Abstract
The Mediterranean Sea is currently experiencing a decline in the abundance of several key species, as a consequence of anthropogenic pressures (e.g., increase in human population, habitat modification and loss, pollution, coastal urbanization, overexploitation, introduction of non-indigenous species and climate change). Herbaria and natural history collections are certainly fundamental for taxonomic studies, but they are also an invaluable, if currently underestimated, resource for understanding ecological and evolutionary responses of species to environmental changes. Macroalgae herbarium collections, which are really consistent (ranging from 200,000 to approximately 500,000 specimens) in some European herbaria (e.g., Muséum National d’Histoire Naturelle in Paris, University of Copenhagen, Natural History Museum in Kensington), can be successfully used as real “witnesses” to biodiversity changes. In this respect, we report some case studies from the Mediterranean Sea which summarize well the potential of macroalgae herbarium specimens to provide useful data on biodiversity changes. Indeed, these data enable the evaluation of the responses of biota, including shifts in species ranges, the detection of the presence of introduced species, and the prediction of changes in species distributions and patterns under future climate scenarios. To increase the use of this invaluable tool of research, their curation, the digitization of collections, and specimen genomics should be even more addressed.
1. Introduction
The Mediterranean Sea, representing the 0.82% of the world’s oceans, is inhabited by an unusually rich and diverse biota (approximately 17,000 species) which makes it a true hotspot of biodiversity [1,2], even by virtue of the high rates of endemic species it supports (25%) [3]. At the same time, the Mediterranean Sea is among the most impacted seas, as a consequence of different anthropogenic pressures that are significantly affecting biodiversity, such as habitat modification and loss, pollution, coastal urbanization, overexploitation, the intentional or unintentional introduction of non-indigenous species (NIS, i.e., organisms introduced outside of their natural range) and climate change [2,4,5,6]. Thus, several valuable species and/or habitats are currently experiencing a severe decline or local extinction, and these consequences are predicted to increase in the future [7,8].
Understanding how human stressors have affected biodiversity over time is crucial for understanding the biological impact they have had in the past and to make provision on how biodiversity will respond to future global environmental changes. In this context, herbarium specimens provide unique information for studying long-term changes [9,10,11,12].
In its original meaning, the term “herbarium” refers to a book on medicinal plants, whereas the beginning of the herbarium as a collection of dried specimens is attributed to Luca Ghini (1490–1556). Since then herbaria have been fundamental as taxonomic repositories [13], and nowadays, with the possibility to extract DNA from herbarium specimens for genetic studies, practiced since the 1990s [14], the importance of these repositories has increased even more. However, in the late 20th century the use of herbaria diversified a lot. As an example, in the 1960s, historical data available through the herbarium specimens were first used to study global change [15] to which they are currently considered real ‘witnesses’ [16]. Funk [17] reports that: “Herbaria, dried pressed plant specimens and their associated data… are remarkable and irreplaceable sources of information about plants and the world they inhabit”, emphasizing the scientific value they have for a broad range of studies (e.g., taxonomy, systematics, morphology, phenology, biodiversity, paleobiology, ecology and ethnobotany). As a veritable gold mine of information even after a long time, herbaria may also be essential and invaluable resources for studying biodiversity for conservation and ecological purposes. Thus, they can provide information about species habitat, the presence of a species in a certain locality and at a certain time, and the loss of habitat and species. They can highlight changes in species ranges, the introduction but also the expansion in space and time of NIS, and enable the prediction of future changes in species richness and distributions under future climate scenarios [16,18]. In fact, they can facilitate the detection of spatial-temporal biodiversity changes at regional, national and global scale over a longer period than field-based observation studies can do. They can also provide information to define conservation priorities, improve decisions taken on rare and/or threatened species and apply conservation efforts with as much efficiency as possible [19,20]. Even the IUCN (International Union for Conservation of Nature) Committee indicates their importance as a crucial step for the elaboration of red lists [21]. On the other hand, herbarium specimens can facilitate the identification of hotspots, ecoregions and centers of biodiversity, as well as the establishment of priority areas [22].
Recently, the increasing specimen digitization [23,24] and the development of online platforms (e.g., Encyclopedia of Life, GBIF, Map of Life, iNaturalist, JSTOR’s Global Plants), which facilitate access to collections via the Internet, freely and free of charge, has greatly enhanced the use of herbarium data in scientific research [25]. One of the most important initiatives is GBIF—the Global Biodiversity Information Facility, (https://www.gbif.org/what-is-gbif)—which is an international network and research infrastructure funded by the world’s governments and aimed at providing open access to data about all types of life on Earth. The final objective of this network is to support scientific research, promote biological conservation and sustainable development. Another initiative is the Federation of European Phycological Societies (FEPS) which aims to create a “European Database of Algal and Cyanobacterial Archived Materials for Analytical and Molecular Biology Research”. The FEPS initiative will lead to more complete data on the algal collections present in Europe.
However, it should be noted that taxonomic errors may be present in herbaria, and information may be not detailed in very old specimens; moreover, the absence of a species in a certain locality may be explained in different ways, for instance the species may not be present, was not collected, or was not detected. Instead, a high number of specimens in a certain area may be due to a specific interest for the area, or the presence of experts working on those taxonomic groups. In addition, common species are undercollected whereas rare or unusual species are overcollected [26].
