Microbial Communities in the Fynbos Region of South Africa: What Happens during Woody Alien Plant Invasions
Abstract
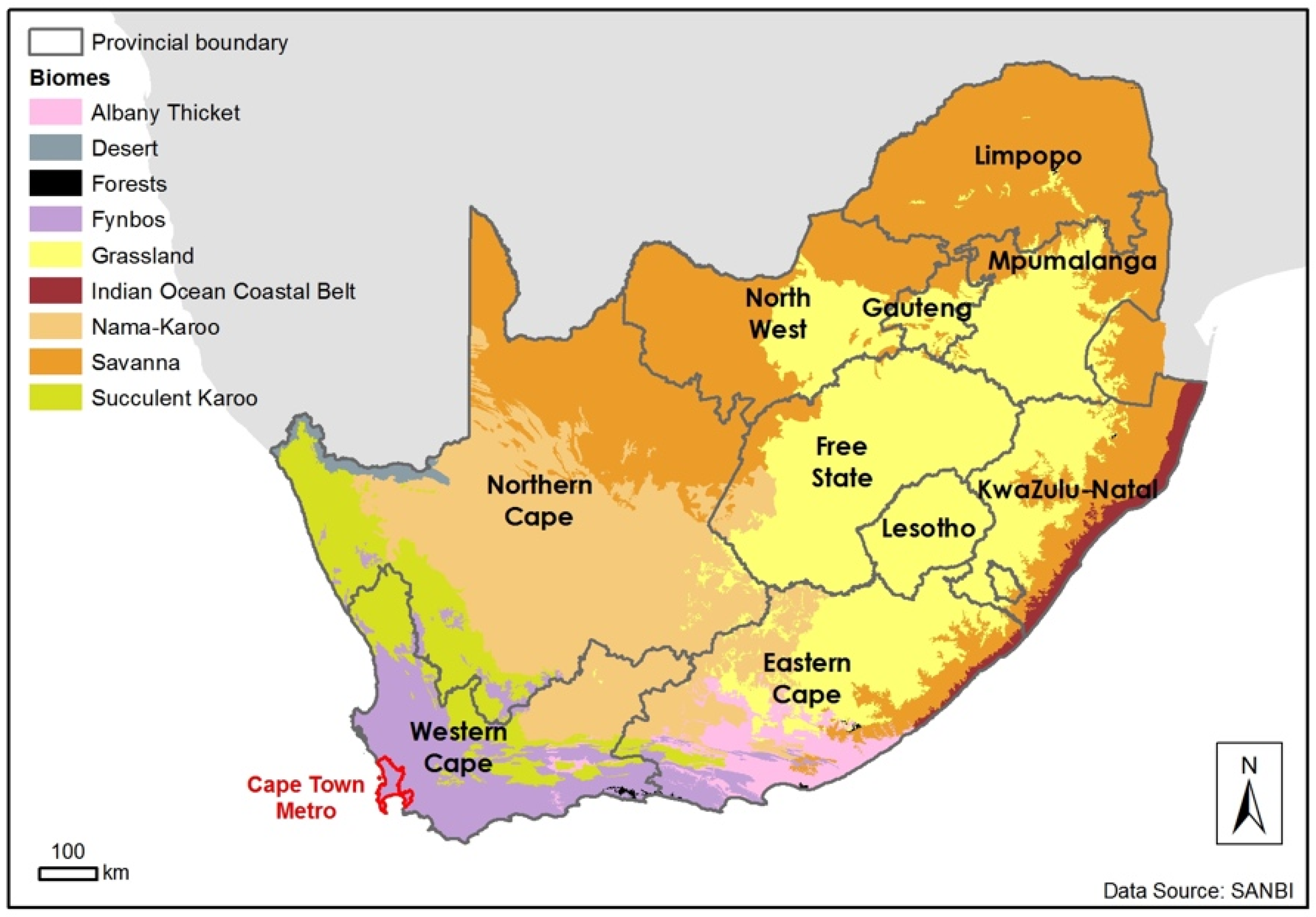
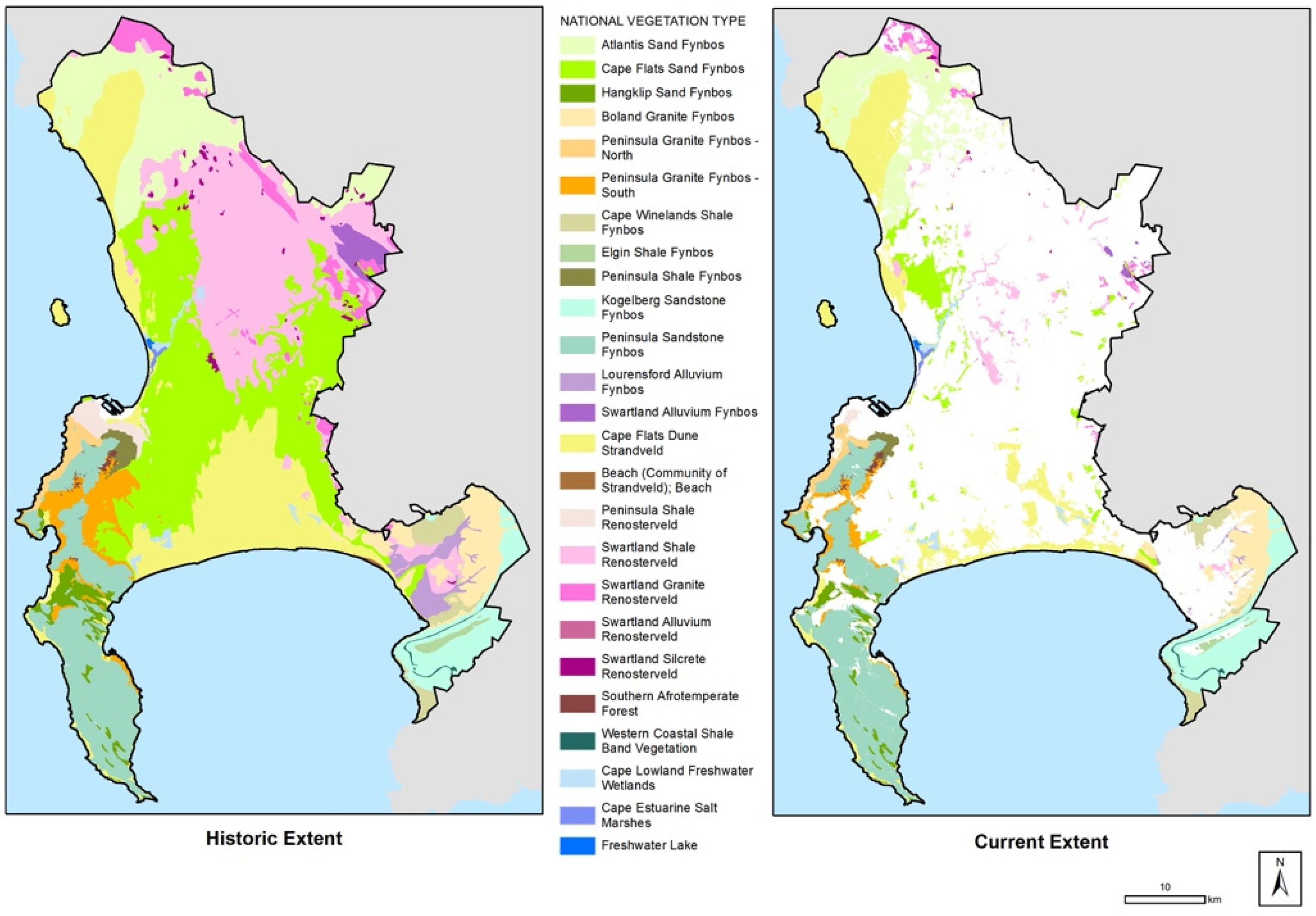
1. Introduction (to the Fynbos Biome)
2. Plant–Soil–Microbial Interactions
3. Microorganisms in Natural Fynbos
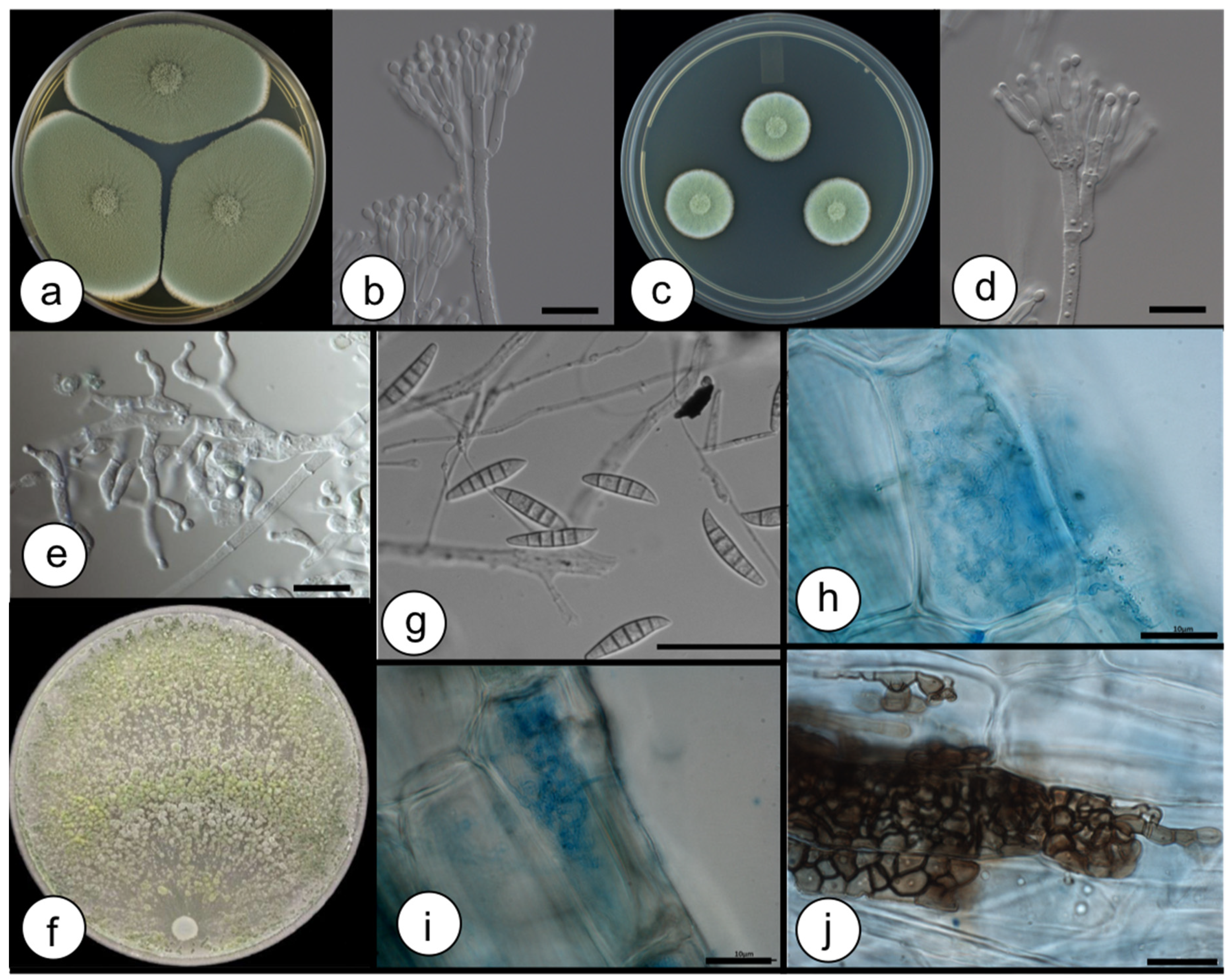
3.1. Fungi
3.2. Bacteria
3.3. Viruses
4. Alien Plant Invasion in the Fynbos Biome
5. Changes in Soil Properties
6. The Role of Microorganisms in Invasion
| Hypothesis | Definition | Example | Reference |
|---|---|---|---|
| Enemy release hypothesis | Absence of an antagonist during colonization results in the successful establishment of invaded plant species. | Sirex noctilio(woodwasp), together with its symbiotic fungus, Amylostereum areolatum infested pine trees in the fynbos region, South Africa decades after establishment of invasive pine as plantations. | [148] |
| The rust fungus Uromycladium tepperianum was only introduced in 1987 into South Africa as a biocontrol measure on Acacia. | [149] | ||
| Enhanced mutualism hypothesis (novel mutualism) | Invasive plant species associate with native soil mutualists in its introduced ranges which leads to successful invasion. | Acacia has been shown to recruit non-specific rhizobia that are native to the fynbos for nodule forming. | [146] |
| Eucalyptus has been shown to recruit native ectomycorrhizae in other areas of Africa. This has, however, not been shown for species invasive in fynbos, but are likely to occur. | [147] | ||
| Degraded mutualism hypothesis | The invasion of an area by non-mycorrhizal plants reduces the abundance of arbuscular mycorrhizal (AM) fungi. | The invasion of an area by nonmycorrhizal plants reduces the abundance of arbuscular mycorrhizal fungi (AMF). However, a change in the nutritional status or the absence of important fynbos species such as the Proteaceae may disproportionately select for the re-establishment of AMF-plants, to the detriment of the ECM (Ectomycorrhizal)-plants. | |
| Accumulation of local pathogen hypothesis | This suggests that invasive alien plant species gather native soil pathogens that restrict native plant spread and growth. | No evidence. | |
| Novel weapon hypothesis | This postulates that invasive plants possess new biochemical weapons that function as strong allelopathic agents for new plant–soil–microbe interactions. | Slash and burn of Eucalypt during removal of invasive alien plants had a lasting legacy effect on the recovery of native fynbos and changed the soil bacterial communities over an extended time period, most likely as a result of allelochemicals released during decomposition, exacerbated by fire. | [139,150] |
7. Effect of Restoration on Microbial Communities
8. Looking Forward
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myers, N. Biodiversity hotspots revisited. Bioscience 2003, 53, 916–917. [Google Scholar]
- Goldblatt, P.; Manning, J. Plant Diversity of the Cape Region of Southern Africa. Ann. Missouri Bot. Gard. 2002, 89, 281–302. [Google Scholar] [CrossRef]
