Old Brains in Alcohol: The Usability of Legacy Collection Material to Study the Spider Neuroarchitecture
Abstract
:1. Introduction
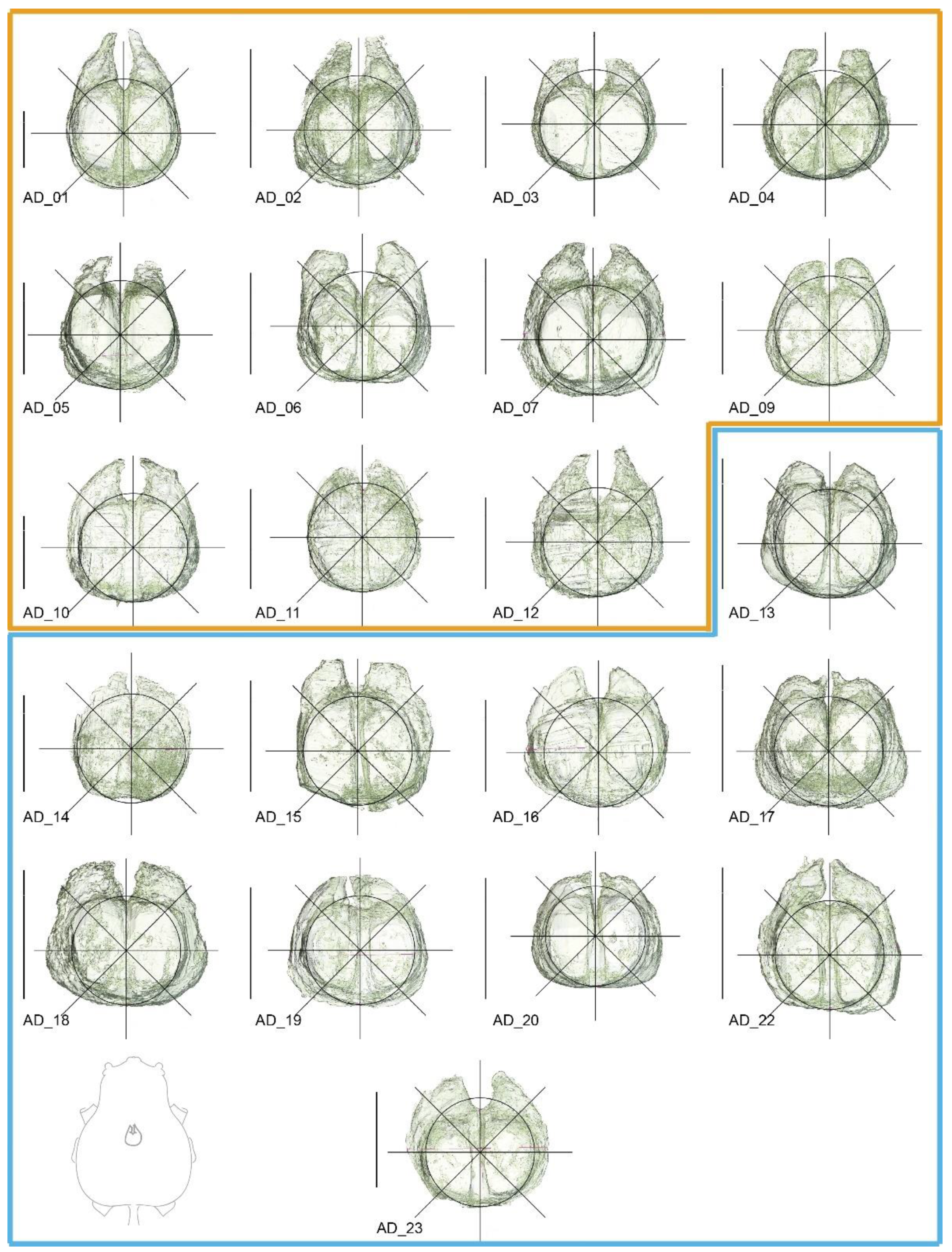
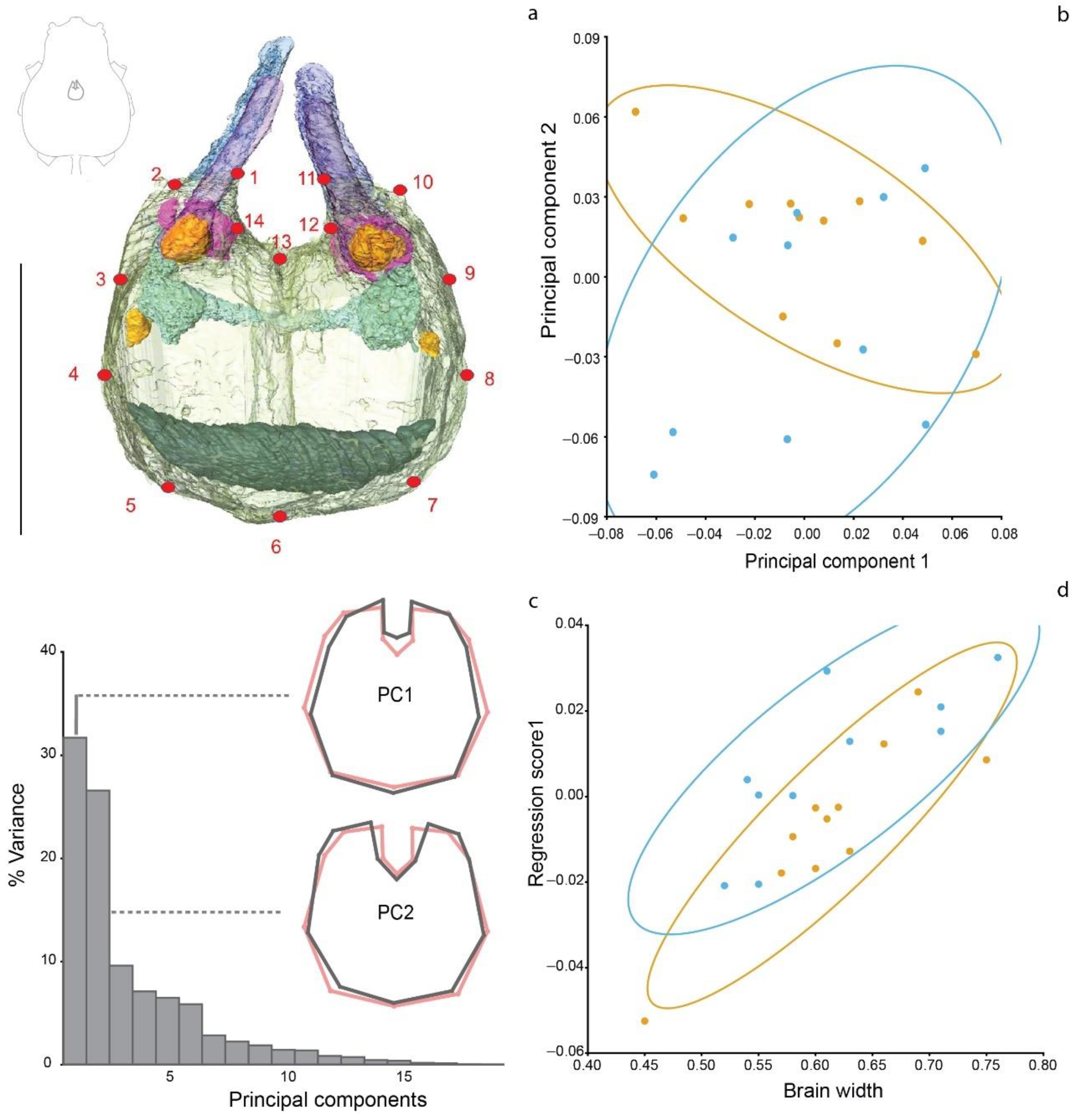
2. Materials and Methods
3. Results
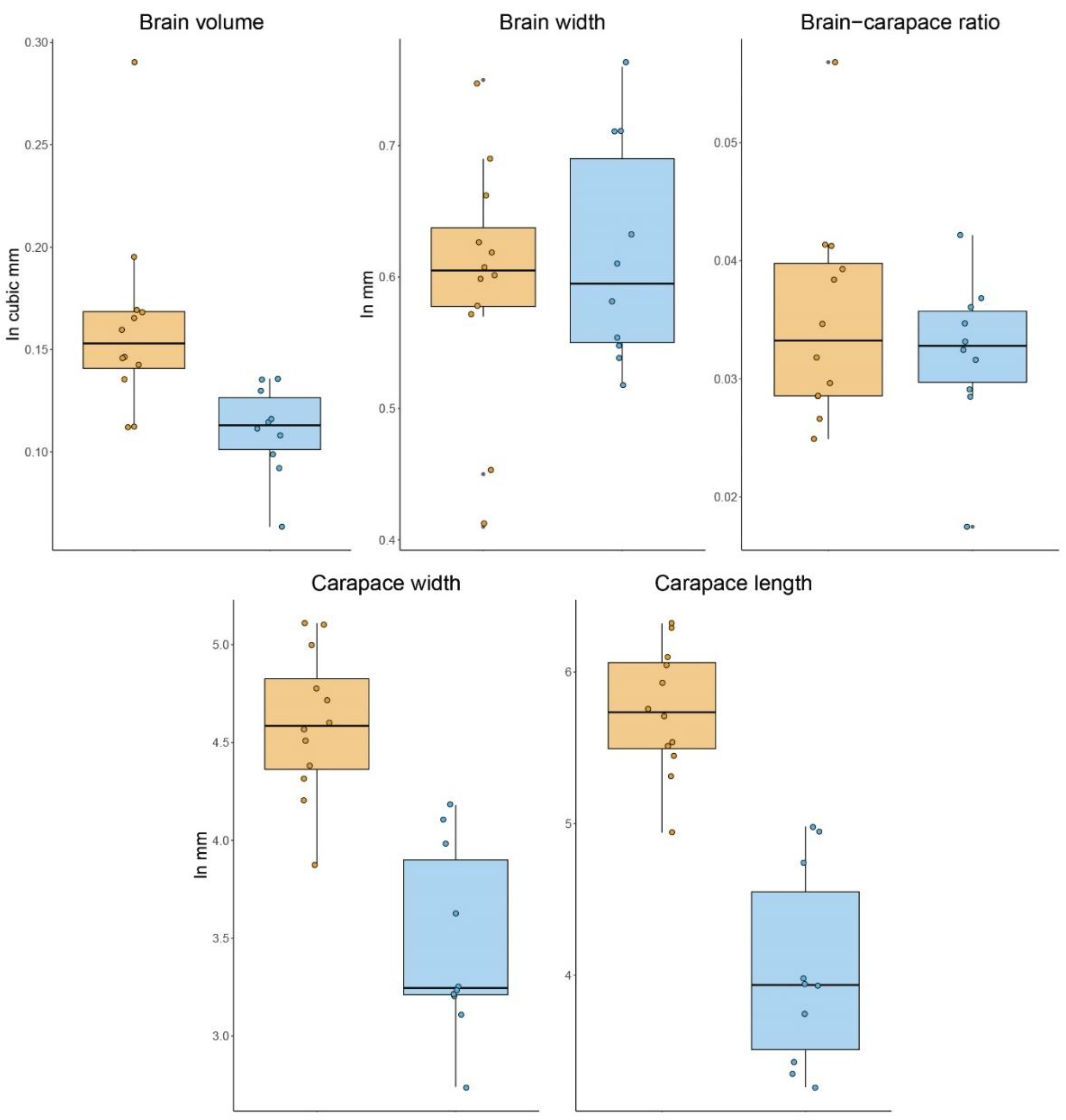
3.1. Size and Volumetric Analyses
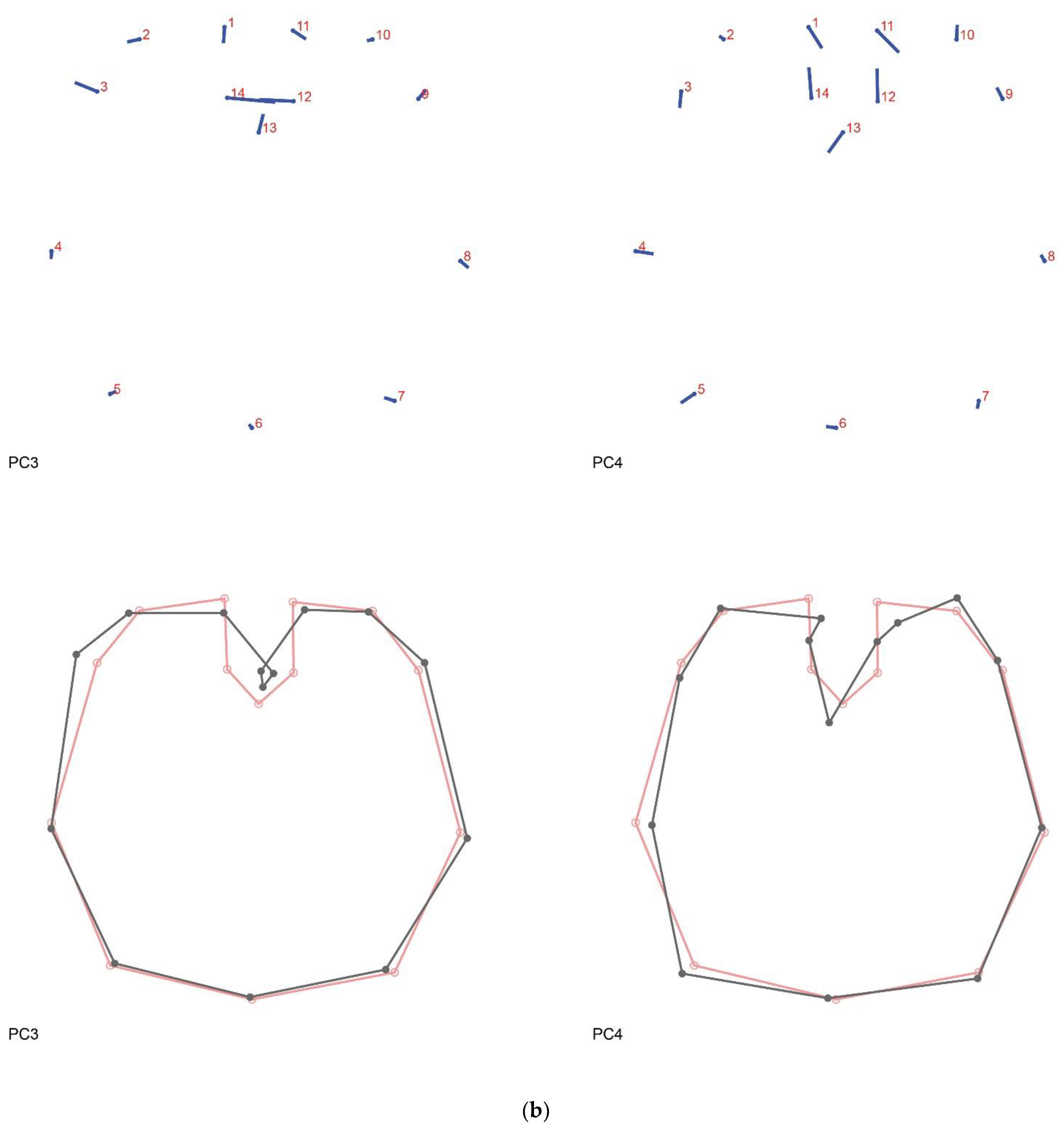
3.2. Shape Variation
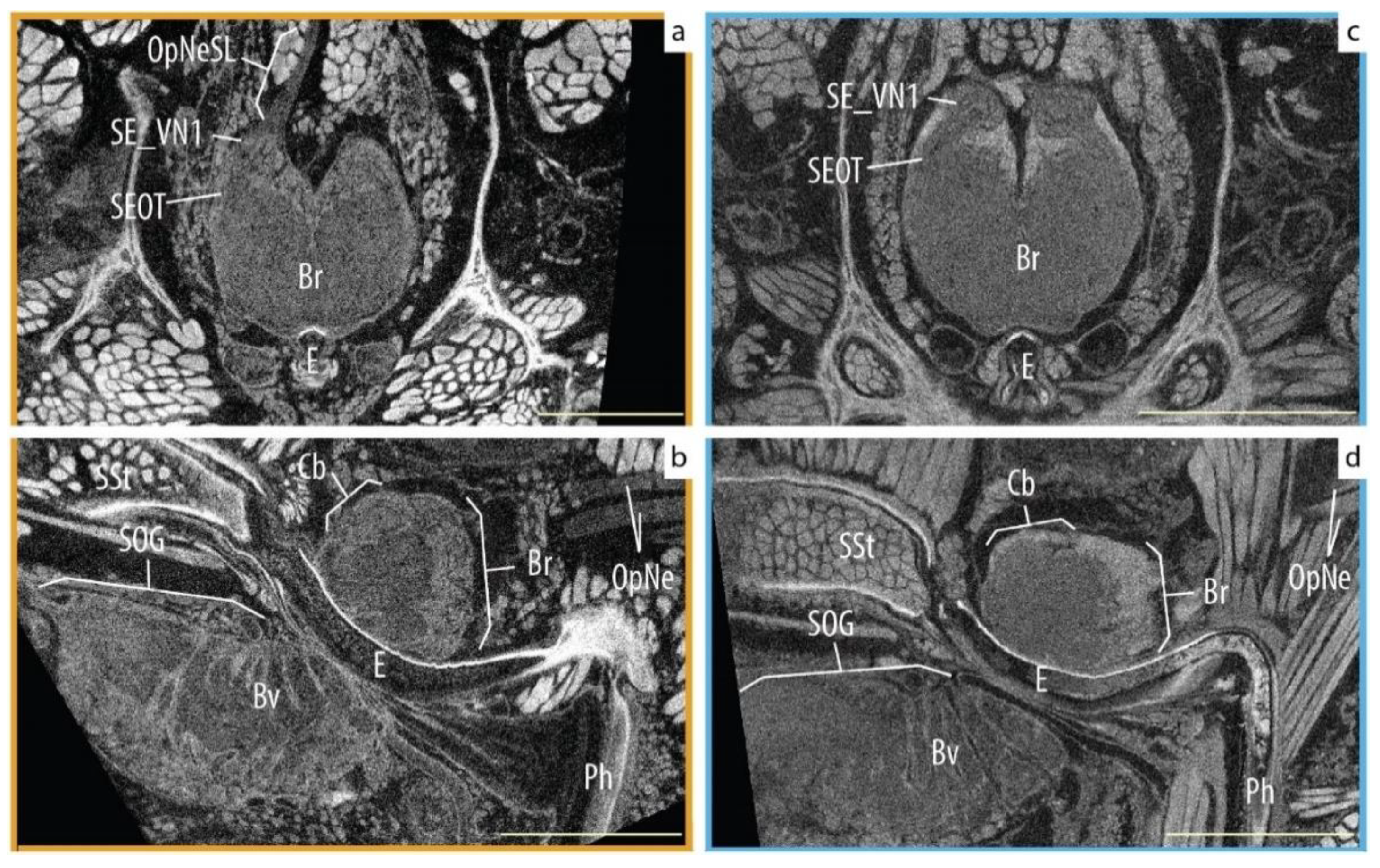
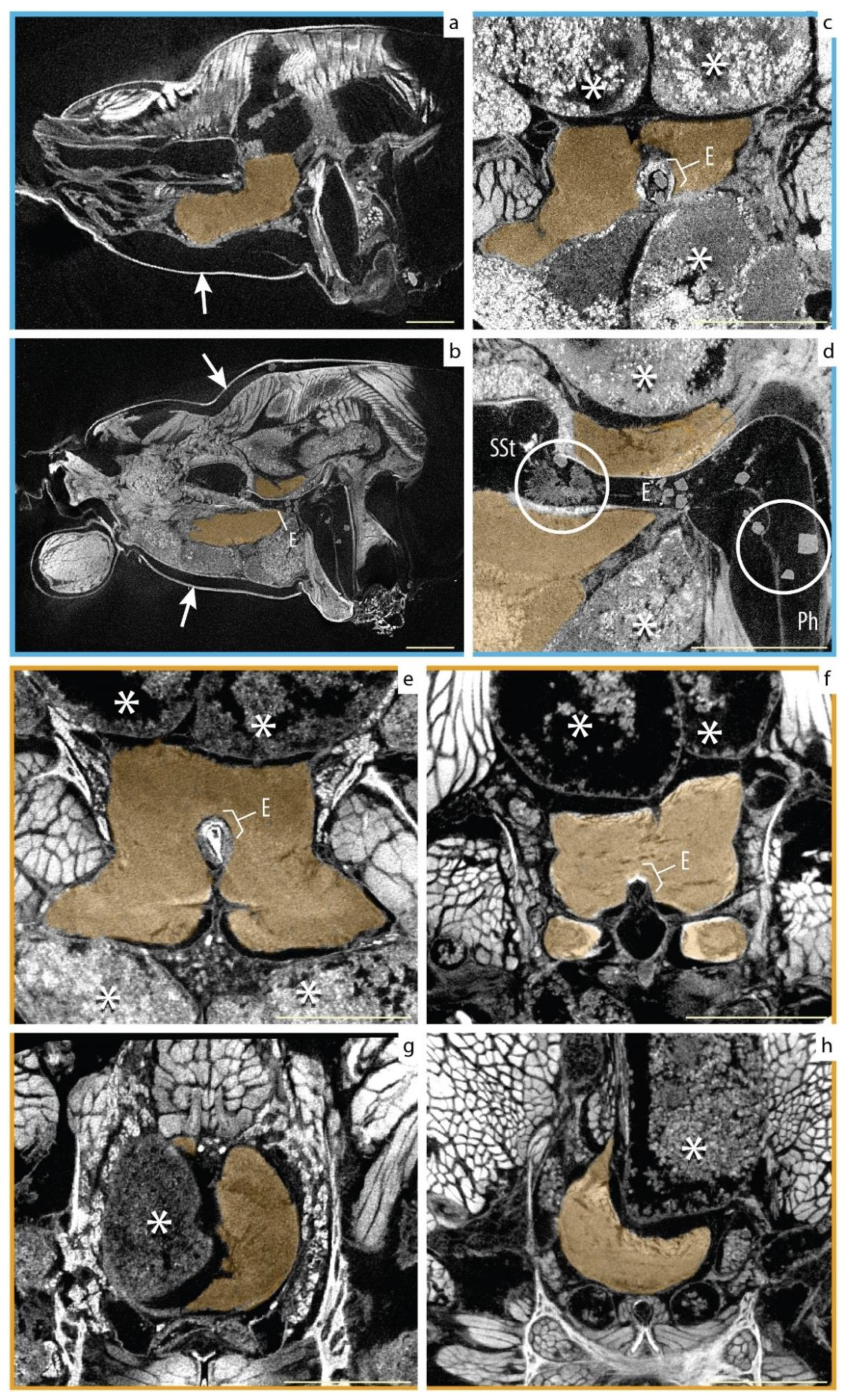
3.3. Qualitative Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Meineke, E.K.; Davies, T.J.; Daru, B.H.; Davis, C.C. Biological collections for understanding biodiversity in the Anthropocene. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20170386. [Google Scholar] [CrossRef] [Green Version]
- Derkarabetian, S.; Benavides, L.R.; Giribet, G. Sequence capture phylogenomics of historical ethanol-preserved museum specimens: Unlocking the rest of the vault. Mol. Ecol. Resour. 2019, 19, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Kvist, S.; Lenihan, J.; Giribet, G.; Ziegler, A. Sine systemate chaos? A versatile tool for earthworm taxonomy: Non-destructive imaging of freshly fixed and museum specimens using micro-computed tomography. PLoS ONE 2014, 9, e96617. [Google Scholar] [CrossRef] [PubMed]
