DNA Barcoding of Penaeidae (Decapoda; Crustacea): Non-Distance-Based Species Delimitation of the Most Economically Important Shrimp Family
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction, Amplification, and Sequencing
2.3. DNA Barcoding and MOTU Delimitation Analyses
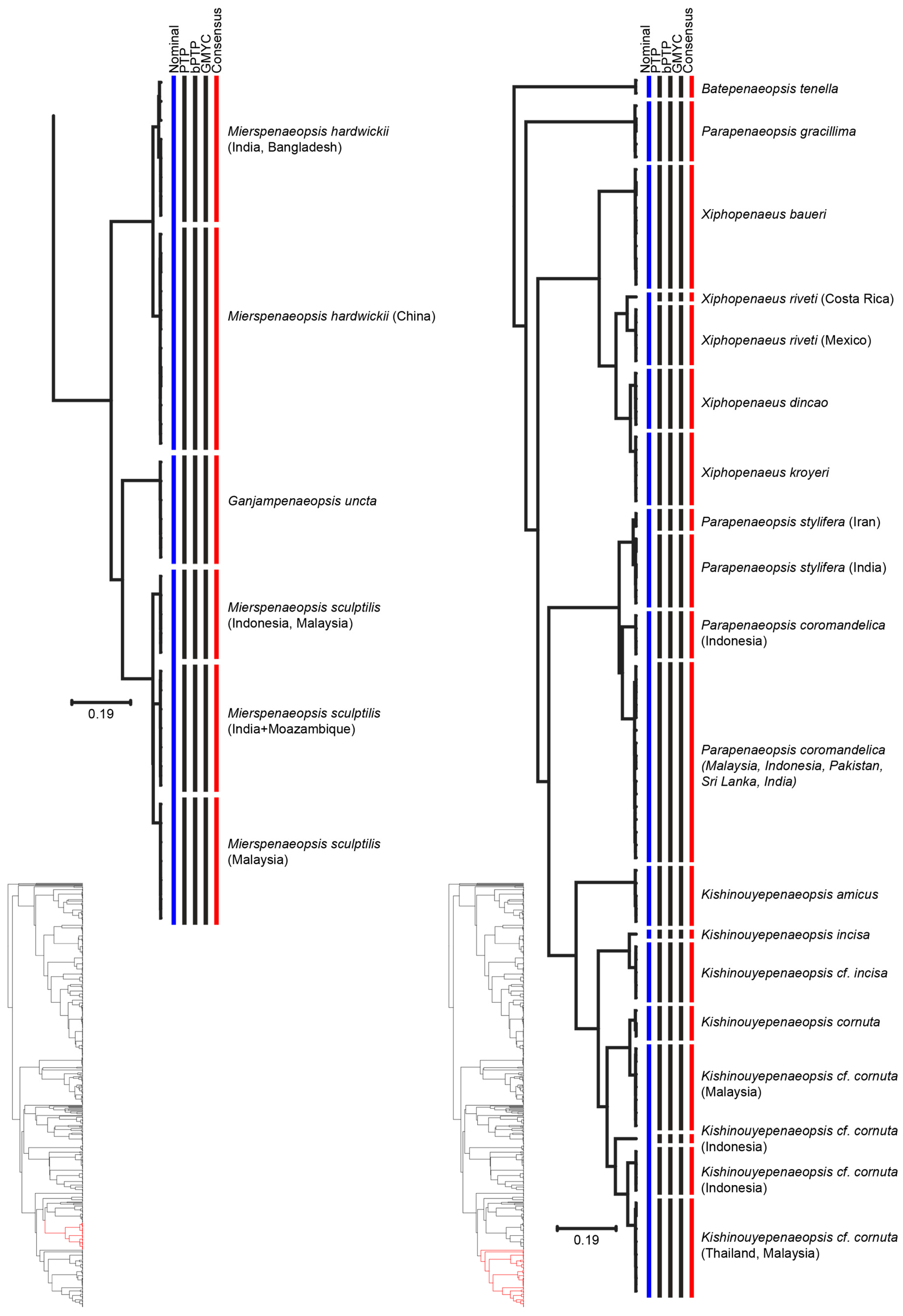
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurzaid, A.; Chan, T.; Nor, S.A.M.; Muchlisin, Z.A.; Chen, W. Molecular phylogeny and diversity of penaeid shrimps (Crustacea: Decapoda) from South-East Asian waters. Zool. Scr. 2020, 49, 596–613. [Google Scholar] [CrossRef]
- Dall, W.; Hill, B.J.; Rothlisberg, P.C.; Sharples, D.J. The Biology of the Penaeidae; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; pp. 1–227. [Google Scholar]
- De Grave, S.; Fransen, C.H.J.M. Carideorum Catalogus: The Recent Species of the Dendrobranchiate, Stenopodidean, Procarididean and Caridean Shrimps (Crustacea: Decapoda). Zoologische Mededelingen 2011, 9, 85. [Google Scholar]
- De Francisco, A.K.; Pinheiro, A.P.; Silva, T.; Galetti, P.M., Jr. Isolation and characterization of microsatellites in three overexploited penaeid shrimp species along the Brazilian coastline. Conserv. Genet. 2009, 10, 563–566. [Google Scholar] [CrossRef][Green Version]
- Gillert, R. Global Study of Shrimp Fisheries; FAO: Rome, Italy, 2008. [Google Scholar]
- Borrell, Y.; Espinosa, G.; Romo, J.; Blanco, G. DNA microsatellite variability and genetic differentiation among natural populations of the Cuban white shrimp Litopenaeus schmitti. Mar. Biol. 2003, 144, 327–333. [Google Scholar] [CrossRef]
- D’Incao, F.; Valentini, H.; Rodrigues, L.F. Avaliação da pesca de camarões nas regiões sudeste e sul do Brasil. Atlantica 2002, 40, 103–116. [Google Scholar]
- Maheswarudu, G.; Sreeram, M.P.; Dhanwanthari, E.; Varma, J.B.; Sajeev, C.K.; Rao, S.S.; Rao, K.N. Trends in penaeid shrimp landings by sona boats at Visakhapatnam Fishing Harbour, Andhra Pradesh. Indian J. Fish. 2018, 65, 58–65. [Google Scholar] [CrossRef]
- Galindo-bect, M.S.; Glenn, E.P.; Page, H.M.; Fitzsimmons, K.; Galindo-Bect, L.A.; Hernandez-Ayon, J.M.; Petty, R.L.; Garcia-Hernandez, J.; Moore, D. Penaeid shrimp landings in the upper Gulf of California in relation to Colorado River freshwater discharge. Fish Bull 2000, 98, 222–225. [Google Scholar]
- Hartono, S.; Riani, E.; Yulianda, F.; Puspito, G. Pemanfaatan Sumber Daya Udang Penaeid di Teluk Ciletuh, Palabuhanratu Berdasarkan Analisis Kesesuaian Kawasan. J. Ilmu Teknol. Kelaut. Trop. 2020, 12, 195–209. [Google Scholar] [CrossRef]
- Blaber, S.J.M.; Cyrus, D.P.; Albaret, J.-J.; Ching, C.V.; Day, J.W.; Elliott, M.; Fonseca, M.S.; Hoss, D.E.; Orensanz, J.; Potter, I.C.; et al. Effects of fishing on the structure and functioning of estuarine and nearshore ecosystems. ICES J. Mar. Sci. 2000, 57, 590–602. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.A.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Marco, J.; Valderrama, D.; Rueda, M. Evaluating management reforms in a Colombian shrimp fishery using fisheries performance indicators. Mar. Policy 2021, 125, 104258. [Google Scholar] [CrossRef]