- Manning, J.; Goldblatt, P. Plants of the Greater Cape Floristic Region. 1: The Core Cape flora; Strelitzia 29: South African National Biodiversity Institute: Pretoria, South Africa, 2012; ISBN 978-1-919976-74-7. [Google Scholar]
- Goldblatt, P. Floristic diversity in the Cape Flora of South Africa. Biodivers. Conserv. 1997, 6, 359–377. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Rebelo, A.G.; Boucher, C.; Helme, N.; Mucina, L.; Rutherford, M.C. Fynbos Biome. In The vegetation of South Africa, Lesotho and Swaziland; Mucina, L., Rutherford, M.C., Eds.; Strelitzia 19: South African National Biodiversity Institute: Pretoria, South Africa, 2006; pp. 52–219. ISBN 1978-1-919976-21-1. [Google Scholar]
- Van Wilgen, B.W. The evolution of fire management practices in savanna protected areas in South Africa. S. Afr. J. Sci. 2009, 105, 343–349. [Google Scholar] [CrossRef]
- Moran, V.C.; Hoffmann, J.H. Conservation of the fynbos biome in the Cape Floral Region: The role of biological control in the management of invasive alien trees. BioControl 2012, 57, 139–149. [Google Scholar] [CrossRef]
- City of Cape Town. City of Cape Town Biodiversity Report 2018; City of Cape Town: Cape Town, South Africa, 2018. [Google Scholar]
- Postma, A.; Slabbert, E.; Postma, F.; Jacobs, K. Soil bacterial communities associated with natural and commercial Cyclopia spp. FEMS Microbiol. Ecol. 2016, 92, 1–10. [Google Scholar] [CrossRef]
- Slabbert, E.; Jacobs, S.M.; Jacobs, K. The soil bacterial communities of South African fynbos riparian ecosystems invaded by Australian Acacia species. PLoS ONE 2014, 9, e86560. [Google Scholar] [CrossRef]
- Visagie, C.M.; Roets, F.; Jacobs, K. A new species of Penicillium, P. ramulosum sp. nov., from the natural environment. Mycologia 2009, 101, 888–895. [Google Scholar] [CrossRef]
- Visagie, C.M.; Seifert, K.A.; Houbraken, J.; Samson, R.A.; Jacobs, K. Diversity of Penicillium section Citrina within the fynbos biome of South Africa, including a new species from a Protea repens infructescence. Mycologia 2014, 106, 537–552. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Seifert, K.A.; Samson, R.A.; Jacobs, K. Four new Penicillium species isolated from the fynbos biome in South Africa, including a multigene phylogeny of section Lanata-Divaricata. Mycol. Prog. 2015, 14, 1–23. [Google Scholar] [CrossRef]
- Visagie, C.M.; Seifert, K.A.; Houbraken, J.; Samson, R.A.; Jacobs, K. A phylogenetic revision of Penicillium sect. Exilicaulis, including nine new species from fynbos in South Africa. IMA Fungus 2016, 7, 75–117. [Google Scholar] [CrossRef] [PubMed]
- Keet, J.H.; Ellis, A.G.; Hui, C.; Le Roux, J.J. Strong spatial and temporal turnover of soil bacterial communities in South Africa’s hyperdiverse fynbos biome. Soil Biol. Biochem. 2019, 136, 107541. [Google Scholar] [CrossRef]
- South African National Biodiversity Institute (SANBI). Biodiversity GIS Spatial Dataset. Available online: http://bgis.sanbi.org/SpatialDataset (accessed on 4 May 2020).
- Slabbert, E.; Kongor, R.Y.; Esler, K.J.; Jacobs, K. Microbial diversity and community structure in Fynbos soil. Mol. Ecol. 2010, 19, 1031–1041. [Google Scholar] [CrossRef]