- Beaman, R.S.; Cellinese, N. Mass digitization of scientific collections: New opportunities to transform the use of biological specimens and underwrite biodiversity science. Zookeys 2012, 209, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Ellis, S. The history and impact of digitization and digital data mobilization on biodiversity research. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20170391. [Google Scholar] [CrossRef] [Green Version]
- Short, A.E.Z.; Dikow, T.; Moreau, C.S. Entomological Collections in the Age of Big Data. Annu. Rev. Entomol. 2018, 63, 513–530. [Google Scholar] [CrossRef]
- Hofreiter, M. Nondestructive DNA extraction from museum specimens. In Ancient DNA: Methods and Protocols, Methods in Molecular Biology; Shapiro, B., Hofreiter, M., Eds.; Humana Press: New York, NY, USA, 2012; Volume 840, pp. 93–100. [Google Scholar] [CrossRef]
- Gilbert, M.T.P.; Moore, W.; Melchior, L.; Worebey, M. DNA extraction from dry museum beetles without conferring external morphological damage. PLoS ONE 2007, 2, e272. [Google Scholar] [CrossRef] [Green Version]
- Castalanelli, M.A.; Severtson, D.L.; Brumley, C.J.; Szito, A.; Foottit, R.G.; Grimm, M.; Munyard, K.; Groth, D.M. A rapid non-destructive DNA extraction method for insects and other arthropods. J. Asia-Pac. Entomol. 2010, 13, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.A.; Beentjes, K.K.; van Helsdingen, P.; Ijland, S. Which specimens from a museum collection will yield DNA barcodes? A time series study of spiders in alcohol. Zookeys 2013, 365, 245–261. [Google Scholar] [CrossRef] [Green Version]