- Suradi, W.S.; Solichin, A.; Taufani, W.T.; Djuwito; Sabdono, A. Population dynamics of exploited species west shrimps Parapenaeopsis coromandelica H. Milne. Edwards 1837 from the Teluk Penyu coastal waters, Indonesian ocean. Egypt. J. Aquat. Res. 2017, 43, 307–312. [Google Scholar] [CrossRef]
- Willems, T.; De Backer, A.; Kerkhove, T.; Dakriet, N.N.; De Troch, M.; Vincx, M.; Hostens, K. Trophic ecology of Atlantic seabob shrimp Xiphopenaeus kroyeri: Intertidal benthic microalgae support the subtidal food web off Suriname. Estuarine Coast. Shelf Sci. 2016, 182, 146–157. [Google Scholar] [CrossRef]
- Yudhistira, A.; Arisuryanti, D.T. Preliminary findings of cryptic diversity of the giant tiger shrimp (Penaeus monodon Fabricius, 1798) in Indonesia inferred from COI mitochondrial DNA. Genettika 2019, 51, 251–260. [Google Scholar] [CrossRef]
- Tavares, C.; Gusmao, J. Description of a new Penaeidae (Decapoda: Dendrobranchiata) species, Farfantepenaeus isabelae sp. nov. Zootaxa 2016, 4171, 505–516. [Google Scholar] [CrossRef]
- Costa, F.; Dewaard, J.R.; Boutillier, J.; Ratnasingham, S.; Dooh, R.T.; Hajibabaei, M.; Hebert, P. Biological identifications through DNA barcodes: The case of the Crustacea. Can. J. Fish. Aquat. Sci. 2007, 64, 272–295. [Google Scholar] [CrossRef]
- Radulovici, A.E.; Sainte-Marie, B.; Dufresne, F. DNA barcoding of marine crustaceans from the Estuary and Gulf of St Lawrence: A regional-scale approach. Mol. Ecol. Resour. 2009, 9, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.M.; Abdelsalam, K.M.; Mohammed-Geba, K.; Ahmed, H.O.; Kato, M. Genetic and morphological identification of some crabs from the Gulf of Suez, Northern Red Sea, Egypt. Egypt. J. Aquat. Res. 2016, 42, 319–329. [Google Scholar] [CrossRef]
- Apreshgi, K.P.; Dhaneesh, K.V.; Radhakrishnan, T.; Kumar, A.B. DNA barcoding of fiddler crabs Uca annulipes and U. perplexa (Arthropoda, Ocypodidae) from the southwest coast of India. J. Mar. Biol. Assoc. India 2016, 58, 101–104. [Google Scholar] [CrossRef]
- Govender, A.; Groeneveld, J.; Singh, S.; Willows-Munro, S. The design and testing of mini-barcode markers in marine lobsters. PLoS ONE 2019, 14, e0210492. [Google Scholar] [CrossRef]
- Jamaluddin, J.A.F.; Akib, N.A.M.; Ahmad, S.Z.; Halim, S.A.A.A.; Hamid, N.K.A.; Nor, S.A.M. DNA barcoding of shrimps from a mangrove biodiversity hotspot. Mitochondrial DNA Part A 2019, 30, 618–625. [Google Scholar] [CrossRef]
- Cheng, J.; Sha, Z.-L.; Liu, R.-Y. DNA barcoding of genus Metapenaeopsis (Decapoda: Penaeidae) and molecular phylogeny inferred from mitochondrial and nuclear DNA sequences. Biochem. Syst. Ecol. 2015, 61, 376–384. [Google Scholar] [CrossRef]
- Kundu, S.; Rath, S.; Tyagi, K.; Chakraborty, R.; Kumar, V. Identification of penaeid shrimp from Chilika Lake through DNA barcoding. Mitochondrial DNA Part B 2018, 3, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, J.; Lazoski, C.; Monteiro, F.A.; Solé-Cava, A.M. Cryptic species and population structuring of the Atlantic and Pacific seabob shrimp species, Xiphopenaeus kroyeri and Xiphopenaeus riveti. Mar. Biol. 2006, 149, 491–502. [Google Scholar] [CrossRef]