- City of Cape Town Open Data portal. Available online: https://web1.capetown.gov.za/web1/OpenDataPortal/AllDatasets (accessed on 4 May 2020).
- Cowling, R.M.; Procheş, Ş.; Partridge, T.C. Explaining the uniqueness of the Cape flora: Incorporating geomorphic evolution as a factor for explaining its diversification. Mol. Phylogenet. Evol. 2009, 51, 64–74. [Google Scholar] [CrossRef]
- Cowling, R.M.; Potts, A.J.; Bradshaw, P.L.; Colville, J.; Arianoutsou, M.; Ferrier, S.; Forest, F.; Fyllas, N.M.; Hopper, S.D.; Ojeda, F.; et al. Variation in plant diversity in mediterranean-climate ecosystems: The role of climatic and topographical stability. J. Biogeogr. 2015, 42, 552–564. [Google Scholar] [CrossRef]
- Pirie, M.D.; Oliver, E.G.H.; Gehrke, B.; Heringer, L.; De Kuppler, A.M.; Le Maitre, N.C.; Bellstedt, D.U. Underestimated regional species diversity in the Cape Floristic Region revealed by phylogenetic analysis of the Erica abietina/E. viscaria clade (Ericaceae). Bot. J. Linn. Soc. 2017, 184, 185–203. [Google Scholar] [CrossRef]
- Allsopp, N.; Colville, J.F.; Verboom, G.A. Fynbos: Ecology, Evolution and Conservation of a Megadiverse Region; Allsopp, N., Colville, J.F., Verboom, G.A., Cowling, R.M., Eds.; Oxford University Press: London, UK, 2014; ISBN 978-0-199679-58-4. [Google Scholar]
- Maistry, P.M.; Muasya, A.M.; Valentine, A.J.; Chimphango, S.B.M. Increasing nitrogen supply stimulates phosphorus acquisition mechanisms in the fynbos species Aspalathus linearis. Funct. Plant Biol. 2015, 42, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, P.; Mehrabi, Z.; Bezemer, T.M.; De Deyn, G.B.; Kulmatiski, A.; Drigo, B.; Veen, G.F.; van der Heijden, M.G.A.; Kardol, P. Plant–Soil Feedback: Bridging Natural and Agricultural Sciences. Trends Ecol. Evol. 2018, 33, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Curiel Yuste, J.; Fernandez-Gonzalez, A.J.; Fernandez-Lopez, M.; Ogaya, R.; Penuelas, J.; Sardans, J.; Lloret, F. Strong functional stability of soil microbial communities under semiarid Mediterranean conditions and subjected to long-term shifts in baseline precipitation. Soil Biol. Biochem. 2014, 69, 223–233. [Google Scholar] [CrossRef]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Maseko, S.T.; Dakora, F.D. Plant Enzymes, Root Exudates, Cluster Roots and Mycorrhizal Symbiosis are the Drivers of P Nutrition in Native Legumes Growing in P Deficient Soil of the Cape Fynbos in South Africa. J. Agric. Sci. Technol. 2013, 3, 331–340. [Google Scholar]
- Maseko, S.T.; Dakora, F.D. Rhizosphere acid and alkaline phosphatase activity as a marker of P nutrition in nodulated Cyclopia and Aspalathus species in the Cape fynbos of South Africa. S. Afr. J. Bot. 2013, 89, 289–295. [Google Scholar] [CrossRef]
- Brink, C.; Postma, A.; Jacobs, K. Rhizobial diversity and function in rooibos (Aspalathus linearis) and honeybush (Cyclopia spp.) plants: A review. S. Afr. J. Bot. 2017, 110, 80–86. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant-microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Tkacz, A.; Cheema, J.; Chandra, G.; Grant, A.; Poole, P.S. Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME J. 2015, 9, 2349–2359. [Google Scholar] [CrossRef]