- Vink, C.J.; Thomas, S.M.; Paquin, P.; Hayashi, C.Y.; Hedin, M. The effects of preservatives and temperatures on arachnid DNA. Invertebr. Syst. 2005, 19, 99–104. [Google Scholar] [CrossRef]
- Marquina, D.; Buczek, M.; Ronquist, F.; Lukasik, P. The effect of ethanol concentration on the morphological and molecular preservation of insects for biodiversity studies. PeerJ 2021, 9, e10799. [Google Scholar] [CrossRef] [PubMed]
- King, J.R.; Porter, S.D. Recommendations on the use of alcohols for preservation of ant specimens (Hymenoptera, Formicidae). Insectes Soc. 2004, 51, 197–202. [Google Scholar] [CrossRef]
- Sombke, A.; Lipke, E.; Michalik, P.; Uhl, G.; Harzsch, S. Potential and limitations of X-ray micro-computed tomography in arthropod neuroanatomy: A methodological and comparative survey. J. Comp. Neurol. 2015, 523, 1281–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisbecker, V. Distortion in formalin-fixed brains: Using geometric morphometrics to quantify the worst-case scenario in mice. Brain Struct. Funct. 2012, 217, 677–685. [Google Scholar] [CrossRef]
- Ziegler, A.; Menze, B.H. Accelerated acquisition, visualization, and analysis of zoo-anatomical data. In Computation for Humanity; Zander, J., Mosterman, J., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 233–261. [Google Scholar]
- Keklikoglou, K.; Faulwetter, S.; Chatzinikolaou, E.; Wils, P.; Brecko, J.; Kvaček, J.; Metscher, B.; Arvanitidis, C. Micro-computed tomography for natural history specimens: A handbook of best practice protocols. Eur. J. Taxon. 2019, 522, 1–55. [Google Scholar] [CrossRef]
