Algal Diversity in Paramecium bursaria: Species Identification, Detection of Choricystis parasitica, and Assessment of the Interaction Specificity
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Cultivation
2.2. Observation and Size Determination of Intracellular Algae
2.3. DNA Extraction and Amplification of Molecular Markers
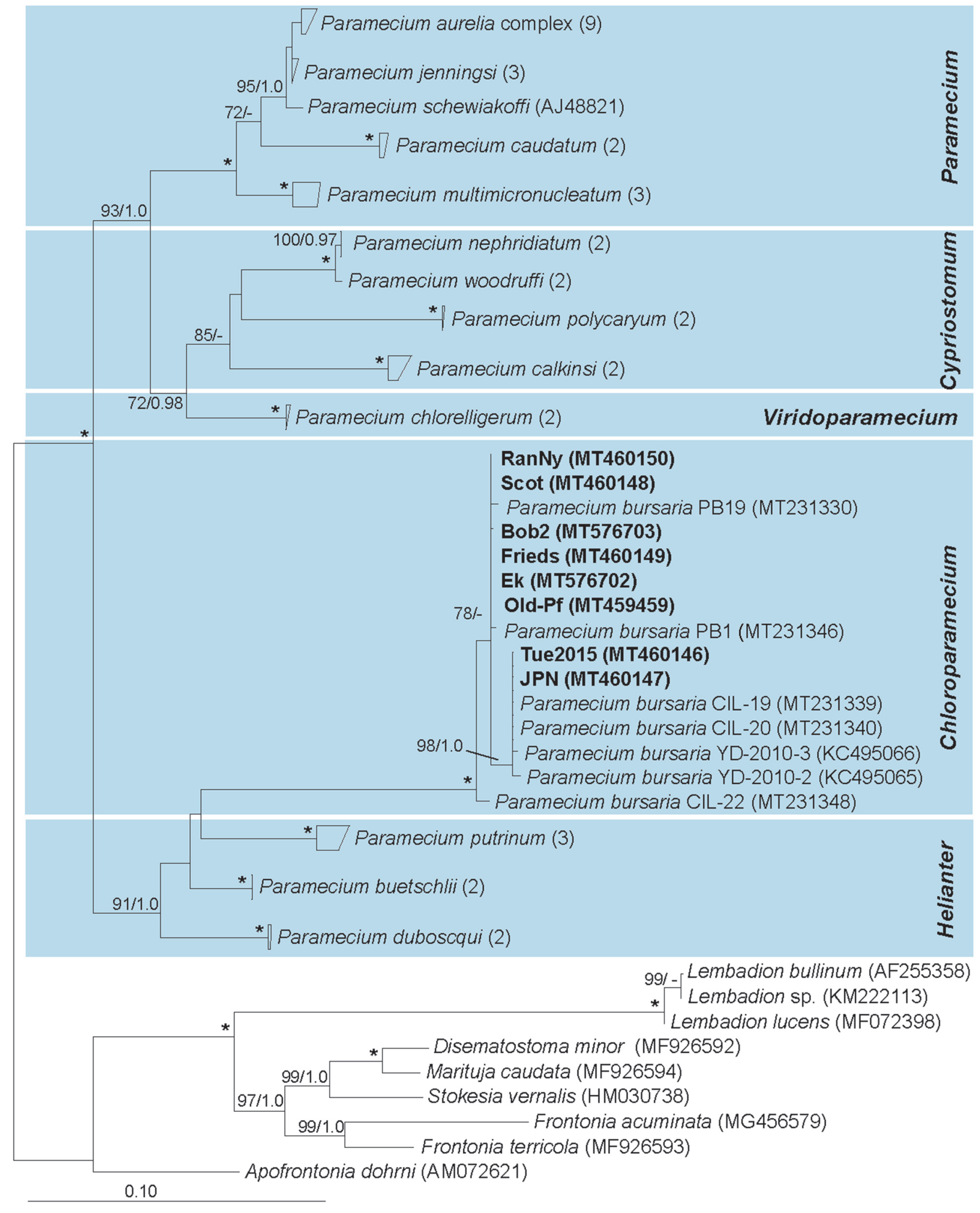
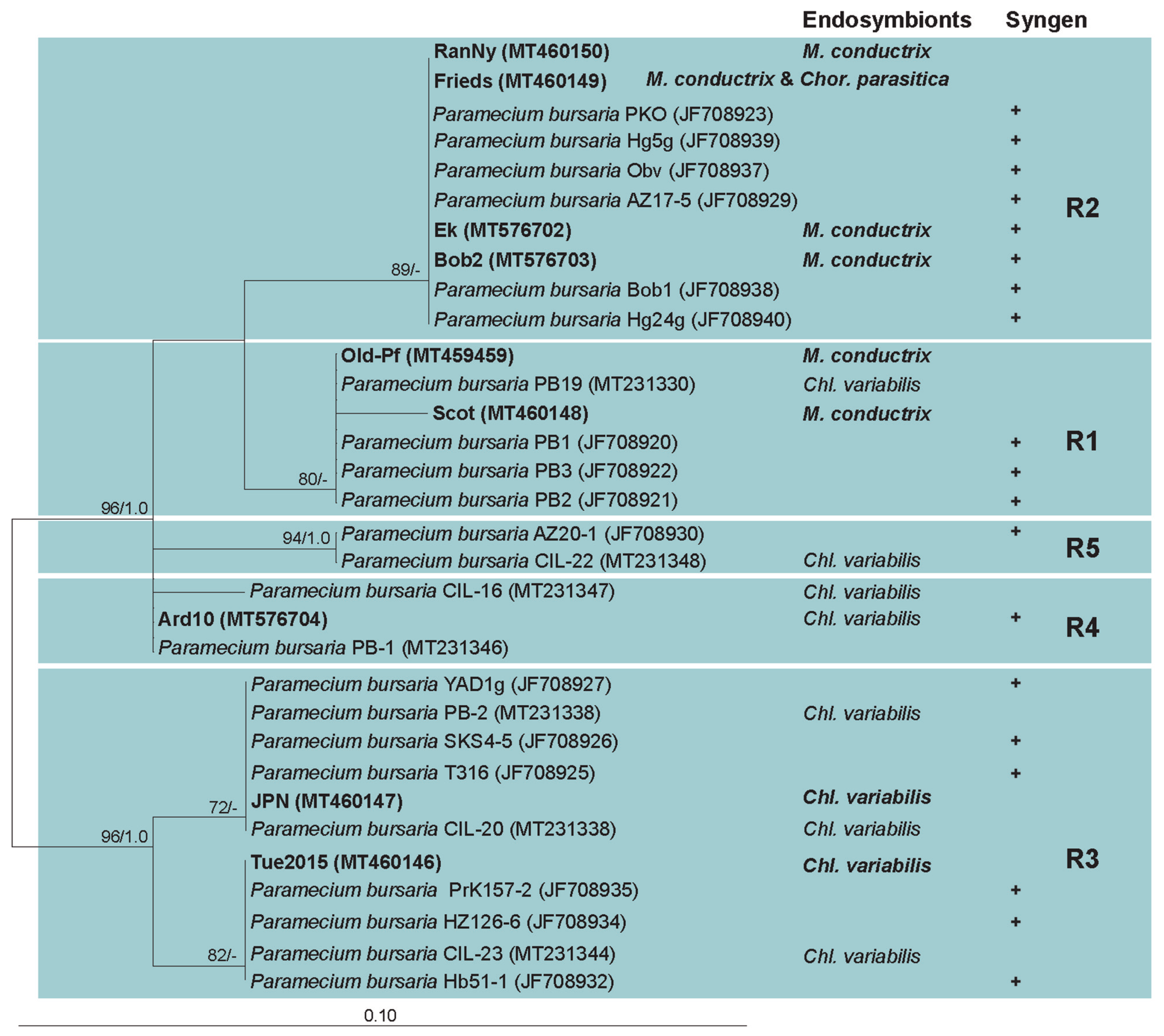
2.4. Molecular Characterization of Paramecium bursaria
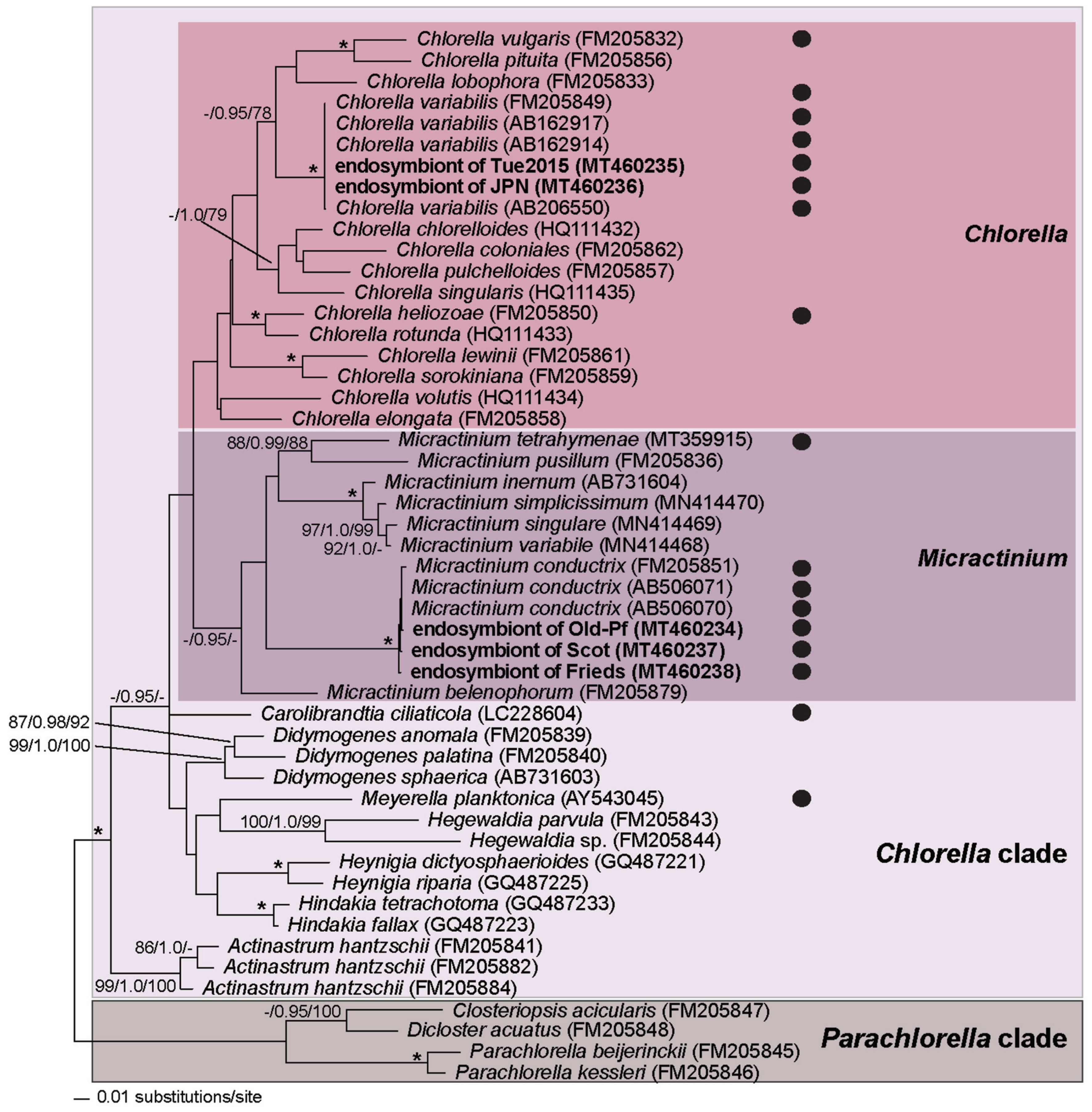
2.5. Molecular Characterization of Green Algal Endosymbionts in Paramecium bursaria
2.6. Establishment of Aposymbiotic Paramecium bursaria
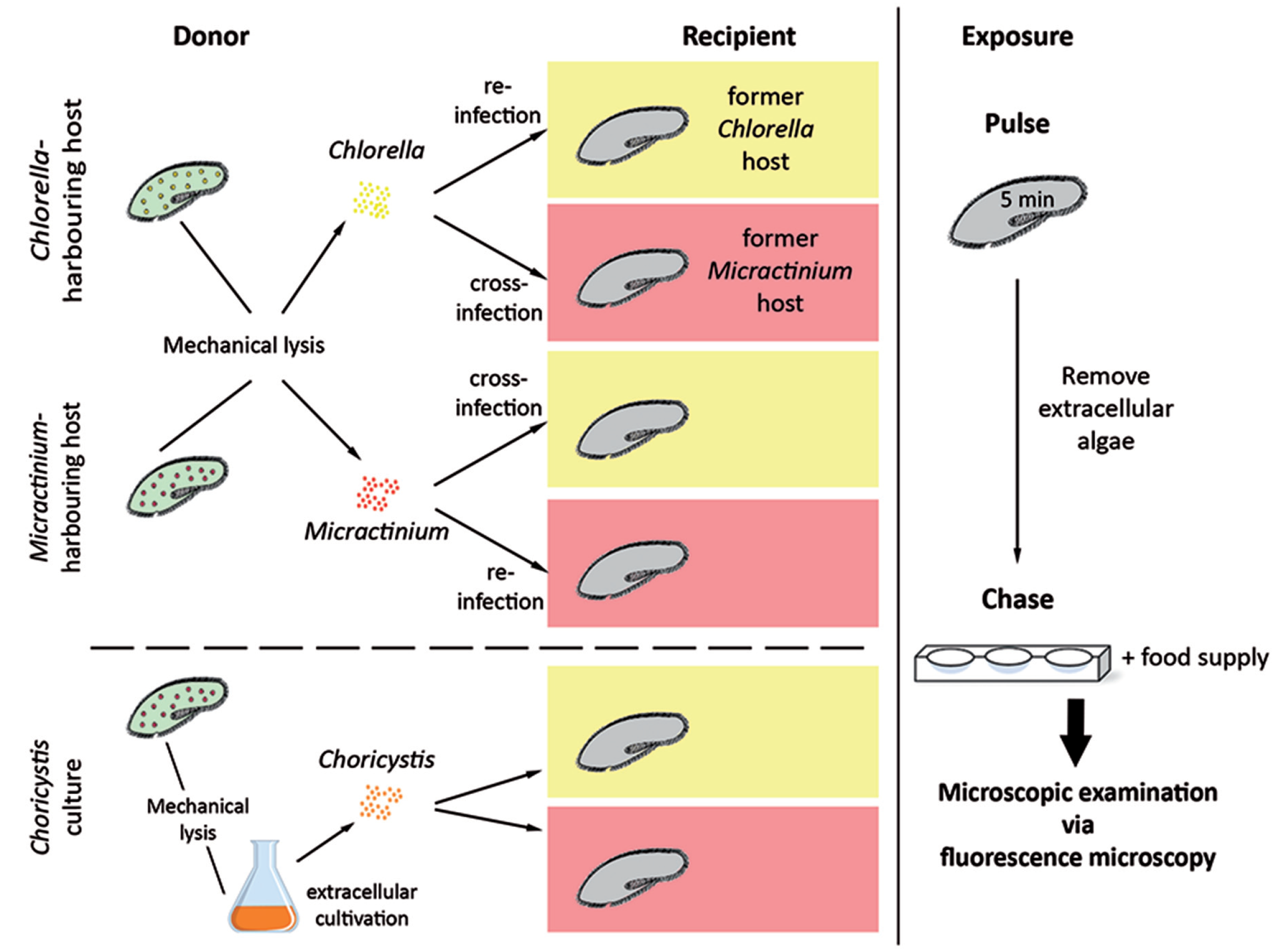
2.7. Pulse-Chase Infection Experiments
3. Results
3.1. Microscopic Investigation of the Intracellular Algae
3.2. Paramecium bursaria and the Five Syngens
3.3. Chlorella variabilis, Micractinium conductrix, and Choricystis parasitica as Paramecium’s Endosymbionts
3.4. Establishment of Symbioses
4. Discussion
4.1. Symbiotic Relationships and Specificity of Paramecium bursaria and Its Green Algal Endosymbionts
4.2. Molecular Characterization of Paramecium bursaria and Its Green Algal Endosymbionts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, O.F. Vermium Terrestrium et Fluviatilium, seu Animalium Infusoriorum, Helminthicorum et Testaceorum non Marinorum Succincta Historia; Heineck et Faber: Havniae et Lipsiae, Germany, 1773; Volume 1, p. 74. [Google Scholar]
- Kahl, A. Urtiere oder Protozoa. I. Wimpertiere oder Ciliata (Infusoria). 4. Peritricha und Chonotricha. Die Tierwelt der angrenzenden Meeresteile 1935, 30, 651–886. [Google Scholar]
- Kreutz, M.; Stoeck, T.; Foissner, W. Morphological and molecular characterization of Paramecium (Viridoparamecium nov. subgen.) chlorelligerum Kahl (Ciliophora). J. Eukaryot. Microbiol. 2012, 59, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Fawley, M.W.; Fawley, K.P.; Owen, H.A. Diversity and ecology of small coccoid green algae from Lake Itasca, Minnesota, USA, including Meyerella planktonica, gen. et sp. nov. Phycologia 2005, 44, 35–48. [Google Scholar] [CrossRef]
- Lanzoni, O.; Fokin, S.I.; Lebedeva, N.; Migunova, A.; Petroni, G.; Potekhin, A. Rare freshwater ciliate Paramecium chlorelligerum Kahl, 1935 and its macronuclear symbiotic bacterium “Candidatus Holospora parva”. PLoS ONE 2016, 11, e0167928. [Google Scholar] [CrossRef]
- Focke, G. Ueber einige Organisationsverhältnisse bei polygastrischen Infusorien und Räderthieren. Oken. Isis 1836, 10, 785–787. [Google Scholar]
- Fujishima, M. Endosymbionts in Paramecium; Springer Science and Business Media: Berlin, Germany, 2009; Volume 12. [Google Scholar]