The aim of this paper is to highlight the important role of phycological herbaria not only for taxonomic research but also as witnesses to changes of macroalgae diversity, providing some case studies from the Mediterranean Sea. To reach this aim we also provide an overview of the European collections of macroalgae. Data from the European phycological herbaria, with a look also at Mediterranean specimens, were obtained by consulting the Index Herbariorum (http://sweetgum.nybg.org/science/ih) [27], the Algae Directory [28], GIBF (https://www.gbif.org/what-is-gbif), the CoRIMBo network of Italy, e-ReColNat infrastructure (https://www.recolnat.org/en/), and the webpages and/or the curators of the different herbaria. With respect to the Italian collections, we referred also to Giaccone et al. [29].
2. Materials and Methods
The search for relevant literature, updated till June 2020, was carried out using standard electronic databases. Data from the European phycological herbaria, with a look also at Mediterranean specimens, were obtained by consulting the Index Herbariorum (http://sweetgum.nybg.org/science/ih) [27], the Algae Directory [28], GIBF (https://www.gbif.org/what-is-gbif), the CoRIMBo network of Italy, e-ReColNat infrastructure (https://www.recolnat.org/en/), and the webpages and/or the curators of the different herbaria. With respect to the Italian collections, we referred also to Giaccone et al. [29].
3. Results
3.1. Overview of European Phycological Collections
According to the Index Herbariorum, 688 herbaria are present in 33 European countries (Figure 1), with France, the UK, and Germany hosting the highest number of herbarium specimens (25.96, 22.31, and 22.16 million specimens, respectively) [27]. With respect to the phycological collections, more than one million, seven thousand specimens are currently preserved in the European herbaria. This figure is substantially smaller than that of vascular plants, as a consequence of the considerably smaller number of seaweed species, but also to the lower number of specialists on the subject, probably due also to the greater difficulty in sampling.
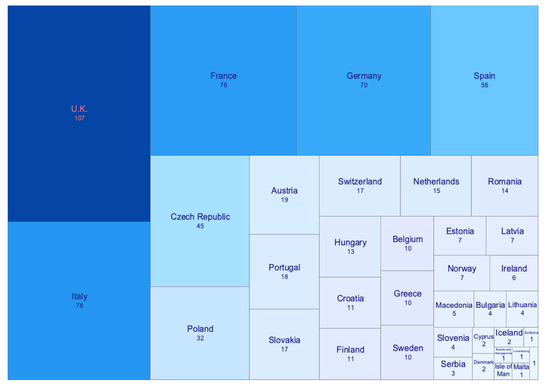
Figure 1.
Number of herbaria reported for each European country [27].
In Table 1 we report the largest collections of marine macroalgae hosted in the European phycological herbaria. In particular, the biggest macroalgae collections are hosted in the herbarium of the University of Copenhagen (C), with 260,000 specimens, the Natural History Museum of London (BM), with 205,000 specimens (9000 types), the herbarium of the Naturalis Biodiversity Center at Leiden (L) with 148,000 specimens, and the herbarium of Lund University (LD), with 102,000 specimens, among which, the Agardh Herbarium (50,000 samples and 6000 types) represents one of the most important collections of algae in the world. These are also outstanding: the Muséum National d’Histoire Naturelle of Paris (PC), with 500,000 specimens (more than 10,000 types) and the Naturhistorisches Museum Wien (W), with 200,000 specimens, although these herbaria are not totally digitized (PC) or not digitized (W), and the figures provided by the herbaria curators include freshwater algae and marine Bacillariophyta.

Table 1.
European herbaria hosting consistent marine macroalgae collections. The acronym of the herbaria in the Index Herbariorum [27] is also included. The number of specimens of herbaria labelled with *, includes freshwater algae and marine Bacillariophyta.
Few data are published on the Mediterranean macroalgae collections deposited at the European herbaria, with the latest update going back to the list reported in the Algae Directory [28]. The number of Mediterranean macroalgae specimens currently preserved and quantified in European herbaria ranges between 95,000 and 124,000. However, in addition to these, we estimate that there would be about 91,000 more specimens hosted in those herbaria that are not yet partially or totally digitized. Apart from the big Mediterranean macroalgae collection (between 20,000 and 40,000 specimens) hosted at the Muséum National d’Histoire Naturelle, Paris (PC), there are other consistent collections at the Centre d’Océanologie de Marseille (HCOM, ~15,000–20,000 specimens), at the University of Girona (HGI, 17,700 specimens), at Ghent University (GENT, 8075 specimens), and at the University of Messina (MS, 8000). Finally, besides these big collections we can find other relevant collections hosted at Universidad de Barcelona (BCN, 6500 specimens), Università di Catania (CAT, 5590 specimens), Trinity College (TCD, 4000–5000 specimens), Università di Trieste (TSB, ~4190 specimens) and University of Zagreb (ZA and ZAHO, 4000 specimens).
3.2. Case Studies from the Mediterranean Sea
Herbaria specimens can be a crucial tool for reconstructing distribution maps and variations in species ranges through time, analyzing the regression of some species and assessing their conservation status (stable, in decline, extinct), depicting the introduction and/or the geographic expansion of non-indigenous species. In this context, large brown fucoid algae (Fucales, Phaeophyceae) and kelps (Laminariales and Tilopteridales, Phaeophyceae), but also introduced species, such as Dictyota cyanoloma (Dictyotales, Phaeophyceae), are effective examples (Figure 2).

Figure 2.
Specimens of Carpodesmia mediterranea (Sauvageau) Orellana and Sansón (A) and Laminaria rodriguezii Bornet (B) from the algae section of the herbarium of Universitat de Girona (HGI-A).