- Kerkhove, T.R.H.; Boyen, J.; De Backer, A.; Mol, J.H.; Volckaert, F.A.M.; Leliaert, F.; De Troch, M. Multilocus data reveal cryptic species in the Atlantic seabob shrimp Xiphopenaeus kroyeri (Crustacea: Decapoda). Biol. J. Linn. Soc. 2019, 127, 847–862. [Google Scholar] [CrossRef]
- Chan, T.-Y.; Muchlisin, Z.A.; Hurzaid, A. Verification of a pseudocryptic species in the commercially important tiger prawn Penaeus monodon Fabricius, 1798 (Decapoda: Penaeidae) from Aceh Province, Indonesia. J. Crustac. Biol. 2021, 41. [Google Scholar] [CrossRef]
- Ditty, J.G.; Bremer, J.R.A. Species Discrimination of Postlarvae and Early Juvenile Brown Shrimp (Farfantepenaeus aztecus) and Pink Shrimp (F. duorarum) (Decapoda: Penaeidae): Coupling Molecular Genetics and Comparative Morphology to Identify Early Life Stages. J. Crustac. Biol. 2011, 31, 126–137. [Google Scholar] [CrossRef]
- Shen, Y.; Kang, J.; Chen, W.; He, S. DNA barcoding for the identification of common economic aquatic products in Central China and its application for the supervision of the market trade. Food Control. 2016, 61, 79–91. [Google Scholar] [CrossRef]
- Nicolè, S.; Negrisolo, E.; Eccher, G.; Mantovani, R. DNA barcoding as a reliable method for the authentication of commercial seafood products. Food Technol. Biotechnol. 2012, 50, 387–398. [Google Scholar]
- Alam, M.M.M.; De Croos, M.D.S.T.; Pálsson, S.; Pálsson, S. Mitochondrial DNA variation reveals distinct lineages in Penaeus semisulcatus (Decapoda, Penaeidae) from the Indo-West Pacific Ocean. Mar. Ecol. 2016, 38, e12406. [Google Scholar] [CrossRef]
- Carvalho-Batista, A.; Terossi, M.; Zara, F.J.; Mantelatto, F.L.; Costa, R.C. A multigene and morphological analysis expands the diversity of the seabod shrimp Xiphopenaeus Smith, 1869 (Decapoda: Penaeidae), with descriptions of two new species. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pérez Farfante, I. Western Atlantic shrimps of the genus Penaeus. Fish Bull 1969, 67, 461–591. [Google Scholar]
- Pérez Farfante, I. Illustrated Key to the Penaeoid Shrimps of Commerce in the Americas; Technical Report; National Marine Fisheries Service: Silver Spring, MD, USA, 1988. Available online: https://repository.library.noaa.gov/view/noaa/5793 (accessed on 12 June 2021).
- Santamaría, J.; Carbajal-Enzian, P.; Clemente, S. Guía Ilustrada para Reconocimiento de Langostinos y Otros Crustáceos con Valor Comercial en el Perú. Available online: https://repositorio.imarpe.gob.pe/handle/20.500.12958/3311 (accessed on 12 June 2021).
- Méndez, M. Claves de identificación y distribución de los langostinos y camarones (Crustacea: Decapoda) del mar y rios de la costa del Perú. Boletín Inst Mar Perú 1981, 5, 1–170. [Google Scholar]
- Norma, C.F. Lista de Crustáceos del Perú (Decapoda y Stomatopoda) con Datos de su Distribución Geográfica. 1970. Available online: https://biblioimarpe.imarpe.gob.pe/handle/20.500.12958/263 (accessed on 12 June 2021).