- Strüder-Kypke, M.C.; Wright, A.D.G.; Fokin, S.I.; Lynn, D.H. Phylogenetic relationships of the subclass Peniculia (Oligohymenophorea, Ciliophora) inferred from small subunit rRNA gene sequences. J. Eukaryot. Microbiol. 2000, 47, 419–429. [Google Scholar] [CrossRef]
- Fokin, S.I.; Przyboś, E.; Chivilev, S.M.; Beier, C.L.; Horn, M.; Skotarczak, B.; Wodecka, B.; Fujishima, M. Morphological and molecular investigations of Paramecium schewiakoffi sp. nov. (Ciliophora, Oligohymenophorea) and current status of distribution and taxonomy of Paramecium spp. Eur. J. Protistol. 2004, 40, 225–243. [Google Scholar] [CrossRef]
- Pröschold, T.; Darienko, T.; Silva, P.C.; Reisser, W.; Krienitz, L. The systematics of Zoochlorella revisited employing an integrative approach. Environ. Microbiol. 2011, 13, 350–364. [Google Scholar] [CrossRef]
- Vorobyev, K.; Andronov, E.; Rautian, M.; Skoblo, I.; Migunova, A.; Kvitko, K. An atypical Chlorella symbiont Paramecium bursaria. Protistology 2009, 6, 39–44. [Google Scholar]
- Hoshina, R.; Imamura, N. Phylogenetic position of endosymbiotic green algae in Paramecium bursaria Ehrenberg Japan. Plant Biol. 2004, 6, 447–453. [Google Scholar] [CrossRef]
- Gaponova, I.N.; Andronov, E.E.; Migunova, A.V.; Vorobyev, K.P.; Chizhevskaja, E.P.; Kvitko, K.V. Genomic dactyloscopy of Chlorella sp., symbionts of Paramecium bursaria. Protistology 2007, 4, 311–317. [Google Scholar]
- Migunova, A.; Kvitko, K.; Prokosheva, M.; Litvinov, D. Influence of temperature on duplication Paramecium bursaria, Chlorella, PBCV-1 viruses in system of threefold symbiosis. Vestnic St. Peterburg Univ. 2000, 3, 65–75. [Google Scholar]
- Shihira, I.; Krauss, R.W. Chlorella: Physiology and Taxonomy of Forty-One Isolates; University of Maryland: College Park, MD, USA, 1965; pp. 1–97. [Google Scholar]
- Brandt, K. Ueber das Zusammenleben von Thieren und Algen. Arch. Anat. Physiol. 1881, 1881, 570–574. [Google Scholar]
- Pröschold, T.; Bock, C.; Luo, W.; Krienitz, L. Polyphyletic distribution of bristle formation in Chlorellaceae: Micractinium, Diacanthos, Didymogenes and Hegewaldia gen. nov. (Trebouxiophyceae, Chlorophyta). Phycol. Res. 2010, 58, 1–8. [Google Scholar] [CrossRef]
- Karakashian, S.J.; Rudzinska, M.A. Inhibition of lysosomal fusion with symbiont-containing vacuoles in Paramecium bursaria. Exp. Cell Res. 1981, 131, 387–393. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Infection of Paramecium bursaria by symbiotic Chlorella species. In Endosymbionts in Paramecium; Springer: Berlin, Germany, 2009; pp. 31–55. [Google Scholar]