Large canopy-forming fucoids are dominant species in pristine environments along temperate rocky coasts worldwide, which provide essential ecological goods and services [30,31,32,33]. These valuable and sensitive communities are currently experiencing worldwide a severe decline and/or loss [34,35,36], as a result of a variety of anthropogenic stressors such pollution, coastal urbanization, overexploitation, the intentional or indirect introduction of NIS, and climate changes, i.e., acidification and warming.
Thibaut et al. [37], for instance, compared the state in 2003 of Fucales on the Albères coast, France, by means of historical records. The authors found that only five species of the fourteen reported in the first thirty years of the 20th century by Sauvageau [38] and Feldmann [39,40], or the nine species reported by Gros [41] were still present, showing a decrease in the area of all the populations since the 1940s and the total collapse of the genus Sargassum. Seven taxa (Carpodesmia crinita, Treptacantha barbata, Cystoseira foeniculacea f. tenuiramosa, T. ballesterosii, T. ballesterosii var. compressa, S. hornschuchii and S. vulgare), considered frequent or abundant by Feldmann [39,40] and some of them also dominant species in several phytobenthic communities, resulted extinct in the area. Moreover, only one of the five recorded species (Cy. compressa) showed no signs of regression, two species (Ca. brachycarpa and Ca. zosteroides) were considered as rare, and one (T. elegans) very rare. An increase in water turbidity could be responsible for the progressive replacement of T. ballesterosii var. compressa by Ca. zosteroides between the 1940s and the 1970s [41,42] and the total collapse of T. ballesterosii var. compressa at the late 1970s [41]. Carpodesmia mediterranea, a species that was reported to make a continuous belt along the shores of the Albères coast [39,40], in 2003 was less abundant and has almost disappeared from some areas.
Blanfuné et al. [43] analyzed in detail the distribution of Ca. mediterranea over the Gulf of Lions, France, comparing the current distribution with historical data. The authors observed two minima: 1960s–1970s and 2007, the latter being the most severe. Other minima could have occurred in the past, although they have gone unnoticed. Overall, the general trend over the past century has been a decline, but, in French Catalonia it has been marked by clear-cut ups and downs. Possible causes for the observed decline of the range of Ca. mediterranea, together with its disappearance at its northernmost limit, could be the anthropogenic impact and overgrazing. In the 1960s–1970s, Gros [41] described the invasion of Ca. mediterranea habitat by dense stands of the mussel Mytilus galloprovincialis, possibly due to organic pollution. Mussels could reduce the settlement and survival of Ca. mediterranea recruits because of the instability of the mussels’ support or the lack of light for recruits that grow directly on the rocky substrate. The mussel farming spread from the 1950s to the 1970s could be responsible for the mussels’ larvae invasion of the Ca. mediterranea habitat. Heatwaves and an exceptional storm are plausibly responsible for the 2007 decline of Ca. mediterranea. Between 2007 and 2012, further exceptional storms opened spaces in the mussel beds for the recruitment of Ca. mediterranea, which partially recovered [43].
Recently, Thibaut et al. [34] reconstructed the distribution of Fucales species along the French Riviera coast (north-western Mediterranean), comparing their distribution between 2007 and 2013 with historical data from the 19th century. Of the 18 species historically reported for this area, five were probably extinct in the zone (T. elegans, Cy. foeniculacea f. latiramosa, T. squarrosa, T. ballesterosii and S. hornschuchii). For instance, T. squarrosa, originally described by De Notaris from material collected in Nice [44] and reported till the end of the 19th century, was not found during the survey period (2007–2013). Similarly, S. hornschuchii, first collected in 1821 in Cannes [45], was reported till the 20th century in Cannes (Raphélis in Général Herbarium) and Antibes (J. Feldmann Herbarium), but the authors did not find this species during their survey. In addition, nine taxa suffered a decline (Ca. amentacea, T. barbata f. barbata, Ca. brachycarpa, Ca. crinita, T. sauvageauana and S. vulgare) or became nearly locally extinct (Cy. foeniculacea f. tenuiramosa, T. ballesterosii var. compressa and S. acinarium). Regressions were localized around the entrances to ports and offshore from the cities.
On the other hand, Thibaut et al. [34] showed that Cy. compressa, first collected in 1839 in Nice (C. Agardh in Requien Herbarium, Algues Vertes Herbarium), and Ca. amentacea, collected for the first time in 1826 in Nice by Antoine Risso (in Bory de Saint-Vincent Herbarium), were still abundant and common. Thibaut et al. [46] analyzed the long-term changes in Ca. amentacea populations along the French Mediterranean coasts, comparing the state between 2008 and 2011 with historical records (from the 18th–19th to the early 21st century). A remarkable stability over 1–5 decades was observed, except for four areas along the Western Provence, the French Riviera and Corsica, where the conspicuous decline of Ca. amentacea populations was highlighted. In the vicinity of the Cortiou sewage outfall at Marseilles, the largest French Mediterranean city and one of the largest cities of the whole Mediterranean Sea, a steady decrease of Ca. amentacea has apparently occurred since the 1960s. Likewise, in the Gulf of Fos (western Provence, French Mediterranean coast), which harbors one of the largest French ports and industrial facilities (petrochemical and chemical industries, oil refineries, steelworks, power plant, etc.), the distribution of Ca. amentacea has remained unchanged since 1975, but subsequently the populations were more fragmented and in competition with mussel beds. Instead, T. ballesterosii var. tenuior was first recorded during the survey by Thibaut et al. [46]. Habitat destruction, overgrazing by the sea urchins Paracentrotus lividus and Arbacia lixula, competition with the invasive macroalgae Caulerpa taxifolia and Caulerpa cylindracea, fishing activities and the increase in turbidity were considered the most severe probable causes of the decline of most Fucales in France [37,47,48,49,50].