- Faulwetter, S.; Dailianis, T.; Vasileiadou, A.; Arvanitidis, C. Investigation of contrast enhancing techniques for the application of Micro-CT in marine biodiversity studies. Microsc.Anal. 2013, 27, S4–S7. [Google Scholar]
- Sakurai, Y.; Ikeda, Y. Development of a contrast-enhanced micro computed tomography protocol for the oval squid (Sepioteuthis lessoniana) brain. Microsc. Res. Tech. 2019, 82, 1941–1952. [Google Scholar] [CrossRef]
- Swart, P.; Wicklein, M.; Sykes, D.; Ahmed, F.; Krapp, H.G. A quantitative comparison of micro-CT preparations in Dipteran flies. Sci. Rep. 2016, 6, 39380. [Google Scholar] [CrossRef] [Green Version]
- Alba-Tercedor, J.; Hunter, W.B.; Alba-Alejandre, I. Using micro-computed tomography to reveal the anatomy of adult Diaphorina citri Kuwayama (Insecta: Hemiptera, Liviidae) and how it pierces and feeds within a citrus leaf. Sci. Rep. 2021, 11, 1358. [Google Scholar] [CrossRef]
- Alba-Alejandre, I.; Hunter, W.B.; Alba-Tercedor, J. Micro-CT study of male genitalia and reproductive system of the Asian citrus psyllid, Diaphorina citri Kuwayama, 1908 (Insecta: Hemiptera, Liviidae). PLoS ONE 2018, 13, e0202234. [Google Scholar] [CrossRef]
- Castejón, D.; Alba-Tercedor, J.; Rotllant, G.; Ribes, E.; Durfort, M.; Guerao, G. Micro-computed tomography and histology to explore internal morphology in decapod larvae. Sci. Rep. 2018, 8, 14399. [Google Scholar] [CrossRef] [PubMed]