- Kodama, Y.; Fujishima, M. Secondary symbiosis between Paramecium and Chlorella cells. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 279, pp. 33–77. [Google Scholar]
- He, M.; Wang, J.; Fan, X.; Liu, X.; Shi, W.; Huang, N.; Zhao, F.; Miao, M. Genetic basis for the establishment of endosymbiosis in Paramecium. ISME J. 2019, 13, 1360–1369. [Google Scholar] [CrossRef]
- Reisser, W. Die stoffwechselphysiologischen Beziehungen zwischen Paramecium bursaria Ehrberg und Chlorella spec. der Paramecium bursaria-Symbiose. Arch. Microbiol. 1976, 107, 357–360. [Google Scholar] [CrossRef]
- Brown, J.A.; Nielsen, P.J. Transfer of photosynthetically produced carbohydrate from endosymbiotic Chlorellae Paramecium bursaria. J. Protozool. 1974, 21, 569–570. [Google Scholar] [CrossRef]
- Summerer, M.; Sonntag, B.; Hörtnagl, P.; Sommaruga, R. Symbiotic ciliates receive protection against UV damage from their algae: A test with Paramecium bursaria and Chlorella. Protist 2009, 160, 233–243. [Google Scholar] [CrossRef]
- Dunigan, D.D.; Al-Sammak, M.; Al-Ameeli, Z.; Agarkova, I.V.; DeLong, J.P.; Van Etten, J.L. Chloroviruses lure hosts through long-distance chemical signaling. J. Virol. 2019, 93, e01688-18. [Google Scholar] [CrossRef]
- Coy, S.R.; Alsante, A.N.; Van Etten, J.L.; Wilhelm, S.W. Cryopreservation of Paramecium bursaria Chlorella Virus-1 during an active infection cycle of its host. PLoS ONE 2019, 14, e0211755. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, R.; Imamura, N. Origins of algal symbionts of Paramecium bursaria. In Endosymbionts in Paramecium; Springer: Berlin, Germany, 2009; pp. 1–29. [Google Scholar]
- Sommaruga, R.; Sonntag, B. Photobiological aspects of the mutualistic association between Paramecium bursaria and Chlorella. In Endosymbionts in Paramecium; Springer: Berlin, Germany, 2009; pp. 111–130. [Google Scholar]
- Kodama, Y.; Suzuki, H.; Dohra, H.; Sugii, M.; Kitazume, T.; Yamaguchi, K.; Shigenobu, S.; Fujishima, M. Comparison of gene expression of Paramecium bursaria with and without Chlorella variabilis symbionts. BMC Genom. 2014, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.D.; Minter, E.J.; Cameron, D.D.; Brockhurst, M.A. Shining a light on exploitative host control in a photosynthetic endosymbiosis. Curr. Biol. 2016, 26, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, R.; Fujiwara, Y. Photobiont flexibility in Paramecium bursaria: Double and triple photobiont co-habitation. Adv. Microbiol. 2012, 2, 227. [Google Scholar] [CrossRef][Green Version]
- Tonooka, Y.; Watanabe, T. A natural strain of Paramecium bursaria lacking symbiotic algae. Eur. J. Protistol. 2002, 38, 55–58. [Google Scholar] [CrossRef]