- Sambrook, J.; Fritish, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Lis, J.T.; Schleif, R. Size fractionation of double-stranded DNA by precipitation with polyethylene glycol. Nucleic Acids Res. 1975, 2, 383–390. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Pons, J.; Barraclough, T.; Gómez-Zurita, J.; Cardoso, A.; Duran, D.P.; Hazell, S.; Kamoun, S.; Sumlin, W.D.; Vogler, A.P. Sequence-Based Species Delimitation for the DNA Taxonomy of Undescribed Insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.L.; Birindelli, J.L.; Carvalho, D.C.; Affonso, P.R.A.M.; Venere, P.C.; Ortega, H.; Carrillo-Avila, M.; Rodríguez-Pulido, J.A.; Galetti, P.M.J. Revealing Hidden Diversity of the Underestimated Neotropical Ichthyofauna: DNA Barcoding in the Recently Described Genus Megaleporinus (Characiformes: Anostomidae). Front. Genet. 2017, 8, 149. [Google Scholar] [CrossRef]
- Young, R.G.; Abbott, C.L.; Therriault, T.W.; Adamowicz, S.J. Barcode-based species delimitation in the marine realm: A test using Hexanauplia (Multicrustacea: Thecostraca and Copepoda). Genome 2017, 60, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.P.; Nicholson, K.E.; Luque-Montes, I.R.; Köhler, G.; Cerrato-Mendoza, C.A.; Medina-Flores, M.; Wilson, L.D.; Townsend, J.H. Cryptic Diversity, but to What Extent? Discordance between Single-Locus Species Delimitation Methods within Mainland Anoles (Squamata: Dactyloidae) of Northern Central America. Front. Genet. 2019, 10, 11. [Google Scholar] [CrossRef]
- Ma, K.Y.; Chan, T.-Y.; Chu, K.H. Refuting the six-genus classification of Penaeus s.l. (Dendrobranchiata, Penaeidae): A combined analysis of mitochondrial and nuclear genes. Zool. Scr. 2011, 40, 498–508. [Google Scholar] [CrossRef]
