | 45 | 0.5 |
| 3 | A. grevillei (Berk.) Rabenh. | 26 | 0.5 |
| 4 | A. muscicola (Menegh.) Wille | 12, 21, 25, 26, 40, 48 | 2.6 |
| 5 | * A. parietina Näg. | 4, 12, 21, 22, 26 | 2.2 |
| 6 | * A. rivularis (Carm.) Rabenh. | 4, 22, 25, 47 | 1.7 |
| 7 | Aphanocapsa sp. | 8, 12, 14, 17, 21, 22, 25, 26, 34, 35, 43, 44, 47, 48, 50, 51 | 7.0 |
| 8 | * Aphanothece pallida (Kütz.) Rabenh. | 26 | 0.5 |
| 9 | A. saxicola Näg. | 12, 14, 21, 22, 25, 26, 34, 42, 44 | 3.9 |
| 10 | A. stagnina (Spreng.) A. Braun | 22 | 0.4 |
| 11 | Aphanothece sp. | 22, 48 | 0.9 |
| 12 | * Calothrix breviarticulata W. West et G. S. West | 4, 49 | 0.9 |
| 13 | C. parietina Thur. ex Born. et Flah. | 4, 5, 10, 11, 13, 14, 15, 17, 20, 21, 22, 25, 26, 27, 29, 30, 31, 32, 34, 35, 38, 39, 41, 42, 43, 44, 45, 47, 48, 50, 51 | 18.7 |
| 14 | Chamaesiphon polonicus (Rost.) Hansg. | 8, 13, 14, 20, 23, 31, 32, 33, 35, 36, 43, 45 | 9.6 |
| 15 | * Chlorogloea purpurea Geitl. | 22 | 0.4 |
| 16 | * Chroococcopsis epiphytica Geitl. | 25 | 0.4 |
| 17 | Chroococcus cohaerens (Bréb.) Näg. | 3, 7, 20, 22, 36, 43 | 3.5 |
| 18 | * Ch. ercegovicii Komárek et Anagn. | 44 | 0.4 |
| 19 | * Ch. helveticus Näg. | 44 | 0.4 |
| 20 | Ch. minutus (Kütz.) Näg. | 19, 26, 45 | 1.3 |
| 21 | Ch. minutus (Kütz.) Näg. var. thermalis Copeland | 20 | 0.4 |
| 22 | Ch. pallidus (Näg.) Näg. | 6, 7, 21, 25, 26, 29, 43, 44 | 4.8 |
| 23 | Ch. spelaeus Erceg. | 17, 21, 44, 47 | 2.6 |
| 24 | Ch. tenax (Kirchn.) Hieron. | 22, 26, 44 | 1.3 |
| 25 | Ch. varius A. Braun | 11, 14, 21, 22, 34, 45, 46 | 5.7 |
| 26 | Cyanosarcina chroococcoides (Geitl.) Kováčik | 26, 47 | 0.9 |
| 27 | Cyanosarcina sp. | 22, 44 | 0.9 |
| 28 | Cyanothece aeruginosa (Näg.) Komárek | 12, 17, 22, 23, 25, 27, 28, 35 | 4.8 |
| 29 | Desmonostoc muscorum (C. Ag. ex Born. et Flah.) Hrouzek et Ventura | 21, 22 | 0.9 |
| 30 | Dichothrix gypsophila (Kütz.) Born. et Flah. | 22, 27, 43, 44, 47 | 3.5 |
| 31 | D. orsiniana (Kütz.) Born. et Flah. | 6 | 0.4 |
| 32 | Fischerella muscicola (Thur.) Gom. | 4, 36 | 0.9 |
| 33 | * Gloeocapsa alpina (Näg.) Brand | 44 | 0.4 |
| 34 | G. compacta Kütz. | 22, 30, 34, 35, 43, 44, 47 | 6.1 |
| 35 | * G. fusco-lutea (Näg.) Kütz. | 29 | 0.4 |
| 36 | * G. kuetzingiana Näg. | 12, 14, 15, 17, 18, 21, 22, 25, 27, 29, 34, 35, 42, 43, 44 | 10.9 |
| 37 | * G. ralfsii (Harv.) Kütz. | 14 | 0.9 |
| 38 | * G. rupestris Kütz. | 27 | 0.4 |
| 39 | G. sanguinea (C. Ag.) Kütz. | 12, 14, 15, 17, 18, 21, 22, 26, 27, 29, 34, 35, 37, 39, 43, 44, 45, 46 | 13.0 |