Blanfune et al. [36] compared the current distribution of Ca. crinita, an ecologically important species and a regionally protected marine algae (Barcelona Convention; UNEP/MAP, 2009), over the entire French Mediterranean coast, including Corsica, with the historical data (e.g., herbarium vouchers since 1700). A drastic decline of Ca. crinita on the continental coast, up to local extinction (French Catalonia), near extinction (Languedoc and western Provence), or functional extinction (French Riviera) was observed, while a still stable and healthy population was found in Eastern Provence and in Corsica. The authors also assessed the conservation status of Ca. crinita, according to the IUCN Red List criteria [51]. On the basis of the comparison between historical and current data, and the knowledge of anthropogenic pressures, the authors propose the classification of Ca. crinita as ‘Critically Endangered’ (CR), near to ‘Regionally Extinct’ (RE) in French Catalonia and Languedoc, as ‘Vulnerable’ (VU) in the region Provence-Alpes-Côte-d’Azur, as ‘Least Concern’ (LC) in Corsica.
Furthermore, Žuljević et al. [52], comparing historical data (e.g., herbarium collections) [53,54] and recent records of the endemic deep-water Mediterranean kelp Laminaria rodriguezii in the Adriatic Sea, found that L. rodriguezii had three principal areas of distribution, located in the central part of the Adriatic Sea: Jabuka Pit and the islands of Biševo and Palagruža. Recent data (since 1996 data from MEDITS expeditions, ROV surveys and benthic surveys) on the distribution of L. rodriguezii in the Adriatic Sea revealed that the distribution range of the species has drastically declined within the last 40 years, reducing by more than 85%. Since 2000, the species was recorded only at Palagruža. It can be said with high confidence that L. rodriguezii is no longer present at Jabuka Pit, where it was repeatedly found in the late 1940s and 1950s, and Biševo. The most probable reason for its disappearance is the direct and indirect impact of trawling (physical collecting and decrease of water transparency).
Historic collections have played a very important role in tracing the introduction of Dictyota cyanoloma (Dictyotales) into the Mediterranean [55]. Dictyota cyanoloma was described in 2010, based on specimens from Palamós, NW Mediterranean [56], although the species was first reported from the Iberian Peninsula as D. ciliolata by Rull Lluch et al. [57]. The species is widely distributed in the Mediterranean Sea and recent records have proven its expansion to the European Atlantic (Portugal, Galicia, the SW England), and the Macaronesia [56,58,59,60,61,62,63], while Steen et al. [55] reported it from Australia and New Zealand. Steen et al. [55] obtained molecular sequence information from historical herbarium samples of Dictyota spp. which proved the presence of D. cyanoloma in the Adriatic Sea as early as 1935. Molecular data obtained from herbarium samples collected in 1947, 1958 and 1976 confirmed its presence in the Split area. Other herbarium records also confirmed the presence of D. cyanoloma from Cannes (1999) and Barcelona (2005). However, the question remains unanswered whether D. cyanoloma was ubiquitous but unrecognized within the Mediterranean throughout the 20th century, or whether it was an introduction that remained contained during a lag phase of several decades before expanding its range. To answer this question, it would be necessary to screen other specimens from European herbaria.
Molecular studies on herbaria specimens can also be a useful tool to understand the ancient species composition, structure and diversity of a community. Herbaria host many inadequately identified species that have been used in ecological studies to characterize marine communities. Nowadays, molecular data may allow us to distinguish these species and, consequently, improve the knowledge about the community. As an example, several deep detritic communities of the Balearic Islands were described between 2012 and 2016 [64,65,66]. Phylogenetic analyses of Mediterranean and European Atlantic herbarium specimens of a species usually thriving in these bottoms, Halymenia latifolia (Halymeniales, Rhodophyta), supported by morphological data, indicated that this taxon encompasses at least five species belonging to three cryptic genera, four of which are present in the Mediterranean Sea. Accordingly, the true H. latifolia was transferred to a new genus, Nesoia, as N. latifolia [67], some Mediterranean specimens were transferred also to the genus Nesoia, as N. mediterranea [68], other were maintained inside Halymenia, as H. ballesterosii [69], and other were transferred to a new genus, Neofolia, as N. rosea [70]. Consequently, future ecological work on macroalgae communities off deep detritic bottoms should take into account these changes.
4. Discussion
A key challenge for today is to understand how species are responding to habitat degradation, the spread of invasive species, pollution, overexploitation, and climate change. In this respect, herbarium specimens, due to their temporal and geographical extent, have great potential for the study of biodiversity changes and for the planning of conservation actions.
Within the past decade, the recognition of the role of herbaria as an invaluable tool for observing biodiversity changes has greatly increased [37,63,71]. The case studies reported here highlight well the important role of phycological herbaria to monitor both key native species changes and alien species over a long time period. As already stated, they may help in detecting shifts in species ranges and the presence of introduced species, and predicting future changes, e.g., retreat or extinction, in species distributions and richness under future climate scenarios [11,72,73]. They may help in the assessment of native and alien species distribution also thanks to the support of molecular data [54].
Since specimens can also capture trait shifts through time, historical collections can provide the opportunity to reconstruct past environments. They may also depict the past history of introduced algal species, showing for instance that they were already present many years before they were noticed, and also in reconstructing the evolutionary and biogeographical history of extant, as well as extinct taxa [74]. Certainly, reconstructing past distributions from historical records is often challenging [75,76], and herbarium records can provide biased information if particular taxa have been insufficiently or partially recorded [77]. Even though there are some limitations in using herbarium specimens as they are qualitative rather than quantitative data, herbarium specimens have a great potential for monitoring and conservation purposes.
