Additions to Occultibambusaceae (Pleosporales, Dothideomycetes): Unrevealing Palmicolous Fungi in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Morphological Examination
2.2. DNA Extraction, PCR Amplification and Sequencing
2.3. Phylogenetic Analyses
3. Results
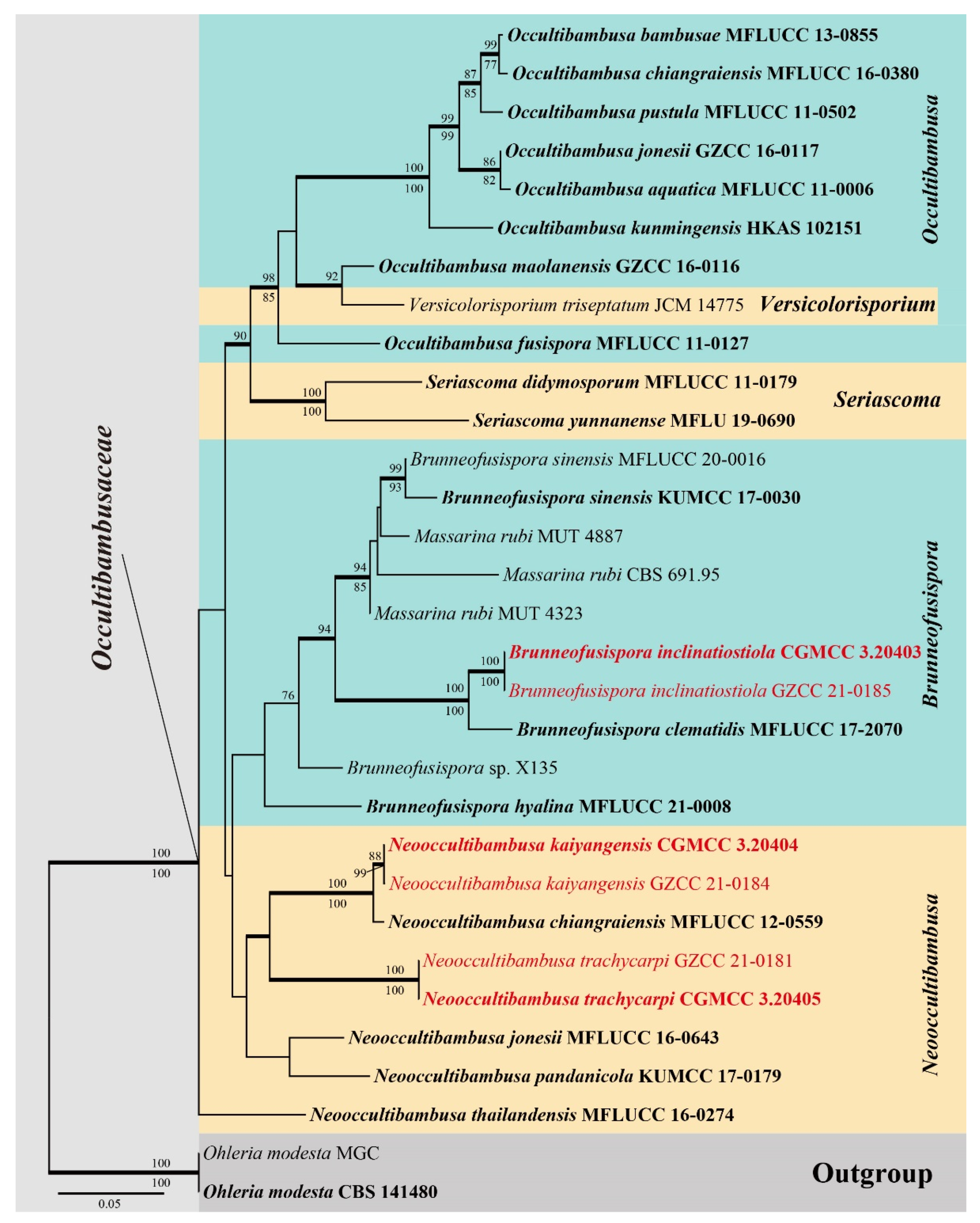
3.1. Phylogenetic Analyses
3.2. Taxonomy
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, D.Q.; Phookamsak, R.; Wijayawardene, N.N.; Li, W.J.; Bhat, D.J.; Xu, J.C.; Taylor, J.E.; Hyde, K.D.; Chukeatirote, E. Bambusicolous fungi. Fungal Divers. 2017, 82, 1–105. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Tanaka, K.; Harada, Y. Bambusicolous fungi in Japan (7): A new coelomycetous genus, Versicolorisporium. Mycoscience 2008, 49, 211–214. [Google Scholar] [CrossRef]
- Doilom, M.; Dissanayake, A.J.; Wanasinghe, D.N.; Boonmee, S.; Liu, J.K.; Bhat, D.J.; Taylor, J.E.; Bahkali, A.H.; Mckenzie, E.H.C.; Hyde, K.D. Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers. 2017, 82, 107–182. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Raspé, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal diversity notes 929–1035: Taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Wang, B.; Hyde, K.D.; McKenzie, E.H.C.; Raja, H.A.; Tanaka, K.; Abdel-Wahab, M.A.; Abdel-Aziz, F.A.; Doilom, M.; Phookamsak, R.; et al. Freshwater Dothideomycetes. Fungal Divers. 2020, 105, 319–575. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Wijayawardene, N.N.; Xu, J.C.; Cheewangkoon, R.; Mortimer, P.E. Taxonomic novelties in Magnolia-associated pleosporalean fungi in the Kunming Botanical Gardens (Yunnan, China). PLoS ONE 2020, 15, e0235855. [Google Scholar] [CrossRef]
- Rathnayaka, A.; Dayarathne, M.; Maharachchikumbura, S.S.N.; Liu, J.K.J.; Hyde, K.D. Introducing Seriascoma yunnanense sp. nov. (Occultibambusaceae, Pleosporales) based on evidence from morphology and phylogeny. Asian J. Mycol. 2019, 2, 245–253. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Jeewon, R.; Bhat, J.D.; Camporesi, E.; Kang, J.C. Neooccultibambusa jonesii, a novel taxon within Occultibambusaceae. Mycosphere 2016, 7, 1458–1472. [Google Scholar] [CrossRef]
- Hyde, K.D.; Chaiwan, N.; Norphanphoun, C.; Boonmee, S.; Camporesi, E.; Chethana, K.W.T.; Dayarathne, M.C.; de Silva, N.I.; Dissanayake, A.J.; Ekanayaka, A.H.; et al. Mycosphere notes 169–224. Mycosphere 2018, 9, 271–430. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; McKenzie, E.H.C.; Bhat, D.J.; Phillips, A.J.L.; Wanasinghe, D.N.; Samarakoon, M.C.; Jayawardena, R.S.; Dissanayake, A.J.; Tennakoon, D.S.; et al. Fungal diversity notes 840–928: Micro-fungi 4 associated with Pandanaceae. Fungal Divers. 2018, 93, 1–160. [Google Scholar] [CrossRef]