| 40 | G. violascea (Corda) Rabenh. | 5, 7, 14, 15, 17, 18, 19, 21, 22, 25, 26, 27, 29, 30, 34, 39, 43, 44, 45, 46, 51 | 23.0 |
| 41 | * Gloeocapsopsis chroococcoides (Nováček) Komárek | 29 | 0.9 |
| 42 | * G. cyanea (Krieger) Komárek et Anagn. | 44 | 0.4 |
| 43 | G. magma (Bréb.) Komárek et Anagn. | 3, 4, 5, 7, 10, 11, 14, 15, 16, 19, 21, 22, 25, 26, 29, 30, 34, 35, 36, 38, 39, 40, 43, 45 | 16.1 |
| 44 | * G. pleurocapsoides (Nováček) Komárek et Anagn. | 43 | 0.4 |
| 45 | Gloeocapsopsis sp. | 31, 32, 45 | 1.3 |
| 46 | * Gloeothece confluens Näg. | 25, 45, 47 | 1.3 |
| 47 | * G. fusco-lutea Näg. | 44 | 0.4 |
| 48 | * G. heufleri Grunov | 17, 44 | 0.9 |
| 49 | * G. palea (Kütz.) Rabenh. | 44 | 0.4 |
| 50 | * G. rupestris (Lyngb.) Born. | 26, 29 | 1.3 |
| 51 | * G. tepidariorum (A. Braun) Lagerh. | 27, 42 | 1.7 |
| 52 | * Leptolyngbya “Albertano\Kováčik-green” | 25 | 0.4 |
| 53 | Leptolyngbya cf. gracillima (Hansg.) Anagn. et Komárek | 12, 13, 14, 19, 21, 25, 35, 44, 45, 47 | 5.2 |
| 54 | * L. sieminskae Richt. et Matula | 12, 14, 15, 21, 22, 27, 31, 32, 34, 35, 38, 43, 44, 47, 51 | 9.6 |
| 55 | Leptolyngbya sp. | 46, 48, 49, 50, 51 | 3.5 |
| 56 | Merismopedia glauca (Ehrenb.) Kütz. | 44 | 0.4 |
| 57 | Microcoleus autumnalis (Trev. ex Gom.) Strunecky et al. | 7, 9, 13, 19, 20, 27, 33, 34, 35, 41, 44, 45 | 10.4 |
| 58 | * M. vaginatus Gom. ex Gom. | 10, 15, 22, 27, 30, 35, 38, 39, 42, 43, 48, 51 | 9.1 |
| 59 | * Nostoc caeruleum Lyngb. ex Born. et Flah. | 1, 17, 45, 47, 48, 49, 50, 51 | 4.8 |
| 60 | N. commune Vauch. ex Born. et Flah. | 5, 7, 10, 12, 14, 21, 22, 25, 26, 27, 35, 38, 39, 41, 42, 43, 44, 45, 46, 47, 48, 49, 51 | 13.9 |
| 61 | Nostoc paludosum Kütz. ex Born. et Flah. | 4, 25, 41 | 1.7 |
| 62 | Nostoc pruniforme [L.] C. Ag. ex Born. et Flah. | 40 | 0.4 |
| 63 | Nostoc sp. | 2, 4, 15, 22, 26, 35, 44, 48 | 4.8 |
| 64 | Oscillatoria sancta Kütz. ex Gom. | 50, 51 | 1.3 |
| 65 | O. tenuis C. Ag. ex Gom. | 12, 14, 24, 26, 35, 38, 44 | 4.8 |
| 66 | Petalonema incrustans [Kütz.] Komárek | 14, 18, 22, 26, 27, 29, 34, 39, 42, 43, 44, 46, 47, 48, 51 | 12.2 |
| 67 | Phormidesmis sp. | 4, 7, 12, 14, 16, 17, 18, 20, 21, 22, 25, 26, 27, 29, 32, 33, 34, 35, 36, 43, 44, 45, 46 | 24.3 |
| 68 | * Phormidiochaete nordstedtii (Born. et Flah. ex De Toni) Komárek | 19, 45 | 1.7 |
| 69 | Phormidium ambiguum Gom. | 26 | 0.9 |
| 70 | * P. kuetzingianum (Kirchn. ex Gom.) Anagn. et Komárek | 36, 49, 50 | 2.2 |
| 71 | Phormidium uncinatum Gom. ex Gom. | 12, 44 | 0.9 |
| 72 | * Pseudanabaena minima (G. S. An) Anagn. | 30 | 0.4 |