However, the value of herbaria for monitoring study is strictly linked to their curation, continuity through time, digitization and sequencing data. All this prevents an agile and easy search and acquisition of data, also sorting them by specific fields, such as geographic area, collector, or taxonomic group. The search carried out on European phycological herbaria highlighted some gaps and scarcity of information mainly due to the partial or total lack of cataloguing and digitization of the majority of collections. Moreover, the greatest part of the digitized collections is not accessible online. Thus, for instance, some herbaria e.g., the ones of the Real Jardín Botánico from Madrid (MA) and the Natural History Museum of Kensington (BM) are already available online at GIBF, while e.g., the Herbarium of the University of Girona (HGI-A), is totally digitized, but not yet available online.
5. Conclusions
The examples presented here summarized well the potential of herbarium specimens as witnesses to biodiversity changes, one of the possible use of herbaria data. However, the potential of the herbaria collections is not fully exploited. Indeed, to increase the use of herbaria data it is essential their digitization, to ensure the continued support of herbaria and their staff. Investment is necessary to maintain the infrastructure of the herbaria, to ensure the conservation of the specimens, and to guarantee regular updating of the databases making them easily accessible to society and researchers.
Author Contributions
Conceptualization, methodology, validation, data curation, writing—original draft preparation, writing—review and editing, A.M.M., S.A.M., C.R.-P.; supervision, A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We want to thank the curators of the European phycological herbaria which provided information on Mediterranean specimens: Roser Guàrdia (BCN), Christian Lange and Nina Lundholm (C), Chiara Nepi and Lorenzo Cecchi (FI), Claes Persson (GB), Olivier De Clerck (GENT), Marc Verlaque (HCOM), Roxali Bijmoer (L), Arne Thell (LD), Margarita Dueñas (MA), Raffaella Trabucco (MCVE), José García Sánchez (MGC), Rossella Marcucci (PAD), Line Le Gall (PC), Mauro Iberite (RO), David García San Leon (SANT), John Parnell (TCD), Annalisa Falace and Marisa Vidali (TSB), Christian Bräuchler (W), Marija Gligora Udovič (ZA and ZAHO), and the Museo di Biologia Marina “Pietro Parenzan” (Anna Maria Miglietta). We thank the Ateneu de Maó for provinding the information about the Herbari d’Algues J.J. Rodríguez y Femenías. We also thank the Italian CoRIMBo (Coordinamento della Rete Italiana dei Musei Botanici) network and GIBF for the information on BM (https://doi.org/10.15468/dl.a84xjw, https://doi.org/10.15468/dl.7wyfyb and https://doi.org/10.15468/dl.2wbahp), MA (https://doi.org/10.15468/dl.uk4xb9, https://doi.org/10.15468/dl.s2b8uv and https://doi.org/10.15468/dl.7errz3) and SANT (https://doi.org/10.15468/dl.2wa2fz, https://doi.org/10.15468/dl.75re49, https://doi.org/10.15468/dl.hynp9g).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bianchi, C.N.; Morri, C. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for Future Research. Mar. Poll. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Sci. Rep. Port-Cros Natl. Park 2004, 20, 97–146. [Google Scholar]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Cinar, M.E.; Almogi- Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Patterns in introduction trends and pathways. Medit. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast. Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Corsini-Foka, M.; Morri, C.; Zenetos, A. Thirty years after: Dramatic change in the coastal marine ecosystems of Kos Island (Greece), 1981–2013. Mediterr. Mar. Sci. 2014, 15, 482–497. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; Ben Rais Lasram, F.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean under siege: Spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–481. [Google Scholar] [CrossRef]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef]
- Primack, R.B.; Miller-Rushing, A.J. The role of botanical gardens in climate change research. New Phytol. 2009, 182, 303–313. [Google Scholar] [CrossRef]
- Lavoie, C. Biological collections in an ever-changing world: Herbaria as tools for biogeographical and environmental studies. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 68–76. [Google Scholar] [CrossRef]
- Vellend, M.; Baeten, L.; Myers-Smith, I.H.; Elmendorf, S.C.; Beauséjour, R.; Brown, C.D.; De Frenne, P.; Verheyen, K.; Wipf, S. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. USA 2013, 110, 19456–19459. [Google Scholar] [CrossRef] [PubMed]
- Meineke, E.K.; Davies, T.J.; Daru, B.H.; Davis, C.C. Biological collections for understanding biodiversity in the Anthropocene. Phil. Trans. R. Soc. B 2018, 374, 20170386. [Google Scholar] [CrossRef] [PubMed]