- Nakajima, T.; Matsubara, T.; Ohta, Y.; Miyake, D. Exploitation or cooperation? Evolution of a host (ciliate)-benefiting alga in a long-term experimental microcosm culture. Biosystems 2013, 113, 127–139. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yuyama, I.; Shimizu, H.; Nozawa, M.; Ikeo, K.; Gojobori, T. Different endosymbiotic interactions in two hydra species reflect the evolutionary history of endosymbiosis. Genome Biol. Evol. 2016, 8, 2155–2163. [Google Scholar] [CrossRef]
- Esteban, G.F.; Fenchel, T.; Finlay, B.J. Mixotrophy in ciliates. Protist 2010, 161, 621–641. [Google Scholar] [CrossRef]
- Rautian, M.S.; Wackerow-Kouzova, N.D. Phylogenetic placement of two previously described intranuclear bacteria from the ciliate Paramecium bursaria (Protozoa, Ciliophora): ‘Holospora acuminata’ and ‘Holospora curviuscula’. Int. J. Syst. Evol. Microbiol. 2013, 63, 1930–1933. [Google Scholar] [CrossRef]
- Gong, J.; Qing, Y.; Guo, X.; Warren, A. “Candidatus Sonnebornia yantaiensis”, a member of candidate division OD1, as intracellular bacteria of the ciliated protist Paramecium bursaria (Ciliophora, Oligohymenophorea). Syst. Appl. Microbiol. 2014, 37, 35–41. [Google Scholar] [CrossRef]
- Görtz, H. Infections of Paramecium bursaria with bacteria and yeasts. J. Cell. Sci. 1982, 58, 445–453. [Google Scholar] [PubMed]
- Suzaki, T.; Omura, G.; Görtz, H. Infection of symbiont-free Paramecium bursaria with yeasts. Jpn. J. Protozool. 2003, 36, 17–18. [Google Scholar]
- Nakahara, M.; Handa, S.; Watanabe, S.; Deguchil, H. Choricystis minor as a new symbiont of simultaneous two-species association with Paramecium bursaria and implications for its phylogeny. Symbiosis 2004, 36, 127–151. [Google Scholar]
- Szokoli, F.; Sabaneyeva, E.; Castelli, M.; Krenek, S.; Schrallhammer, M.; Soares, C.A.; da Silva-Neto, I.D.; Berendonk, T.U.; Petroni, G. “Candidatus Fokinia solitaria”, a novel “stand-alone” symbiotic lineage of Midichloriaceae (Rickettsiales). PLoS ONE 2016, 11, e0145743. [Google Scholar] [CrossRef]
- Krenek, S.; Berendonk, T.U.; Fokin, S.I. New Paramecium (Ciliophora, Oligohymenophorea) congeners shape our view its biodiversity. Org. Divers. Ecol. 2015, 15, 215–233. [Google Scholar] [CrossRef]
- Da Silva Paiva, T.; do Nascimento Borges, B.; Harada, M.L.; da Silva-Neto, I.D. Description and molecular phylogeny of Paramecium grohmannae sp. nov. (Ciliophora, Peniculida) from a wastewater treatment plant in Brazil. Rev. Bras. De Zoociências 2016, 17, 7–19. [Google Scholar]
- Castelli, M.; Serra, V.; Senra, M.V.; Basuri, C.K.; Soares, C.A.; Fokin, S.I.; Modeo, L.; Petroni, G. The hidden world of Rickettsiales symbionts: “Candidatus Spectririckettsia obscura,” a novel bacterium found in Brazilian and Indian Paramecium caudatum. Microb. Ecol. 2019, 77, 748–758. [Google Scholar] [CrossRef]