- van de Kamp, T.; Schwermann, A.H.; dos Santos Rolo, T.; Lösel, P.D.; Engler, T.; Etter, W.; Faragó, T.; Göttlicher, J.; Heuveline, V.; Kopmann, A.; et al. Parasitoid biology preserved in mineralized fossils. Nat. Commun. 2018, 9, 3325. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Quiroz, F.A.; Miller, J.A. Micro-CT visualization of the CNS: Performance of different contrast-enhancing techniques for documenting the spider brain. J. Comp. Neurol. 2021. under review. [Google Scholar]
- Steinhoff, P.O.M.; Sombke, A.; Liedtke, J.; Schneider, J.M.; Harzsch, S.; Uhl, G. The synganglion of the jumping spider Marpissa muscosa (Arachnida: Salticidae): Insights from histology, immunohistochemistry and microCT analysis. Arthropod Struct. Dev. 2017, 46, 156–170. [Google Scholar] [CrossRef]
- Stafstrom, J.A.; Michalik, P.; Hebets, E.A. Sensory system plasticity in a visually specialized, nocturnal spider. Sci. Rep. 2017, 7, 46627. [Google Scholar] [CrossRef]
- Steinhoff, P.O.M.; Liedtke, J.; Sombke, A.; Schneider, J.M.; Uhl, G. Early environmental conditions affect the volume of higher-order brain centers in a jumping spider. J. Zool. 2018, 304, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, P.O.M.; Uhl, G.; Harzsch, S.; Sombke, A. Visual pathways in the brain of the jumping spider Marpissa muscosa. J. Comp. Neurol. 2020, 528, 1883–1902. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-W.; Lopardo, L.; Uhl, G. Diversification through sexual selection on gustatorial courtship traits in dwarf spiders. bioRxiv 2021. [Google Scholar] [CrossRef]