- Timm, L.; Browder, J.A.; Simon, S.; Jackson, T.L.; Zink, I.C.; Bracken-Grissom, H.D. A tree money grows on: The first inclusive molecular phylogeny of the economically important pink shrimp (Decapoda: Farfantepenaeus) reveals cryptic diversity. Invertebr. Syst. 2019, 33, 488–500. [Google Scholar] [CrossRef]
- Lukhtanov, V.A. Species Delimitation and Analysis of Cryptic Species Diversity in the XXI Century. Entomol. Rev. 2019, 99, 463–472. [Google Scholar] [CrossRef]
- Alam, M.M.M.; Westfall, K.M.; Pálsson, S. Mitochondrial DNA variation reveals cryptic species in Fenneropenaeus indicus. Bull. Mar. Sci. 2014, 91, 15–31. [Google Scholar] [CrossRef]
- Alam, M.M.M. Recent Phylogeographic Studies Revealed Distinct lineages In Penaeid Shrimps. Oceanogr. Fish. Open Access J. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Hualkasin, W.; Sirimontaporn, P.; Chotigeat, W.; Querci, J.; Phongdara, A. Molecular phylogenetic analysis of white prawns species and the existence of two clades in Penaeus merguiensis. J. Exp. Mar. Biol. Ecol. 2003, 296, 1–11. [Google Scholar] [CrossRef]
- Wanna, W.; Phongdara, A. Genetic variation and differentiation of Fenneropenaeus merguiensis in the Thai Peninsula. In Phylogeography and Population Genetics in Crustacea, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 175–189. [Google Scholar]
- Simbine, L.; Marques, C.G.; Freitas, P.D.; Samucidine, K.E.; Gusmão, J.; Tavares, C.; Junior, P.G. Metapenaeus dobsoni (Miers, 1878), an alien Penaeidae in Mozambican coastal waters: Confirmation by mtDNA and morphology analyses. WIO J. Mar. Sci. 2018, 17, 1–12. [Google Scholar]