- Sonneborn, T.M. Mating types in Paramecium aurelia: Diverse conditions for mating in different stocks; occurrence, number and interrelations of the types. Proc. Am. Philosoph. Soc. 1938, 79, 411–434. [Google Scholar]
- Bomford, R. The syngens of Paramecium bursaria: New mating types and intersyngenic mating reactions. J. Protozool. 1966, 13, 497–501. [Google Scholar] [CrossRef]
- Greczek-Stachura, M.; Potekhin, A.; Przyboś, E.; Rautian, M.; Skoblo, I.; Tarcz, S. Identification of Paramecium Bursaria syngens through molecular markers–comparative analysis of three loci in the nuclear and mitochondrial DNA. Protist 2012, 163, 671–685. [Google Scholar] [CrossRef]
- Strüder-Kypke, M.C.; Lynn, D.H. Comparative analysis of the mitochondrial cytochrome c oxidase subunit I (COI) gene in ciliates (Alveolata, Ciliophora) and evaluation of its suitability as a biodiversity marker. Syst. Biodivers. 2010, 8, 131–148. [Google Scholar] [CrossRef]
- Tasneem, F.; Shakoori, F.R.; Ilyas, M.; Shahzad, N.; Potekhin, A.; Shakoori, A.R. Genetic diversity of Paramecium species on the basis of multiple loci analysis and ITS secondary structure models. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Spanner, C.; Darienko, T.; Biehler, T.; Sonntag, B.; Pröschold, T. Endosymbiotic green algae in Paramecium Bursaria: A new isolation method and a simple diagnostic PCR approach for the identification. Diversity 2020, 12, 240. [Google Scholar] [CrossRef]
- Potekhin, A.; Mayén-Estrada, R. Paramecium diversity and a new member of the Paramecium Aurelia species complex described from Mexico. Diversity 2020, 12, 197. [Google Scholar] [CrossRef]
- Hoshina, R.; Fujiwara, Y. Molecular characterization of Chlorella cultures of the National Institute for Environmental Studies Culture Collection with description of Micractinium inermum sp. nov., Didymogenes sphaerica sp. nov., and Didymogenes soliella sp. nov. (Chlorellaceae, Trebouxiophyceae). Phycol. Res. 2013, 61, 124–132. [Google Scholar]
- Krienitz, L.; Bock, C. Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia 2012, 698, 295–326. [Google Scholar] [CrossRef]
- Luo, W.; Pröschold, T.; Bock, C.; Krienitz, L. Generic concept in Chlorella-related coccoid green algae (Chlorophyta, Trebouxiophyceae). Plant Biol. 2010, 12, 545–553. [Google Scholar] [CrossRef]
- Pröschold, T.; Pitsch, G.; Darienko, T. Micractinium tetrahymenae (Trebouxiophyceae, Chlorophyta), a new endosymbiont isolated from ciliates. Diversity 2020, 12, 200. [Google Scholar] [CrossRef]