- Saint-Remy, G. Contribution a L’étude du Cerveau Chez les Arthropodes Trachéates; Faculté des Sciences de Paris: Paris, France, 1890. [Google Scholar]
- Long, S.M. Spider Brain Morphology & Behavior; University of Massachusetts: Amherst, MA, USA, 2016. [Google Scholar]
- Long, S.M. Variations on a theme: Morphological variation in the secondary eye visual pathway across the order of Araneae. J. Comp. Neurol. 2021, 529, 259–280. [Google Scholar] [CrossRef]
- Hill, D.E. The Structure of the Central Nervous System of Jumping Spiders of the Genus Phidippus (Araneae: Salticidae). Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2006. [Google Scholar]
- Wickham, H. ggplot2 Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. 2021. Available online: https://cran.r-project.org/package=dplyr (accessed on 27 September 2021).
- Rohlf, F.J. tpsUtil, v1.81; Department of Anthropology, and Ecology & Evolution, Stony Brook University: New York, NY, USA, 2021. [Google Scholar]
- Rohlf, F.J. tpsDig2, v2.32; Department of Anthropology, and Ecology & Evolution, Stony Brook University: New York, NY, USA, 2021. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Theska, T.; Sieriebriennikov, B.; Wighard, S.S.; Werner, M.S.; Sommer, R.J. Geometric morphometrics of microscopic animals as exemplified by model nematodes. Nat. Protoc. 2020, 15, 2611–2644. [Google Scholar] [CrossRef]