- Greve, M.; Lykke, A.M.; Fagg, C.W.; Gereau, R.E.; Lewis, G.P.; Marchant, R.; Marshall, A.R.; Ndayishimiye, J.; Bogaert, J.; Svenning, J.-C. Realising the potential of herbarium records for conservation biology. S. Afr. J. Bot. 2016, 105, 317–323. [Google Scholar] [CrossRef]
- Taylor, J.W.; Swann, E.C. DNA from herbarium specimens. In Ancient DNA; Hermann, B., Hummel, S., Eds.; Springer-Berlag: Berlin, Germany, 1994; pp. 167–181. [Google Scholar]
- Ruhling, A.; Tyler, G. Ecology of heavy metals—A regional and historical study. Bot. Not. 1969, 122, 248–259. [Google Scholar]
- Lang, P.L.M.; Willems, F.M.; Scheepens, J.F.; Burbano, H.A.; Bossdorf, O. Using herbaria to study global environmental change. New Phytol. 2019, 221, 110–122. [Google Scholar] [CrossRef]
- Funk, V.A. The importance of Herbaria. Plant Sci. Bull. 2003, 49, 94–95. [Google Scholar]
- Johnson, K.G.; Brooks, S.J.; Fenberg, F.B.; Glover, A.G.; James, K.E.; Lister, A.M.; Michel, E.; Spencer, M.; Todd, J.A.; Valsami-Jones, E.; et al. Climate change and biosphere response: Unlocking the collections vault. BioScience 2011, 61, 147–153. [Google Scholar] [CrossRef]
- Krupnick, G.A.; Kress, W.J.; Wagner, W.L. Achieving Target 2 of the Global Strategy for Plant Conservation: Building a preliminary assessment of vascular plant species using data from herbarium specimens. Biodivers. Conserv. 2009, 18, 1459–1474. [Google Scholar] [CrossRef]
- Kricsfalusy, V.V.; Trevisan, N. Prioritizing regionally rare plant species for conservation using herbarium data. Biodivers. Conserv. 2014, 23, 39–61. [Google Scholar] [CrossRef]
- IUCN. An Introduction to the IUCN Red List of Ecosystems: The Categories and Criteria for Assessing Risks to Ecosystems; IUCN: Gland, Switzerland, 2016. [Google Scholar] [CrossRef]
- Davy, A.J. Museum specimens breathe life into plant conservation? Trends Ecol. Evol. 2005, 20, 285–286. [Google Scholar] [CrossRef]
- Seregin, A.P. Making the Russian flora visible: Fast digitisation of the Moscow University herbarium (MW) in 2015. Taxon 2016, 65, 203–209. [Google Scholar] [CrossRef]
- Armeli Minicante, A.; Birello, G.; Sigovini, M.; Minuzzo, T.; Perin, A.; Ceregato, A. Building a Natural and Cultural Heritage Repository for the Storage and Dissemination of Knowledge: The Algarium Veneticum and the Archivio di Studi Adriatici Case Study. J. Libr. Metadata 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Anderson, J.G.T.; Bakker, J.D.; Billo, T.J.; Dunwiddie, P.W.; Groom, M.J.; Hampton, S.E.; Herman, S.G.; Levey, D.J.; Machnicki, N.J.; et al. Natural history’s place in science and society. BioScience 2014, 64, 300–310. [Google Scholar] [CrossRef]
- Nelson, W.A.; Dalen, J.; Neill, K.F. Insights from natural history collections: Analysing the New Zealand macroalgal flora using herbarium data. PhytoKeys 2013, 30, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Thiers, B. The World’s Herbaria 2018: A Summary Report Based on Data from Index Herbariorum. 2019. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 15 May 2020).
- COST 49. Algae Directory: Algologists, Companies, Culture Collections and Herbaria in European Countries; Office for Official Publications of European Communities: Luxembourg, 1997; p. 235.
- Giaccone, T.; Catra, M.; Serio, D.; Giaccone, G. A review of Mediterranean macrophytobenthos collections present in Italy: A contribution to the Mediterranean Initiative on Taxonomy. Chem. Ecol. 2008, 24, 175–184. [Google Scholar] [CrossRef]
- Sales, M.; Ballesteros, E. Long-term comparison of algal assemblages dominated by Cystoseira crinita (Fucales, Heterokontophyta) from Cap Corse (Corsica, North Western Mediterranean). Eur. J. Phycol. 2010, 45, 404–412. [Google Scholar] [CrossRef]
- Sales, M.; Ballesteros, E.; Anderson, M.J.; Ivesa, I.; Cardona, E. Biogeographical patterns of algal communities in the Mediterranean Sea: Cystoseira crinita-dominated assemblages as a case study. J. Biogeogr. 2012, 39, 140–152. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meinesz, A.; Thibaut, T.; Mangialajo, L. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of marine protected areas. Adv. Oceanogr. Limnol. 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Mineur, F.; Arenas, F.; Assis, J.; Davies, A.J.; Engelen, A.H.; Fernandes, F.; Malta, E.-J.; Thibaut, T.; Van Nguyen, T.; Vaz-Pinto, F.; et al. European seaweeds under pressure: Consequences for communities and ecosystem functioning. J. Sea Res. 2015, 98, 91–108. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Verlaque, M. Decline and local extinction of Fucales in French Riviera: The harbinger of future extinctions? Medit. Mar. Sci. 2015, 16, 206–224. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The fate of Cystoseira crinita, a forest-forming Fucale (Phaeophyceae, Stramenopiles), in France (North Western Mediterranean Sea). Estuar. Coast. Shelf Sci. 2016, 181, 196–208. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira, Sargassum) in the Albères coast (north-western Mediterranean). Mar. Poll. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Sauvageau, C. A propos des Cystoseira de Banyuls et de Guéthary. Bull. Stat. Biol. Arcachon 1912, 14, 1–424. [Google Scholar]