- Heeg, J.S.; Wolf, M. ITS2 and 18S rDNA sequence-structure phylogeny of Chlorella and allies (Chlorophyta, Trebouxiophyceae, Chlorellaceae). Plant Gene 2015, 4, 20–28. [Google Scholar] [CrossRef]
- Krienitz, L.; Hegewald, E.H.; Hepperle, D.; Huss, V.A.; Rohr, T.; Wolf, M. Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycology 2004, 43, 529–542. [Google Scholar] [CrossRef]
- Krienitz, L.; Bock, C.; Kotut, K.; Proschold, T. Genotypic diversity of Dictyosphaerium-morphospecies (Chlorellaceae, Trebouxiophyceae) in African inland waters, including the description of four new genera. Fottea 2012, 12, 231–253. [Google Scholar] [CrossRef]
- Bella, C.; Koehler, L.; Grosser, K.; Berendonk, T.U.; Petroni, G.; Schrallhammer, M. Fitness impact of obligate intranuclear bacterial symbionts depends on host growth phase. Front. Microbiol. 2016, 7, 2084. [Google Scholar] [CrossRef] [PubMed]
- Zagata, P.; Greczek-Stachura, M.; Tarcz, S.; Rautian, M. The evolutionary relationships between endosymbiotic green algae of Paramecium bursaria syngens originating from different geographical locations. Folia Biol. 2016, 64, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fokin, S.; Andreoli, I.; Verni, F.; Petroni, G. Apofrontonia dohrni sp. n. and the phylogenetic relationships within Peniculia (Protista, Ciliophora, Oligohymenophorea). Zool. Scr. 2006, 35, 289–300. [Google Scholar] [CrossRef]
- Darienko, T.; Rad-Menéndez, C.; Campbell, C.; Pröschold, T. Are there any true marine Chlorella species? Molecular phylogenetic assessment and ecology of marine Chlorella-like organisms, including a description of Droopiella gen. nov. Syst. Biodivers. 2019, 17, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Castro, B.; Gaitan, A.; Preisig, O.; Wingfield, B.; Wingfield, M. Relationships of Ceratocystis fimbriata isolates from Colombian coffee-growing regions based on molecular data and pathogenicity. J. Phytopathol. 2003, 151, 395–405. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Swofford, D. PAUP*. Phylogenetic analysis using parsimony (* and other, odels). Version 4.0 b10 for Macintosh; Sinauer Associates Inc.: Sunderland, MA, USA, 2002. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Pröschold, T. Species concept and nomenclatural changes within the genera Elliptochloris and Pseudochlorella (Trebouxiophyceae) based on an integrative approach. J. Phycol. 2016, 52, 1125–1145. [Google Scholar] [CrossRef]
- Chae, H.; Lim, S.; Kim, H.S.; Choi, H.G.; Kim, J.H.; Chae, H.; Lim, S.; Kim, H.S.; Choi, H.G.; Kim, J.H. Morphology and phylogenetic relationships of Micractinium (Chlorellaceae, Trebouxiophyceae) taxa, including three new species from Antarctica. Algae 2019, 34, 267–275. [Google Scholar] [CrossRef]