- Loesel, R.; Seyfarth, E.A.; Bräunig, P.; Agricola, H.J. Neuroarchitecture of the arcuate body in the brain of the spider Cupiennius salei (Araneae, Chelicerata) revealed by allatostatin-, proctolin-, and CCAP-immunocytochemistry and its evolutionary implications. Arthropod Struct. Dev. 2011, 40, 210–220. [Google Scholar] [CrossRef]
- Babu, K.S.; Barth, F.G. Neuroanatomy of the central nervous system of the wandering spider, Cupiennius salei (Arachnida, Araneida). Zoomorphology 1984, 104, 344–359. [Google Scholar] [CrossRef]
- Schmid, A.; Becherer, C. Distribution of histamine in the CNS of different spiders. Microsc. Res. Tech. 1999, 44, 81–93. [Google Scholar] [CrossRef]
- Lipke, E.; Hammel, J.U.; Michalik, P. First evidence of neurons in the male copulatory organ of a spider (Arachnida, Araneae). Biol. Lett. 2015, 11, 20150465. [Google Scholar] [CrossRef] [PubMed]
- Dederichs, T.M.; Müller, C.H.G.; Sentenská, L.; Lipke, E.; Uhl, G.; Michalik, P. The innervation of the male copulatory organ of spiders (Araneae)—A comparative analysis. Front. Zool. 2019, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Quiroz, F.A.; Petcharad, B.; Miller, J.A. First records and three new species of the family Symphytognathidae (Arachnida, Araneae) from Thailand, and the circumscription of the genus Crassignatha Wunderlich, 1995. Zookeys 2021, 1012, 21–53. [Google Scholar] [CrossRef]
- iNaturalist: Cross Orbweaver (Araneus diadematus). Available online: https://www.inaturalist.org/taxa/52628-Araneus-diadematus (accessed on 27 September 2021).