- Richards, M.B.; Stock, W.D.; Cowling, R.M. Soil nutrient dynamics and community boundaries in the Fynbos vegetation of South Africa. Plant Ecol. 1997, 130, 143–153. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Brink, C.; Postma, A.; Slabbert, E.; Postma, F.; Muasya, A.; Jacobs, K. Bacterial communities associated with natural and commercially grown rooibos (Aspalathus linearis). Pedosphere. in press.
- Jacobs, S.; Naude, M.; Slabbert, E.; Kambaj, O.; Fourie, M.; Esler, K.; Jacobs, K.; Mantlana, B.; Rozanov, A.; Cowan, D. Identifying Relationships Between Soil Processes and Biodiversity to Improve Restoration of Riparian Ecotones Invaded by Invasive Acacias; WRC Report, No. 1927/1/131; Water Research Commission: Pretoria, South Africa, 2013; p. 151. ISBN 978-1-4312-0445-7. [Google Scholar]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, N.; Stock, W.D. Mycorrhizal status of plants growing in the Cape Floristic Region, South Africa. Bothalia 1993, 23, 91–104. [Google Scholar] [CrossRef]
- Berliner, R.; Mitchell, D.T.; Allsopp, N. The vesicular-arbuscular mycorrhizal infectivity of sandy soils in the south-western Cape, South Africa. S. Afr. J. Bot. 1989, 55, 310–313. [Google Scholar] [CrossRef]
- Allsopp, N.; Stock, W.D. VA Mycorrhizal Infection in Relation to Edaphic Characteristics and Disturbance Regime in Three Lowland Plant Communities in the South-Western Cape, South Africa. J. Ecol. 1994, 82, 271–279. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Cloete, K.J.; Valentine, A.J.; Blomerus, L.M.; Botha, A.; Pèrez-Fernández, M.A. Nutritional effects of indigenous arbuscular mycorrhizal associations on the sclerophyllous species Agathosma betulina. Web Ecol. 2007, 7, 77–86. [Google Scholar] [CrossRef]
- Allsopp, N.; Stock, W.D. Density dependent interactions between VA mycorrhizal fungi and even-aged seedlings of two perennial Fabaceae species. Oecologia 1992, 91, 281–287. [Google Scholar] [CrossRef]
- Slabbert, E. Microbial Communities of Riparian Ecotone Invaded by Non-Indigenous Acacias; Stellenbosch University: Stellenbosch, South Africa, 2012. [Google Scholar]
- Allsopp, N.; Holmes, P.M. The impact of alien plant invasion on mycorrhizas in mountain fynbos vegetation. S. Afr. J. Bot. 2001, 67, 150–156. [Google Scholar] [CrossRef][Green Version]
- Malloch, D.W.; Pirozynski, K.A.; Raven, P.H. Ecological and evolutionary significance of mycorrhizal symbioses in vascular plants (A Review). Proc. Natl. Acad. Sci. USA 1980, 77, 2113–2118. [Google Scholar] [CrossRef]
- Robinson, R.K. Mycorrhiza in certain Ericaceae native to southern Africa. J. S. Afr. Bot. 1973, 39, 123–129. [Google Scholar]
- Pirie, M.D.; Oliver, E.G.H.; Bellstedt, D.U. A densely sampled ITS phylogeny of the Cape flagship genus Erica L. suggests numerous shifts in floral macro-morphology. Mol. Phylogenet. Evol. 2011, 61, 593–601. [Google Scholar] [CrossRef]