- Crosnier, A.; Machordom, A.; Boisselier-Dubayle, M.-C. Les espèces du genre Trachypenaeopsis (Crustacea, Decapoda, Penaeidae). Approches morphologiques et moléculaires. Zoosystema 2007, 29, 471–489. [Google Scholar]
- Han, Z.; Zhu, W.; Zheng, W.; Li, P.; Shui, B. Significant genetic differentiation between the Yellow Sea and East China Sea populations of cocktail shrimp Trachypenaeus curvirostris revealed by the mitochondrial DNA COI gene. Biochem. Syst. Ecol. 2015, 59, 78–84. [Google Scholar] [CrossRef]
- Sakaji, H.; Hayashi, K.-I. A Review of the Trachysalambria curvirostris Species Group (Crustacea: Decapoda: Penaeidae) with Description of a New Species. Species Divers. 2003, 8, 141–174. [Google Scholar] [CrossRef]
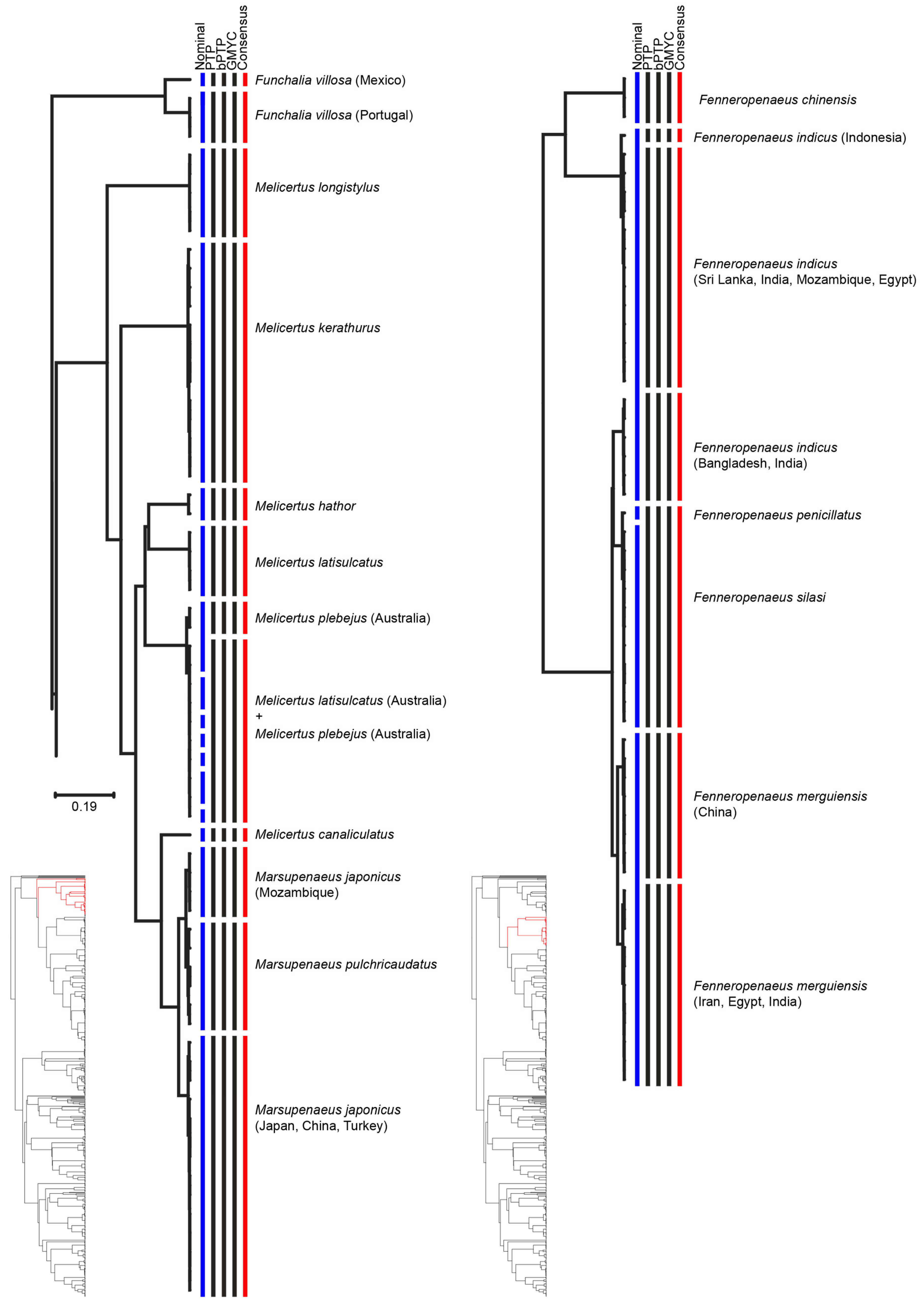
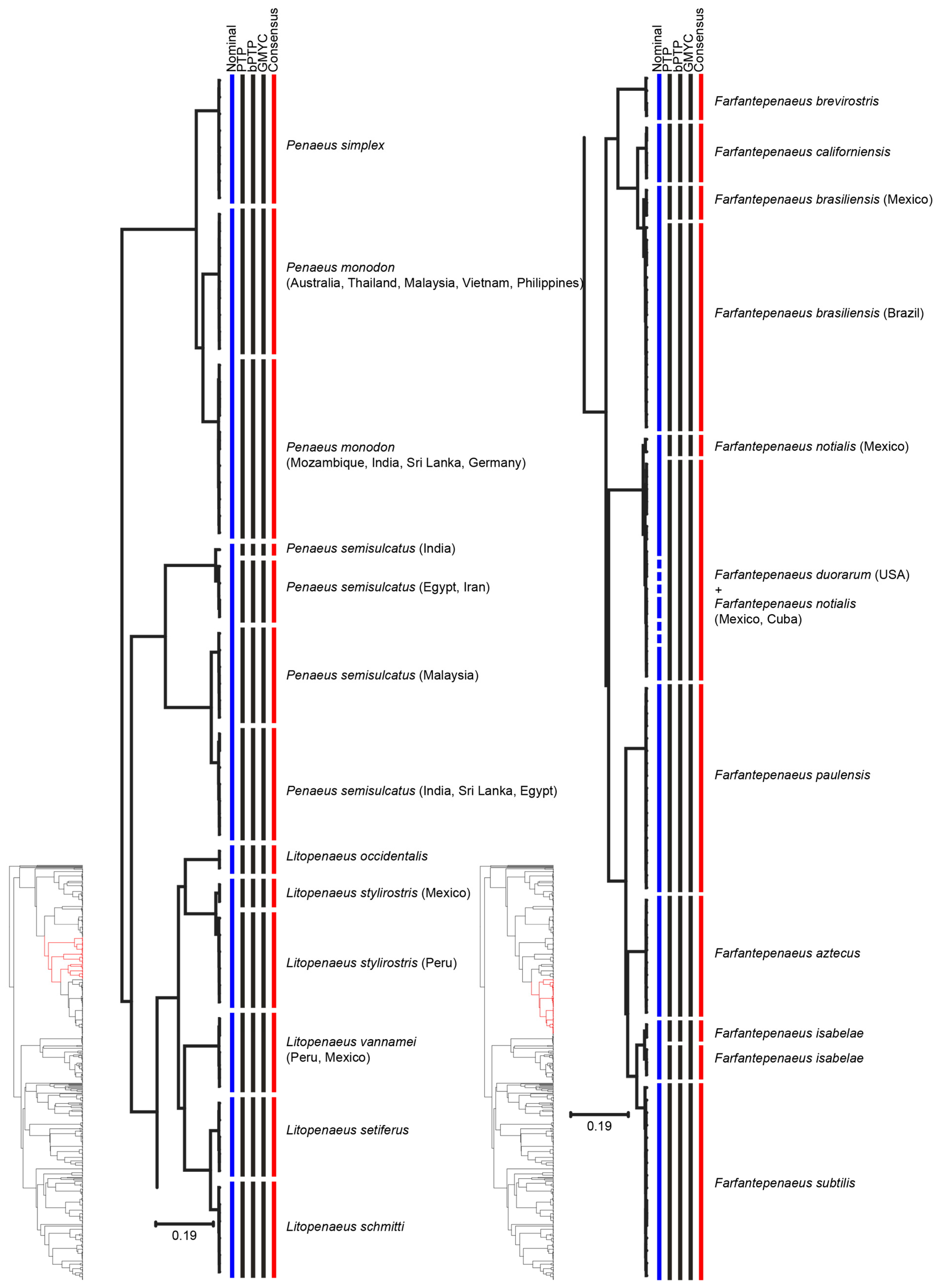
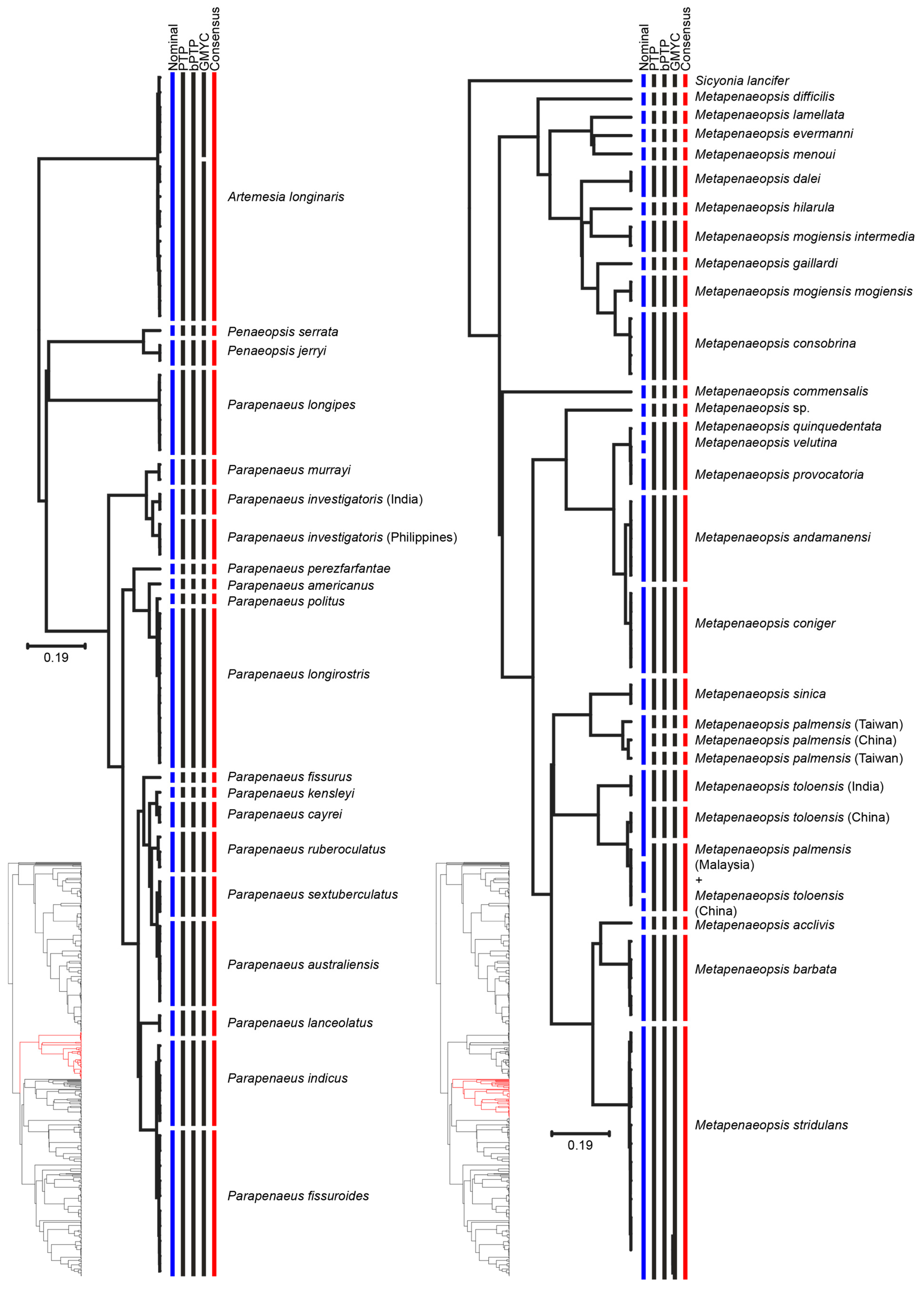
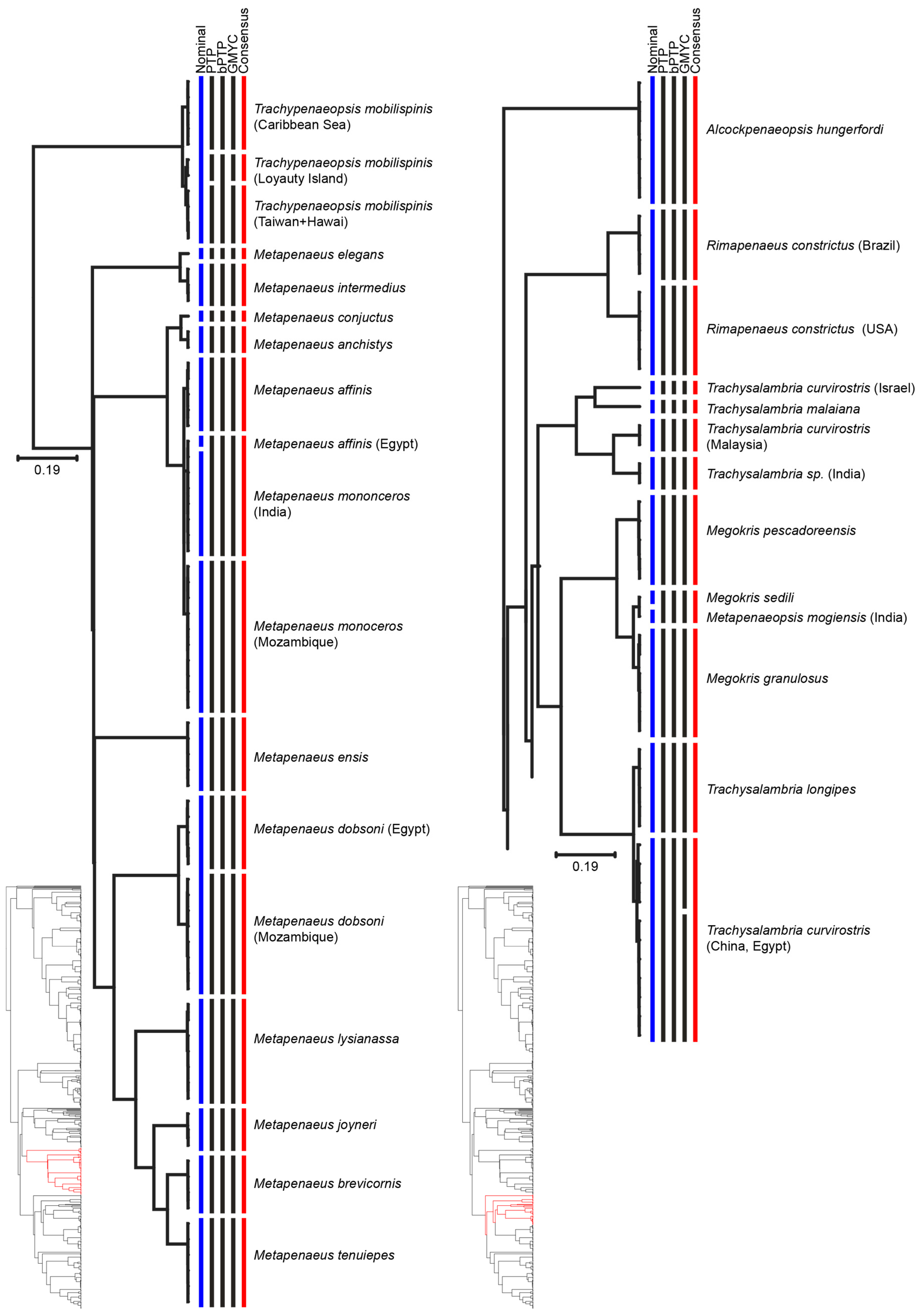
| Species | Consensus MOTUs | Previous Genetic Studies | References |
|---|---|---|---|
| Farfantepenaeus brasiliensis | MOTU 1: South West Atlantic | South West Atlantic | [54] |
| MOTU 2: North West Atlantic | North West Atlantic | ||
| Farfantepenaeus isabelae | MOTU 1: Brazil | One single clade | [54] |
| MOTU 2: Brazil | |||
| Fenneropenaeus indicus | MOTU 1: West Indian | West Indian | [56,57] |
| MOTU 2: East Indian Ocean | East Indian Ocean | ||
| Fenneropenaeus merguiensis | MOTU 1: China Sea | China Sea | [1,58,59] |
| MOTU 2: Andaman Sea, Arabian Sea, and Persian Gulf | Andaman Sea | ||
| Funchalia villosa | MOTU 1: Gulf of Mexico | - | - |
| MOTU 2: Portugal | - | - | |
| Kishinouyepenaeopsis cf. cornuta | MOTU1: Indian Ocean | Indian Ocean | [1] |
| MOTU 2: Strait of Malacca | Strait of Malacca | ||
| MOTU 3: Andaman Sea and Strait of Malacca | Andaman Sea and Strait of Malacca | ||