| 73 | Pseudanabaena sp. | 33, 49 | 0.9 |
| 74 | * Rivularia haematites [DC] C. Ag. ex Born. et Flah. | 49 | 0.4 |
| 75 | * Schizothrix lardacea Gom. | 22 | 0.4 |
| 76 | Scytonema hofmannii C. Ag. ex Born. et Flah. | 45 | 0.4 |
| 77 | S. ocellatum [Dillw.] Lyngb. ex Born. et Flah. | 22 | 0.4 |
| 78 | * Siphononema polonicum (Raciborski) Geitl. | 25, 44 | 0.9 |
| 79 | Stenomitos sp. | 35, 47 | 0.9 |
| 80 | Stigonema hormoides [Kütz.] Born. et Flah. | 7 | 0.4 |
| 81 | S. hormoides [Kütz.] Born. et Flah. var. subarcticum Böcher | 43 | 0.4 |
| 82 | S. informe Kütz. ex Born. et Flah. | 4, 22, 25, 35, 43, 44, 47 | 4.8 |
| 83 | S. mamillosum [Lyngb.] C. Ag. ex Born. et Flah. | 45 | 0.9 |
| 84 | S. minutum [C. Ag.] Hass. ex Born. et Flah. | 4, 5, 7, 13, 16, 18, 19, 20, 21, 22, 25, 28, 29, 30, 34, 35, 39, 42, 45, 46 | 19.1 |
| 85 | * S. mirabile B.-Mann. | 17 | 0.4 |
| 86 | S. ocellatum [Dillw.] Thur. ex Born. et Flah. | 14, 15, 22, 25, 36, 43 | 3.0 |
| 87 | * Symplocastrum friesii [C. Ag.] ex Kirchn. | 2 | 0.4 |
| 88 | Tolypothrix distorta Kütz. ex Born. et Flahault | 12, 13, 14, 21, 22, 41, 44 | 3.5 |
| 89 | T. tenuis Kütz. ex Born. et Flah. | 4, 7, 16, 21, 22, 25, 26, 30, 34, 35, 41, 43, 44, 45, 46, 47, 48 | 13.0 |
| 90 | Tolypothrix sp. | 51 | 0.4 |
| 91 | * Trichocoleus delicatulus (W. West et G.S. West) Anagn. | 34 | 0.4 |
| 92 | * T. sociatus (W. West et G. S. West) Anagn. | 44 | 1.3 |
| 93 | Trichocoleus sp. | 18 | 0.4 |
| PU 1 | Nen | SPU | SV | NZ | FJL | MRt | MRf | Y | |
|---|---|---|---|---|---|---|---|---|---|
| Nen | 54 | - | |||||||
| SPU | 47 | 57 | - | ||||||
| SV | 51 | 44 | 45 | - | |||||
| NZ | 31 | 28 | 32 | 26 | - | ||||
| FJL | 32 | 28 | 37 | 25 | 49 | - | |||
| MRt | 42 | 47 | 46 | 38 | 35 | 29 | - | ||
| MRf | 49 | 53 | 47 | 33 | 27 | 24 | 47 | - | |
| Y | 33 | 32 | 29 | 22 | 24 | 31 | 28 | 21 | - |
| T | 46 | 47 | 43 | 33 | 32 | 35 | 38 | 35 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davydov, D. Cyanobacterial Diversity of the Northern Polar Ural Mountains. Diversity 2021, 13, 607. https://doi.org/10.3390/d13110607
Davydov D. Cyanobacterial Diversity of the Northern Polar Ural Mountains. Diversity. 2021; 13(11):607. https://doi.org/10.3390/d13110607
Chicago/Turabian StyleDavydov, Denis. 2021. "Cyanobacterial Diversity of the Northern Polar Ural Mountains" Diversity 13, no. 11: 607. https://doi.org/10.3390/d13110607
APA StyleDavydov, D. (2021). Cyanobacterial Diversity of the Northern Polar Ural Mountains. Diversity, 13(11), 607. https://doi.org/10.3390/d13110607