- Schwery, O.; Onstein, R.E.; Bouchenak-Khelladi, Y.; Xing, Y.; Carter, R.J.; Linder, H.P. As old as the mountains: The radiations of the Ericaceae. New Phytol. 2015, 207, 355–367. [Google Scholar] [CrossRef]
- Straker, C.J.; Mitchell, D.T. The characterization and estimation of polyphosphates in endomycorrhizas of the Ericaceae. New Phytol. 1985, 99, 431–440. [Google Scholar] [CrossRef]
- Straker, C.J.; Mitchell, D.T. The Activity and Characterization of Acid Phosphatases in Endomycorrhizal Fungi of the Ericaceae. New Phytol. 1986, 104, 243–256. [Google Scholar] [CrossRef]
- Straker, C.J.; Mitchell, D.T. Kinetic Characterization of a Dual Phosphate Uptake System in the Endomycorrhizal Fungus of Erica hispidula L. New Phytol. 1987, 106, 129–137. [Google Scholar] [CrossRef]
- Straker, C.J.; Gianinazzi-Pearson, V.; Gianinazzi, S.; Cleyet-Marel, J.-C.; Bousquet, N. Electrophoretic and immunological studies on acid phosphatase from a mycorrhizal fungus of Erica hispidula L. New Phytol. 1989, 111, 215–221. [Google Scholar] [CrossRef]
- Read, D.J. The structure and function of the ericoid mycorrhizal root. Ann. Bot. 1996, 77, 365–374. [Google Scholar] [CrossRef]
- Cairney, J.W.G.; Ashford, A.E. Biology of mycorrhizal associations of epacrids (Ericaceae). New Phytol. 2002, 154, 305–326. [Google Scholar] [CrossRef]
- Vohník, M.; Albrechtová, J. The Co-occurrence and Morphological Continuum Between Ericoid Mycorrhiza and Dark Septate Endophytes in Roots of Six European Rhododendron Species. Folia Geobot. 2011, 46, 373–386. [Google Scholar] [CrossRef]
- Straker, C.J. Ericoid mycorrhiza: Ecological and host specificity. Mycorrhiza 1996, 6, 215–225. [Google Scholar] [CrossRef]
- Bruzone, M.C.; Fontenla, S.B.; Vohník, M. Is the prominent ericoid mycorrhizal fungus Rhizoscyphus ericae absent in the Southern Hemisphere’s Ericaceae? A case study on the diversity of root mycobionts in Gaultheria spp. from northwest Patagonia, Argentina. Mycorrhiza 2015, 25, 25–40. [Google Scholar] [CrossRef]
- Smith, S.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Pearson, V.; Read, D.J. The Biology of Mycorrhiza in the Ericaceae. II. The Transport of Carbon and Phosphorus by the Endophyte and the Mycorrhiza. New Phytol. 1973, 72, 1325–1331. [Google Scholar] [CrossRef]
- Crous, P.W.; Rong, I.H.; Wood, A.; Lee, S.; Glen, H.; Botha, W.; Slippers, B.; De Beer, W.Z.; Wingfield, M.J.; Hawksworth, D.L. How many species of fungi are there at the tip of Africa? Stud. Mycol. 2006, 55, 13–33. [Google Scholar] [CrossRef]
- Van Wyk, P.S.; Marasas, W.F.O.; Knox-Davies, P.S. Batcheloromyces leucadendri sp. nov. on Leucadendron spp. in South Africa. S. Afr. J. Bot. 1985, 51, 344–346. [Google Scholar] [CrossRef]
- Crous, P.W. New and interesting records of South African fungi. XIII. Foliicolous microfungi. S. Afr. J. Bot. 1993, 59, 602–610. [Google Scholar] [CrossRef]
- Lee, S.; Crous, P.W.; Wingfield, M.J. Pestalotioid fungi from Restionaceae in the Cape Floral Kingdom. Stud. Mycol. 2006, 55, 175–187. [Google Scholar] [CrossRef]
- Swart, L.; Crous, P.W.; Denman, S.; Palm, M.E. Fungi occurring on Proteaceae. I. S. Afr. J. Bot. 1998, 64, 137–145. [Google Scholar] [CrossRef]