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.A.; Boonmee, S.; Zhao, Q.; et al. Fungal diversity notes 367–490: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 80, 1–270. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liu, J.K.; Hyde, K.D.; Yang, W.; Liu, Z.Y. Fungi from Asian Karst formations II. Two new species of Occultibambusa (Occultibambusaceae, Dothideomycetes) from karst landforms of China. Mycosphere 2017, 8, 550–559. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; McKenzie, E.H.C.; Phillips, A.J.L.; Gareth Jones, E.B.; Jayarama Bhat, D.; Marc, S.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Calabon, M.S.; Jones, E.B.G.; Boonmee, S.; Doilom, M.; Lumyong, S.; Hyde, K.D. Five Novel Freshwater Ascomycetes Indicate High Undiscovered Diversity in Lotic Habitats in Thailand. J. Fungi 2021, 7, 117. [Google Scholar] [CrossRef]
- Senanayake, I.; Rathnayake, A.; Marasinghe, D.; Calabon, M.; Gentekaki, E.; Lee, H. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Chomnunti, P.; Hongsanan, S.; Aguirre-Hudson, B.; Tian, Q.; Peršoh, D.; Dhami, M.K.; Alias, A.S.; Xu, J.C.; Liu, X.Z.; Stadler, M. The sooty moulds. Fungal Divers. 2014, 66, 1–36. [Google Scholar] [CrossRef]
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Kazutaka, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Dissanayake, A.J.; Bhunjun, C.S.; Maharachchikumbura, S.S.M.; Liu, J.K. Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 2020, 11, 2652–2676. [Google Scholar] [CrossRef]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2011, 12, 335–337. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods); Sinauer Associates: Sunderland, UK, 2003. [Google Scholar]
- Nylander, J.A.A. MrModeltest V2. Program distributed by the author. In Evolutionary Biology Centre; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.B.; Phookamsak, R.; Hyde, K.D.; Mortimer, P.E.; Xu, J.C.; Kakumyan, P.; Karunarathna, S.C.; Kumla, J. A taxonomic appraisal of bambusicolous fungi in Occultibambusaceae (Pleosporales, Dothideomycetes) with new collections from Yunnan Province, China. Life 2021, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Hyde, K.D. Microfungi of Tropical and Temperate Palms; Fungal Diversity Press, The University of Hong Kong: Hong Kong, 2003; pp. 1–459. [Google Scholar]
- Liu, J.K. Phylogeny of Ascomycetes from Palms; Mae Fah Luang University: Chiang Rai, Thailand, 2014. [Google Scholar]



| Species | Voucher/Strain/Isolate | GenBank Accession Number | ||||
|---|---|---|---|---|---|---|
| SSU | ITS | LSU | RPB2 | TEF1α | ||
| Brunneofusispora clematidis | MFLUCC 17-2070 | MT226685 | MT310615 | MT214570 | MT394692 | MT394629 |