- Hodač, L.; Hallmann, C.; Spitzer, K.; Elster, J.; Faßhauer, F.; Brinkmann, N.; Lepka, D.; Diwan, V.; Friedl, T. Widespread green algae Chlorella and Stichococcus exhibit polar-temperate and tropical-temperate biogeography. FEMS Microbiol. Ecol. 2016, 92, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.H.; Burkard, M.E.; Freier, S.M.; Wyatt, J.R.; Turner, D.H. Predicting oligonucleotide affinity to nucleic acid targets. RNA 1999, 5, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Wiessner, W. Infection of algae-free Paramecium bursaria symbiotic Chlorella sp. isolated from green paramecia: II. A timed study. J. Cell Sci. 1989, 93, 571–579. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Characteristics of the digestive vacuole membrane of the alga-bearing ciliate Paramecium bursaria. Protist 2012, 163, 658–670. [Google Scholar] [CrossRef]
- Hoshina, R.; Kato, Y.; Kamako, S.; Imamura, N. Genetic evidence of “American” and “European” type symbiotic algae of Paramecium bursaria Ehrenberg. Plant Biol. 2005, 7, 526–532. [Google Scholar] [CrossRef]
- Hoshina, R.; Imamura, N. Multiple origins of the symbioses in Paramecium bursaria. Protist 2008, 159, 53–63. [Google Scholar] [CrossRef]
- Hoshina, R.; Iwataki, M.; Imamura, N. Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol. Res. 2010, 58, 188–201. [Google Scholar] [CrossRef]
- Summerer, M.; Sonntag, B.; Sommaruga, R. An experimental test of the symbiosis specificity between the ciliate Paramecium bursaria and strains of the unicellular green alga Chlorella. Environ. Microbiol. 2007, 9, 2117–2122. [Google Scholar] [CrossRef]

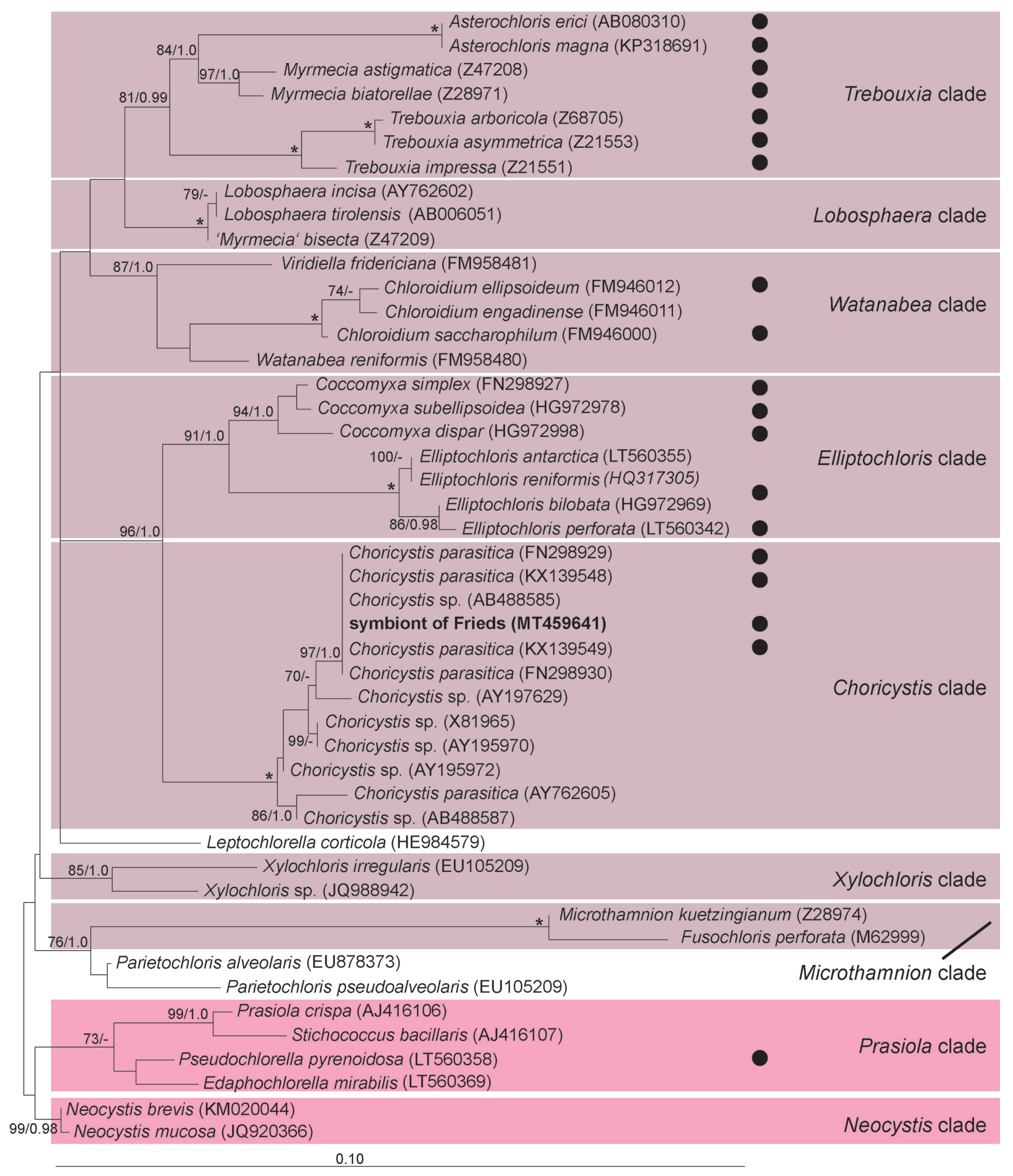
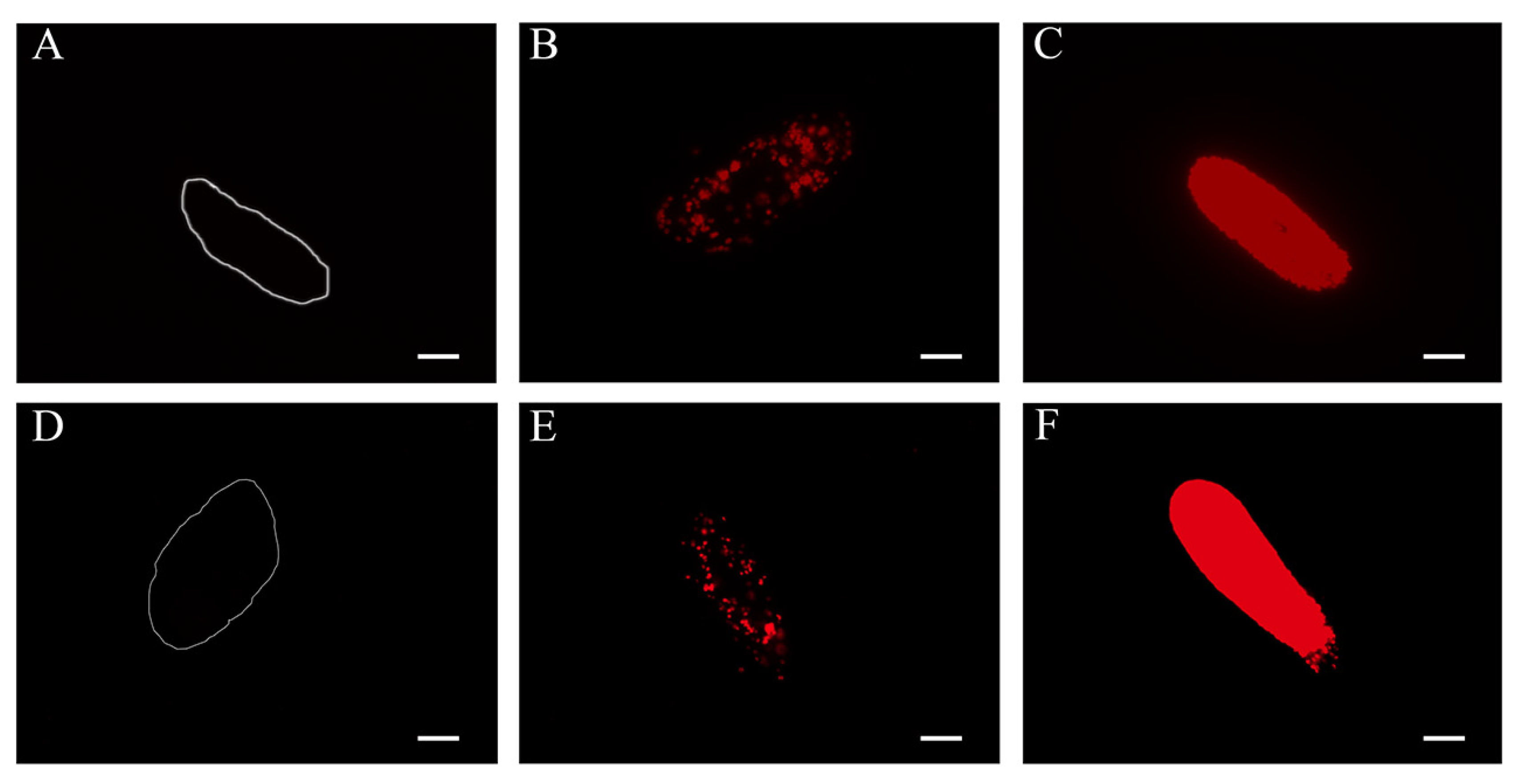
| Strain | Host | Syngen | Acc. Number (Region) | Algal Symbiont | Acc. Number (Region) | Collection Date | Collected by |
|---|---|---|---|---|---|---|---|
| JPN 1 | P. bursaria | R3 | MT460147 (SSU-ITS-LSU part.) | Chlorella variabilis | MT460236 (SSU-ITS) | September 2014 | L. Koehler |
| Tue2015 2 | P. bursaria | R3 | MT460146 (SSU-ITS-LSU part.) | Chlorella variabilis | MT460235 (SSU-ITS) | - | K. Eisler |
| Frieds 3 | P. bursaria | R2 | MT460149 (SSU-ITS-LSU part.) | 1st Micractinium conductrix | MT460238 (SSU-ITS) | August 2014 | M. Witt |
| 2nd Choricystis parasitica | MT459641 (partial SSU) | ||||||
| Old-Pf 4 | P. bursaria | R1 | MT459459 (SSU-ITS-LSU part.) | Micractinium conductrix | MT460234 (SSU-ITS) | August 2014 | K. Grosser |
| RanNy 5 | P. bursaria | R2 | MT460150 (SSU-ITS-LSU part.) | Micractinium conductrix | - (diagn. PCR) | Jul. 2016 | K. Grosser |