- Marais, G.J.; Wingfield, M.J. Fungi associated with infructescences of Protea species in South Africa, including a new species of Ophiostoma. Mycol. Res. 1994, 98, 369–374. [Google Scholar] [CrossRef]
- Marais, G.J.; Wingfield, M.J. Ophiostoma protearum sp. nov. associated with Protea caffra infructescences. Can. J. Bot. 1997, 75, 362–367. [Google Scholar] [CrossRef]
- Lee, S.; Crous, P.W. New species of Anthostomella on fynbos, with a key to the genus in South Africa. Mycol. Res. 2003, 107, 360–370. [Google Scholar] [CrossRef]
- Lee, S.; Crous, P.W. New coelomycetes occurring on Restionaceae. Sydowia 2003, 55, 115–128. [Google Scholar]
- Lee, S.; Groenewald, J.Z.; Taylor, J.E.; Roets, F.; Crous, P.W. Rhynchostomatoid fungi occurring on Proteaceae. Mycologia 2003, 95, 902–910. [Google Scholar] [CrossRef]
- Van Wyk, P.S.; Marasas, W.F.O.; Knox-Davies, P.S. Ascomycetous leaf pathogens of Protea, Leucadendron and Leucospermum in South Africa. Phytophylactica 1975, 7, 91–94. [Google Scholar]
- Knox-Davies, P.S.; van Wyk, P.S.; Marasas, W.F.O. Diseases of Protea, Leucospermum and Leucadendron recorded in South Africa. Phytophylactica 1987, 19, 327–337. [Google Scholar]
- Orffer, S.; Pathology, P. A canker and die-back disease of Protea repens. Phytophylactica 1989, 21, 189–194. [Google Scholar]
- Crous, P.W.; Wingfield, M.J. Additions to Mycosphaerella in the fynbos biome. Mycotaxon 1993, 46, 19–26. [Google Scholar]
- Taylor, J.E.; Denman, S.; Crous, P.W. Endophytes isolated from three species of Protea in a nature reserve in the Western Cape, South Africa. Sydowia 2001, 53, 247–260. [Google Scholar]
- Taylor, J.E.; Crous, P.W. Phaeophleosporafaureae comb. novo associated with leaf spots on Faurea saligna (Proteaceae), with a key to the species of Phaeophleospora. Fungal Divers. 1999, 3, 153–158. [Google Scholar]
- Mostert, L.; Kang, J.; Crous, P.W.; Denman, S. Phomopsis saccharata sp.nov., causing a canker and die-back disease of Protea repens in South Africa. Sydowia 2001, 53, 227–235. [Google Scholar]
- Taylor, J.E.; Lee, S.; Crous, P.W. Biodiversity in the Cape Floral Kingdom: Fungi occurring on Proteaceae. Mycol. Res. 2001, 105, 1480–1484. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Rodriques, C.; Silva Pereira, C.; Dijksterhuis, J.; Seifert, K.A.; Jacobs, K.; Samson, R.A. Five new Penicillium species in section sclerotiora: A tribute to the Dutch Royal family. Persoonia 2013, 31, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Lopez-Quintero, C.A.; Vasco-Palacios, A.M.; Frisvad, J.C.; Theelen, B.; Boekhout, T.; Samson, R.A.; Houbraken, J. Four novel Talaromyces species isolated from leaf litter from Colombian Amazon rain forests. Mycol. Prog. 2016, 15, 1041–1056. [Google Scholar] [CrossRef]
- Roets, F.; Dreyer, L.L.; Crous, P.W. Seasonal trends in colonisation of Protea infructescences by Gondwanamyces and Ophiostoma spp. S. Afr. J. Bot. 2005, 71, 307–311. [Google Scholar] [CrossRef]
- Allsopp, N.; Olivier, D.L.; Mitchell, D.T. Fungal populations associated with root systems of proteaceous seedlings at a lowland fynbos site in South Africa. S. Afr. J. Bot. 1987, 53, 365–369. [Google Scholar] [CrossRef]