- Feldmann, J. Les Algues Marines de ea Côte des Albères. I.-III; Cyanophycées, Chlorophycées et Phéophycées de la Côte des Albères; Imprimerie Wolf: Rouen, France, 1937; p. 197. [Google Scholar]
- Feldmann, J. Recherches sur la Végétation Marine de la Méditerranée. La Côte Des Albères; Imprimerie Wolf: Rouen, France, 1937; p. 339. [Google Scholar]
- Gros, C. Le genre Cystoseira sur côte des Albères. Répartition—Écologie—Morphogenèse. Ph.D. Thesis, Université Pierre et Marie Curie Paris VI, Paris, France, 1978. [Google Scholar]
- Guern, M. Embryologie de quelques espèces du genre Cystoseira Agardh 1821 (Fucales). Vie Milieu 1962, 13, 649–679. [Google Scholar]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The ups and downs of a canopy-forming seaweed over a span of more than one century. Sci. Rep. 2019, 9, 5250. [Google Scholar] [CrossRef] [PubMed]
- Agardh, J.G. Algae Maris Mediterranei et Adriatici, Observationes in Diagnosin specierum et Dispositionem Generum; Apud Fortin, Masson et Cie: Paris, France, 1842; p. 164. [Google Scholar]
- Raphélis, A. Liste des algues récoltées dans les environs de Cannes. Ann. Soc. Sci. Nat. Provence 1907, 1, 1–30. [Google Scholar]
- Thibaut, T.; Blanfumé, A.; Markovic, L.; Verlaque, M.; Boudouresque, C.-F.; Perret-Boudouresque, M.; Maćic, V.; Bottin, L. Unexpected abundance and long-term relative stability of the brown alga Cystoseira amentacea, hitherto regarded as a threatened species, in the north-western Mediterranean Sea. Mar. Pollut. Bull. 2014, 89, 305–323. [Google Scholar] [CrossRef]
- Verlaque, M.; Fritayre, P. Modifications des communautés algales méditerranéennes en présence de l’algue en-vahissante Caulerpa taxifolia (Vahl) C. Agardh. Oceanol. Acta 1994, 17, 659–672. [Google Scholar]
- Boudouresque, C.F.; Meinesz, A.; Ribera, M.A.; Ballesteros, E. Spread of the green alga Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: Possible consequences of a major ecological event. Sci. Mar. 1995, 59 (Suppl. 1), 21–29. [Google Scholar]
- Piazzi, L.; Ceccherelli, G. Persistence of biological invasion effects: Recovery of macroalgal assemblages after removal of Caulerpa racemosa var. cylindracea. Estuar. Coast. Shelf Sci. 2006, 68, 455–461. [Google Scholar] [CrossRef]
- Hereu, B.; Capdevila, P.; Cebrian, E.; Díaz, D.; Garrabou, J.; Kerting, D.; Linares, C.; Navarro, L.; Pauner, O.; Teixido, N. Ecology and perturbations of Mediterranean deep-water algal communities: Linking population biology and community ecology for conservation. In Proceedings of the 5th Mediterranean Symposium on Marine Vegetation, Portorož, Slovenia, 27–28 October 2014; Langar, H., Bouafif, C., Ouerghi, A., Eds.; RAC/SPA publ.: Tunis, Tunisia, 2014; p. 20. [Google Scholar]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2012; p. 32. [Google Scholar] [CrossRef]
- ŽDuljević, A.; Peters, A.F.; Nikolić, V.; Antolić, B.; Despalatović, M.; Cvitković, I.; Isajlović, I.; Mihanović, H.; Matijević, S.; Shewring, D.M.; et al. The Mediterranean deep-water kelp Laminaria rodriguezii is an endangered species in the Adriatic Sea. Mar. Biol. 2016, 163, 69. [Google Scholar] [CrossRef]
- Ercegović, A. La Végétation des Algues sur les Fonds Péchereux de l’Adriatique. Ribarstveno-Bioloska Ekspedicija M/B Hvar Izvjesca 1948–1949. The M.V. Hvar Cruises-Researches into Fisheries Biology Reports 1948–1949; Institut za Oceanografiju i Ribarstvo: Split, Croatia, 1960; p. 32. [Google Scholar]
- Gamulin-Brida, H. Biocenoza muljevitog dna otvorenog srednjeg jadrana. (Biocoenose des fonds vaseux au large de l’Adriatique moyenne). Acta Adriat. 1965, 10, 1–27. [Google Scholar]
- Steen, F.; Aragay, J.; Zuljevic, A.; Verbruggen, H.; Mancuso, F.P.; Bunker, F.; Vitales, D.; Gómez Garreta, A.; De Clerck, O. Tracing the introduction history of the brown seaweed Dictyota cyanoloma (Phaeophyceae, Dictyotales) in Europe. Eur. J. Phycol. 2017, 52, 31–42. [Google Scholar] [CrossRef]
- Tronholm, A.; Steen, F.; Tyberghein, L.; Leliaert, F.; Verbruggen, H.; Siguan, M.A.R.; De Clerck, O. Species delimitation, taxonomy, and biogeography of Dictyota in Europe (Dictyotales, Phaeophyceae). J. Phycol. 2010, 46, 1301–1321. [Google Scholar] [CrossRef]
- Rull Lluch, J.R.; Ballesteros, E.; Barceló, M.C.; Gómez Garreta, A.; Ribera Siguan, M.A. Dictyota ciliolata Sonder ex Kützing (Phaeophyceae, Dictyotales) in the Mediterranean Sea. Cryptogamie Algol. 2007, 28, 89–97. [Google Scholar]
- Afonso-Carrillo, J. Lista actualizada de las Algas Marinas de las Islas Canarias, 2014; Elaborada para la Sociedad Española de Ficología (SEF): Las Palmas, Spain, 2014; p. 64. [Google Scholar]