| Brunneofusispora hyalina | MFLUCC 21-0008 | MW485613 | MW260330 | MW287234 | MW512609 | MW512606 |
| Brunneofusispora inclinatiostiola | CGMCC 3.20403 | MZ964884 | MZ964866 | MZ964875 | OK061075 | OK061069 |
| Brunneofusispora inclinatiostiola | GZCC 21-0185 | MZ964885 | MZ964867 | MZ964876 | OK061076 | OK061070 |
| Brunneofusispora sinensis | KUMCC 17-0030 | MH393556 | MH393558 | MH393557 | _ | MH395329 |
| Brunneofusispora sinensis | MFLUCC 20-0016 | MT159636 | MT159630 | MT159624 | MT159613 | MT159607 |
| Brunneofusispora sp. | X135 | _ | MK304223 | _ | _ | _ |
| Massarina rubi | CBS 691.95 | GU456301 | _ | FJ795453 | FJ795470 | _ |
| Massarina rubi | MUT 4323 | _ | KF636766 | KF636772 | _ | _ |
| Massarina rubi | MUT 4887 | KT587318 | KR014359 | KP671721 | _ | _ |
| Neooccultibambusa chiangraiensis | MFLUCC 12-0559 | KU712458 | KU712442 | KU764699 | _ | KU872761 |
| Neooccultibambusa jonesii | MFLUCC 16-0643 | KY111438 | _ | KY111437 | _ | _ |
| Neooccultibambusa kaiyangensis | CGMCC 3.20404 | MZ964886 | MZ964868 | MZ964877 | OK061077 | OK061071 |
| Neooccultibambusa kaiyangensis | GZCC 21-0184 | MZ964887 | MZ964869 | MZ964878 | OK061078 | OK061072 |
| Neooccultibambusa pandanicola | KUMCC 17-0179 | MG298942 | MG298941 | MG298940 | MG298944 | MG298943 |
| Neooccultibambusa thailandensis | MFLUCC 16-0274 | MH260348 | MH275074 | MH260308 | MH412758 | MH412780 |
| Neooccultibambusa trachycarpi | CGMCC 3.20405 | MZ964888 | MZ964870 | MZ964879 | OK061079 | OK061073 |
| Neooccultibambusa trachycarpi | GZCC 21-0181 | MZ964889 | MZ964871 | MZ964880 | OK061080 | OK061074 |
| Occultibambusa aquatica | MFLUCC 11-0006 | KX698112 | KX698114 | KX698110 | _ | _ |
| Occultibambusa bambusae | MFLUCC 13-0855 | KU872116 | KU940123 | KU863112 | KU940170 | KU940193 |
| Occultibambusa chiangraiensis | MFLUCC 16-0380 | KX655551 | _ | KX655546 | KX655566 | KX655561 |
| Occultibambusa fusispora | MFLUCC 11-0127 | _ | KU940125 | KU863114 | KU940172 | KU940195 |
| Occultibambusa jonesii | GZCC 16-0117 | KY628324 | _ | KY628322 | KY814758 | KY814756 |
| Occultibambusa kunmingensis | HKAS 102151 | MT864342 | MT627716 | MN913733 | MT878453 | MT954407 |
| Occultibambusa maolanensis | GZCC 16-0116 | KY628325 | _ | KY628323 | KY814759 | KY814757 |
| Occultibambusa pustula | MFLUCC 11-0502 | KU872118 | KU940126 | KU863115 | _ | _ |
| Ohleria modesta | CBS 141480 | KX650513 | KX650563 | KX650563 | KX650583 | KX650534 |
| Ohleria modesta | MGC | _ | KX650562 | KX650562 | KX650582 | KX650533 |
| Seriascoma didymosporum | MFLUCC 11-0179 | KU872119 | KU940127 | KU863116 | KU940173 | KU940196 |
| Seriascoma yunnanense | MFLU 19-0690 | MN174694 | _ | MN174695 | MN210324 | MN381858 |