- Uhl, G.; Schmitt, S.; Schäfer, M.A.; Blanckenhorn, W. Food and sex-specific growth strategies in a spider. Evol. Ecol. Res. 2004, 6, 523–540. [Google Scholar] [CrossRef]
- Dowling, J.L.; Luther, D.A.; Marra, P.P. Comparative effects of urban development and anthropogenic noise on bird songs. Behav. Ecol. 2012, 23, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Narango, D.L.; Rodewald, A.D. Urban-associated drivers of song variation along a rural-urban gradient. Behav. Ecol. 2016, 27, 608–616. [Google Scholar] [CrossRef]
- Kerstes, N.A.G.; Breeschoten, T.; Kalkman, V.J.; Schilthuizen, M. Snail shell colour evolution in urban heat islands detected via citizen science. Commun. Biol. 2019, 2, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, A.J.; Adams, B.J.; Bell, K.C.; Ludt, W.B.; Pauly, G.B.; Vendetti, J.E. Natural history collections are critical resources for contemporary and future studies of urban evolution. Evol. Appl. 2021, 14, 233–247. [Google Scholar] [CrossRef] [PubMed]
| Specimen Code | Coll. Date | Collector | Coll. Country | Province | Region | Locality | Coord Lat | Coord Lon |
|---|---|---|---|---|---|---|---|---|
| AD_01 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_02 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_03 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_04 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_05 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_06 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_07 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_09 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_10 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_11 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_12 | 7-Oct-2020 | A. Rivera-Quiroz, D, Arguedas | NL | Zuid Holland | Leiden | Singel Park | 52.16088 | 4.504739 |
| AD_13 | 22-Apr-1940 | B. de Jong | NL | ND | ND | ND | ND | ND |
| AD_14 | 24-Aug-1941 | B. de Jong | NL | Noord Holland | Amsterdam | Kruislaan | ND | ND |
| AD_15 | 9-Oct-1929 | W. B. Begerinck, B. de Jong | NL | Noord Holland | ND | ND | ND | ND |
| AD_16 | 9-Oct-1929 | W. B. Begerinck, B. de Jong | NL | Noord Holland | ND | ND | ND | ND |
| AD_17 | 18-Oct-1946 | B. de Jong | NL | Noord Holland | Hilversum | Lage Vuursche | ND | ND |
| AD_18 | 18-Oct-1946 | B. de Jong | NL | Noord Holland | Hilversum | Lage Vuursche | ND | ND |
| AD_19 | 22-Apr-1940 | B. de Jong | NL | ND | ND | ND | ND | ND |
| AD_20 | 18-Oct-1946 | B. de Jong | NL | Noord Holland | Hilversum | Lage Vuursche | ND | ND |
| AD_22 | 18-Oct-1948 | B. de Jong | NL | Noord Holland | ND | ND | ND | ND |
| AD_23 | 3-Nov-1947 | B. de Jong | NL | Overijssel | Wanneperveen | ND | ND | ND |
| p-Values | [Outliers Removed] | |||
|---|---|---|---|---|
| New | Old | New | Old | |
| Carapace width | 0.854 | 0.2008 | -- | -- |
| Carapace length | 0.8829 | 0.1361 | -- | -- |
| Brain width | 0.3331 | 0.2035 | -- | -- |
| Brain volume | 0.009821 | 0.3665 | 0.688 [1] | -- |
| BR vol. vs. Car. W | 0.1129 | 0.3808 | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Quiroz, F.A.; Miller, J.A. Old Brains in Alcohol: The Usability of Legacy Collection Material to Study the Spider Neuroarchitecture. Diversity 2021, 13, 601. https://doi.org/10.3390/d13110601
Rivera-Quiroz FA, Miller JA. Old Brains in Alcohol: The Usability of Legacy Collection Material to Study the Spider Neuroarchitecture. Diversity. 2021; 13(11):601. https://doi.org/10.3390/d13110601
Chicago/Turabian StyleRivera-Quiroz, F. Andres, and Jeremy Abraham Miller. 2021. "Old Brains in Alcohol: The Usability of Legacy Collection Material to Study the Spider Neuroarchitecture" Diversity 13, no. 11: 601. https://doi.org/10.3390/d13110601
APA StyleRivera-Quiroz, F. A., & Miller, J. A. (2021). Old Brains in Alcohol: The Usability of Legacy Collection Material to Study the Spider Neuroarchitecture. Diversity, 13(11), 601. https://doi.org/10.3390/d13110601