- Bárbara, I.; De Clerk, O.; García-Redondo, V.; Peña, V.; García-Fernández, A.; Peteiro, C.; Sánchez, N. Nuevas citas y adiciones corológicas para la flora bentónica marina del Atlántico Ibérico. Acta Bot. Malac. 2015, 40, 191–198. [Google Scholar] [CrossRef][Green Version]
- Bárbara, I.; Peña, V.; García-Redondo, V.; Díaz-Tapia, P.; García-Fernández, A.; Lugilde, J.; Piñeíro-Corbeira, C. Fragmentos taxonómicos, corológicos, nomenclaturales y fitocenológicos. 237. Nuevas citas y registros corológicos para la flora bentónica marina del Noroeste ibérico. Acta Bot. Malac. 2016, 41, 247–254. [Google Scholar] [CrossRef]
- Bárbara, I.; García-Redondo, V.; Díaz Tapia, P.; García-Fernández, A.; Piñeiro-Corbeira, C.; Peña, V.; Lugilde, J.; Cremades, J. Adiciones y correcciones a la flora bentónica marina del Atlántico ibérico norte. Acta Bot. Malac. 2019, 44, 1–10. [Google Scholar] [CrossRef]
- Gallardo, T.; Bárbara, I.; Afonso-Carrillo, J.; Bermejo, R.; Altamirano, M.; Gómez Garreta, A.; Barceló Martí, M.C.; Rull Lluch, J.; Ballesteros, E.; De la Rosa, J. Nueva lista crítica de las algas bentónicas marinas de España. A new checklist of benthic marine algae of Spain. Algas. Boletín Informativo Sociedad Española Ficología 2016, 51, 7–52. [Google Scholar]
- Bunker, F.; Brodie, J.A.; Maggs, C.A.; Bunker, A.R. Seaweeds of Britain and Ireland, 2nd ed.; Wild Nature Press: Plymouth, UK, 2017; pp. 1–312. [Google Scholar]
- Joher, S.; Ballesteros, E.; Cebrian, E.; Sánchez, N.; Rodríguez-Prieto, C. Deep-water macroalgal-dominated coastal detritic assemblages on the continental shelf off Mallorca and Menorca (Balearic Islands, Western Mediterranean). Bot. Mar. 2012, 55, 485–497. [Google Scholar] [CrossRef]
- Joher, S.; Ballesteros, E.; Rodríguez-Prieto, C. Contribution to the study of deep coastal detritic bottoms: The algal communities of the continental shelf off the Balearic Islands, Western Mediterranean. Med. Mar. Sci. 2015, 16, 573–590. [Google Scholar] [CrossRef]
- Joher, S.; Ballesteros, E.; Rodríguez-Prieto, C. Macroalgal-dominated coastal detritic communities from the Western Mediterranean and the Northeastern Atlantic. Med. Mar. Sci. 2016, 17, 476–495. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, M.S. Female reproductive structures define the novel genus, Nesoia (Halymeniaceae, Rhodophyta). Eur. J. Phycol. 2019, 54, 66–77. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; De Clerck, O.; Huisman, J.M.; Lin, S.-M. Characterization of Nesoia latifolia (Halymeniaceae, Rhodophyta) from Europe with emphasis on cystocarp development and description of Nesoia mediterranea sp. nov. Phycologia 2019, 58, 393–404. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; Afonso-Carrillo, J.; De Clerck, O.; Huisman, J.M.; Lin, S.-M. Systematic revision of the foliose Halymeniaceae (Halymeniales, Rhodophyta) from Europe, with the description of Halymenia ballesterosii sp. nov. from the Mediterranean Sea and Nesoia hommersandii from the Canary Islands. Eur. J. Phycol. 2020, 53, 520–536. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; De Clerck, O.; Huisman, J.M.; Lin, S.-M. Systematics of the red algal genus Halymenia (Halymeniaceae, Rhodophyta): Characterization of the generitype H. floresii and description of Neofolia rosea gen. et sp. nov. Eur. J. Phycol. 2018, 53, 520–536. [Google Scholar] [CrossRef]
- Sáenz-Arroyo, A.; Roberts, C.M.; Torre, J.; Cariño-Olvera, M. Using fishers’ anecdotes, naturalists’ observations and grey literature to reassess marine species at risk: The case of the Gulf grouper in the Gulf of California. Mexico. Fish Fish. 2005, 6, 121–133. [Google Scholar] [CrossRef]
- Husa, V.; Steen, H.; Sjøtun, K. Historical changes in macroalgal communities in Hardangerfjord (Norway). Mar. Biol. Res. 2014, 10, 226–240. [Google Scholar] [CrossRef]
- Graham, C.H.; Ferrier, S.; Huettman, F.; Moritz, C.; Peterson, A.T. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol. Evol. 2004, 19, 497–503. [Google Scholar] [CrossRef]
- Riera, R.; Sangil, C.; Sansón, M. Long-term herbarium data reveal the decline of a temperate-water algae at its southern range. Estuar. Coast. Shelf Sci. 2015, 165, 159–165. [Google Scholar] [CrossRef]
- Provan, J.; Booth, D.; Todd, N.P.; Beatty, G.E.; Maggs, C.A. Tracking biological invasions in space and time: Elucidating the invasive history of the green alga Codium fragile using old DNA. Diversity Distrib. 2008, 14, 343–354. [Google Scholar] [CrossRef]
- Schaffer, H.B.; Fisher, R.N.; Davidson, C. The role of natural history collections in documenting species declines. Trends Ecol. Evol. 1998, 13, 27–30. [Google Scholar] [CrossRef]
- Tingley, M.W.; Beissinger, S.R. Detecting range shifts from historical species occurrences: New perspectives on old data. Trends Ecol. Evol. 2009, 24, 625–633. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).