| Scot 6 | P. bursaria | R1 | MT460148 (SSU-ITS-LSU part.) | Micractinium conductrix | MT460237 (SSU-ITS) | July 2010 | M. Schrall- hammer |
| Ek 7 | P. bursaria | R2 | MT576702 (SSU-ITS-LSU part.) | Micractinium conductrix | - (diagn. PCR) | September 2009 | A. Potekhin |
| Bob2 8 | P. bursaria | R2 | MT576703 (SSU-ITS-LSU part.) | Micractinium conductrix | - (diagn. PCR) | August 2006 | A. Potekhin |
| Ard10 9 | P. bursaria | R4 | MT576704 (ITS-LSU part.) | Chlorella * variabilis | KM203667 (LSU partial) | April 2006 | V. Yashchenko |
| Primer | Sequence [5′-3′] | Literature |
|---|---|---|
| Penic_F82 | GAAACTGCGAATGGCTC | Strüder-Kypke et al., 2000 [8] |
| Penic_F661 | ATAGATGGGGGCATTAGT | mod. from Fokin et al., 2006 [61] |
| Penic_R1280 | CGACACGTCCTAACAAGA | Fokin et al., 2006 [61] |
| 28S_R457 | CTTTCCTTCGYAGTACT | W. Ludwig, pers. commun. |
| AF | TCGACAATCTGGTGGATCCTGCCAGT | Pröschold et al., 2001 [10] |
| Chlo_F238 | GCCCTATCAACTTTCGATG | this study |
| Chlo_G500F | GAATGAGTACAATCTAAACCCCTTAAC | Darienko et al., 2019 [62] |
| Chlo_G800F | CCTGTTGGTCTGTAGGAGTGGAGTAATG | Darienko et al., 2019 [62] |
| Chlo_F1074 | GGGTTGCCTTGTCAGG | this study |
| ITS055R | CTCCTTGGTCCGTGTTTCAAGACGGG | Marin et al., 2003 [63] |
| Chlo_G800R | CATTACTCCGCTCCTACAGACCAACAGG | Darienko et al., 2019 [62] |
| Chlo_R841 | CGGAGTCATCGAAGAAAC | this study |
| Chori_F238 | GCCCTATCAACTTTCAACC | this study |
| Chori_R841 | TGGGGGGGTCATCAAAGG | this study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flemming, F.E.; Potekhin, A.; Pröschold, T.; Schrallhammer, M. Algal Diversity in Paramecium bursaria: Species Identification, Detection of Choricystis parasitica, and Assessment of the Interaction Specificity. Diversity 2020, 12, 287. https://doi.org/10.3390/d12080287
Flemming FE, Potekhin A, Pröschold T, Schrallhammer M. Algal Diversity in Paramecium bursaria: Species Identification, Detection of Choricystis parasitica, and Assessment of the Interaction Specificity. Diversity. 2020; 12(8):287. https://doi.org/10.3390/d12080287
Chicago/Turabian StyleFlemming, Felicitas E., Alexey Potekhin, Thomas Pröschold, and Martina Schrallhammer. 2020. "Algal Diversity in Paramecium bursaria: Species Identification, Detection of Choricystis parasitica, and Assessment of the Interaction Specificity" Diversity 12, no. 8: 287. https://doi.org/10.3390/d12080287
APA StyleFlemming, F. E., Potekhin, A., Pröschold, T., & Schrallhammer, M. (2020). Algal Diversity in Paramecium bursaria: Species Identification, Detection of Choricystis parasitica, and Assessment of the Interaction Specificity. Diversity, 12(8), 287. https://doi.org/10.3390/d12080287