- Postma, A. Soil Microbial Communities Associated with two Commercially Important Plant Species Indigenous to the Fynbos Region of South Africa: Cyclopia spp. (honeybush) and Aspalathus linearis (rooibos); Stellenbosch University: Stellenbosch, South Africa, 2016. [Google Scholar]
- Cloete, K.J.; Valentine, A.J.; Stander, M.A.; Blomerus, L.M.; Botha, A. Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a sclerophyllous medicinal shrub, Agathosma betulina (Berg.) Pillans. Microb. Ecol. 2009, 57, 624–632. [Google Scholar] [CrossRef]
- Vreulink, J.M.; Esterhuyse, A.; Jacobs, K.; Botha, A. Soil properties that impact yeast and actinomycete numbers in sandy low nutrient soils. Can. J. Microbiol. 2007, 53, 1369–1374. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Changing precipitation pattern alters soil microbial community response to wet-up under a Mediterranean-type climate. ISME J. 2015, 9, 946–957. [Google Scholar] [CrossRef]
- Lawal, S.; Lennard, C.; Hewitson, B. Response of southern African vegetation to climate change at 1.5 and 2.0° global warming above the pre-industrial level. Clim. Serv. 2019, 16, 100134. [Google Scholar] [CrossRef]
- Lemaire, B.; Van Cauwenberghe, J.; Verstraete, B.; Chimphango, S.; Stirton, C.; Honnay, O.; Smets, E.; Sprent, J.; James, E.K.; Muasya, A.M. Characterization of the papilionoid-Burkholderia interaction in the Fynbos biome: The diversity and distribution of beta-rhizobia nodulating Podalyria calyptrata (Fabaceae, Podalyrieae). Syst. Appl. Microbiol. 2016, 39, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Magadlela, A.; Vardien, W.; Kleinert, A.; Dreyer, L.L.; Valentine, A.J. The role of phosphorus deficiency in nodule microbial composition, and carbon and nitrogen nutrition of a native legume tree in the Cape fynbos ecosystem. Aust. J. Bot. 2015, 63, 379–386. [Google Scholar] [CrossRef]
- Beukes, C.W.; Steenkamp, E.T.; van Zyl, E.; Avontuur, J.; Chan, W.Y.; Hassen, A.I.; Palmer, M.; Mthombeni, L.S.; Phalane, F.L.; Sereme, T.K.; et al. Paraburkholderia strydomiana sp. nov. and Paraburkholderia steynii sp. nov.: Rhizobial symbionts of the fynbos legume Hypocalyptus sophoroides. Antonie Van Leeuwenhoek 2019, 112, 1369–1385. [Google Scholar] [CrossRef]
- Kanu, S.A.; Dakora, F.D. Symbiotic nitrogen contribution and biodiversity of root-nodule bacteria nodulating Psoralea species in the Cape Fynbos, South Africa. Soil Biol. Biochem. 2012, 54, 68–76. [Google Scholar] [CrossRef]
- Lemaire, B.; Chimphango, S.B.M.; Stirton, C.; Rafudeen, S.; Honnay, O.; Smets, E.; Chen, W. Biogeographical Patterns of Legume-Nodulating Burkholderia spp.: From African Fynbos to Continental Scales. Appl. Environ. Microbiol. 2016, 82, 5099–5115. [Google Scholar] [CrossRef]
- Howieson, J.G.; De Meyer, S.E.; Vivas-Marfisi, A.; Ratnayake, S.; Ardley, J.K.; Yates, R.J. Novel Burkholderia bacteria isolated from Lebeckia ambigua—A perennial suffrutescent legume of the fynbos. Soil Biol. Biochem. 2013, 60, 55–64. [Google Scholar] [CrossRef]