| MOTU 4: South China Sea | South China Sea | ||
| Litopenaeus stylirostris | MOTU 1: Mexico | - | - |
| MOTU 2: Peru | - | - | |
| Marsupenaeus japonicus | MOTU 1: Japan, China, Turkey | Japan | [60] |
| MOTU 2: Mozambique | Mozambique | ||
| Metapenaeopsis palmensis | MOTU 1: China | South China Sea | [1] |
| MOTU 2: Taiwan | East China Sea | ||
| MOTU 3: Taiwan | |||
| Metapenaeopsis toloensis | MOTU 1: India | - | - |
| MOTU 2: China | - | - | |
| Metapenaeopsis toloensis/M. palmensis | MOTU: Malaysia, China | - | - |
| Metapenaeus dobsoni | MOTU 1: Mozambique | Mozambique | [60] |
| MOTU 2: North West Indian | North West Indian | ||
| Metapenaeus monoceros | MOTU 1: East Indian | East Indian | [57] |
| MOTU 2: West Indian Ocean | West Indian Ocean | ||
| Mierspenaeopsis hardwickii | MOTU 1: China | - | - |
| MOTU 2: India and Bangladesh | - | - | |
| Parapenaeus investigatoris | MOTU 1: Philippines | - | - |
| MOTU 2: India | - | - | |
| Penaeus monodon | MOTU 1: Australia | South-East Africa | [57] |
| MOTU 2: Mozambique, India, and Sri Lanka | South and South-East Asia | ||
| Penaeus semisulcatus | MOTU 1: Malaysia | Indian Ocean | [29] |
| MOTU 2: India | Indian Ocean, South China Sea, Strait of Malacca | ||
| MOTU 3: Egypt and Iran | Indian Ocean | ||
| MOTU 4: India, Sri Lanka, and Egypt | Strait of Malacca, South China Sea, Andaman Sea, Celebes Sea | ||
| Rimapenaeus constrictus | MOTU 1: Brazil | - | - |
| MOTU 2: USA | - | - | |
| Trachypenaeopsis mobilispinis | MOTU 1: Pacific | Pacific | [61] |
| MOTU 2: Loyauty Island | Loyauty Island | ||
| MOTU 3: Caribbean Sea | Caribbean Sea | ||
| Trachysalambria curvirostris | MOTU 1: Israel | Yellow Sea | [62] |