| Versicolorisporium triseptatum | JCM 14775 | AB524501 | AB365596 | AB330081 | _ | _ |
| Taxa | Ascomata | Asci (μm) | Ascospores | References | ||
|---|---|---|---|---|---|---|
| Morphology | Size (μm) | Morphology | Size (μm) | |||
| N. chiangraiensis1 | Immersed to erumpent, without a neck, globose to subglobose | (250–)345–355(–400) × (230–)245–295(–325) | (70–)115–160(–207) × 14–21 | Fusoid, hyaline to pale brown, 1–3 septate, with a sheath | (33–)36–37(–43) × 8–13 | [3] |
| N. jonesii2 | Immersed to erumpent, without a neck, globose to subglobose | 104–155 × 130–160 | 47–76 × 8–10 | Fusiform, brown when matrure, 1-septate, submedian, without a sheath | 15–20 × 2.5–4.5 | [8] |
| N. kaiyangensis | Immersed to erumpent, without a neck, depressed globose or irregular | 200–360 × 110–270 | 70–108 × 7.5–11.0 | Fusiform, hyaline to pale brown, 1–3-septate, with a sheath | 16.0–23.5 × 3.0–5.5 | This study |
| N. thailandensis | Superficial, globose to subglobose | 65–80 × 44–61 | 34–51 × 5–8 | Fusiform, yellow–brown, 1-septate, without a sheath | 6–11 × 2–3.5 | [10] |
| N. trachycarpi | Immersed to erumpent, without a neck, depressed globose or irregular | 150–240 × 140–200 | 48–120 × 10–15 | Fusiform, hyaline, 1-septate, becoming light brown and present two pseudosepta when senescent, with a sheath | 12–19 × 3.0–5.0 | This study |
| Conidiophores | Conidiogenous cells size (μm) | Conidia (Chlamydospores) | ||||
| morphology | size (μm) | morphology | size (μm) | |||
| N. pandanicola3 | Pale-brown to brown, 3–5-septate | 13–71 × 3.5–7 | 2.5–5.5 × 4–5.5 | Obclavate, olivaceous brown to mid-brown, 7–17-euseptate | 28–150 × 7–21 | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.-D.; Zhang, S.-N.; Cheewangkoon, R.; Liu, J.-K. Additions to Occultibambusaceae (Pleosporales, Dothideomycetes): Unrevealing Palmicolous Fungi in China. Diversity 2021, 13, 516. https://doi.org/10.3390/d13110516
Yu X-D, Zhang S-N, Cheewangkoon R, Liu J-K. Additions to Occultibambusaceae (Pleosporales, Dothideomycetes): Unrevealing Palmicolous Fungi in China. Diversity. 2021; 13(11):516. https://doi.org/10.3390/d13110516
Chicago/Turabian StyleYu, Xian-Dong, Sheng-Nan Zhang, Ratchadawan Cheewangkoon, and Jian-Kui Liu. 2021. "Additions to Occultibambusaceae (Pleosporales, Dothideomycetes): Unrevealing Palmicolous Fungi in China" Diversity 13, no. 11: 516. https://doi.org/10.3390/d13110516
APA StyleYu, X.-D., Zhang, S.-N., Cheewangkoon, R., & Liu, J.-K. (2021). Additions to Occultibambusaceae (Pleosporales, Dothideomycetes): Unrevealing Palmicolous Fungi in China. Diversity, 13(11), 516. https://doi.org/10.3390/d13110516