| MOTU 2: China and Egypt | South China Sea | ||
| MOTU 3: China | |||
| Xiphopenaeus riveti | MOTU 1: Costa Rica | Costa Rica | [34] |
| MOTU 2: Mexico | Mexico |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, J.L.; Simbine, L.; Marques, C.G.; Zelada-Mázmela, E.; Reyes-Flores, L.E.; López, A.S.; Gusmão, J.; Tavares, C.; Galetti, P.M., Jr.; Freitas, P.D. DNA Barcoding of Penaeidae (Decapoda; Crustacea): Non-Distance-Based Species Delimitation of the Most Economically Important Shrimp Family. Diversity 2021, 13, 460. https://doi.org/10.3390/d13100460
Ramirez JL, Simbine L, Marques CG, Zelada-Mázmela E, Reyes-Flores LE, López AS, Gusmão J, Tavares C, Galetti PM Jr., Freitas PD. DNA Barcoding of Penaeidae (Decapoda; Crustacea): Non-Distance-Based Species Delimitation of the Most Economically Important Shrimp Family. Diversity. 2021; 13(10):460. https://doi.org/10.3390/d13100460
Chicago/Turabian StyleRamirez, Jorge L., Luisa Simbine, Carla G. Marques, Eliana Zelada-Mázmela, Lorenzo E. Reyes-Flores, Adolfo S. López, Jaqueline Gusmão, Carolina Tavares, Pedro M. Galetti, Jr., and Patricia D. Freitas. 2021. "DNA Barcoding of Penaeidae (Decapoda; Crustacea): Non-Distance-Based Species Delimitation of the Most Economically Important Shrimp Family" Diversity 13, no. 10: 460. https://doi.org/10.3390/d13100460
APA StyleRamirez, J. L., Simbine, L., Marques, C. G., Zelada-Mázmela, E., Reyes-Flores, L. E., López, A. S., Gusmão, J., Tavares, C., Galetti, P. M., Jr., & Freitas, P. D. (2021). DNA Barcoding of Penaeidae (Decapoda; Crustacea): Non-Distance-Based Species Delimitation of the Most Economically Important Shrimp Family. Diversity, 13(10), 460. https://doi.org/10.3390/d13100460