- Felix, D. Dakora Root-nodule bacteria isolated from native Amphithalea ericifolia and four indigenous Aspalathus species from the acidic soils of the South African fynbos are tolerant to very low pH. Afr. J. Biotechnol. 2012, 11, 3766–3772. [Google Scholar]
- Human, Z.R.; Crous, C.J.; Roets, F.; Venter, S.N.; Wingfield, M.J.; de Beer, Z.W. Biodiversity and ecology of flower-associated actinomycetes in different flowering stages of Protea repens. Antonie Van Leeuwenhoek 2018, 111, 209–226. [Google Scholar] [CrossRef]
- Barns, S.M.; Takala, S.L.; Kuske, C.R. Wide Distribution and Diversity of Members of the Bacterial Kingdom Acidobacterium in the Environment. Appl. Environ. Microbiol. 1999, 65, 1731–1737. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S rRNA and 16S rRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Damsté, J.S.S. Acidobacteria. In eLS; John Wiley & Sons Ltd: Chichester, UK, 2018. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Trojan, D.; Roux, S.; Herbold, C.; Rattei, T.; Woebken, D. Genomic insights into the Acidobacteria reveal strategies for their success in terrestrial environments. Environ. Microbiol. 2018, 20, 1041–1063. [Google Scholar] [CrossRef]
- Miyambo, T.; Makhalanyane, T.P.; Cowan, D.A.; Valverde, A. Plants of the fynbos biome harbour host species-specific bacterial communities. FEMS Microbiol. Lett. 2016, 363, 1–8. [Google Scholar] [CrossRef]
- Moroenyane, I.; Chimphango, S.B.M.; Dong, K.; Tripathi, B.; Singh, D.; Adams, J.M. Neutral models predict biogeographical patterns of soil microbes at a local scale in Mediterranean heathlands, South Africa. Trans. R. Soc. South Africa 2019, 74, 139–150. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Yilmaz, P. Refining the taxonomic structure of the phylum Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 3796–3806. [Google Scholar] [CrossRef]
- Stafford, W.H.L.; Baker, G.C.; Brown, S.A.; Burton, S.G.; Cowan, D.A. Bacterial diversity in the rhizosphere of Proteaceae species. Environ. Microbiol. 2005, 7, 1755–1768. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Diamond, S.; Butterfield, C.N.; Thomas, B.C.; Banfield, J.F. Novel soil bacteria possess diverse genes for secondary metabolite biosynthesis. Nature 2018, 558, 440–444. [Google Scholar] [CrossRef]
- Diamond, S.; Andeer, P.F.; Li, Z.; Crits-Christoph, A.; Burstein, D.; Anantharaman, K.; Lane, K.R.; Thomas, B.C.; Pan, C.; Northen, T.R.; et al. Mediterranean grassland soil C–N compound turnover is dependent on rainfall and depth, and is mediated by genomically divergent microorganisms. Nat. Microbiol. 2019, 4, 1356–1367. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Segobola, J.; Adriaenssens, E.; Tsekoa, T.; Rashamuse, K.; Cowan, D. Exploring Viral Diversity in a Unique South African Soil Habitat. Sci. Rep. 2018, 8, 111. [Google Scholar] [CrossRef]
- Emerson, J.B. Soil Viruses: A New Hope. mSystems 2019, 4, 1–4. [Google Scholar] [CrossRef]
- Trubl, G.; Jang, H.B.; Roux, S.; Emerson, J.B.; Solonenko, N.; Vik, D.R.; Solden, L.; Ellenbogen, J.; Runyon, A.T.; Bolduc, B.; et al. Soil Viruses Are Underexplored Players in Ecosystem Carbon Processing. mSystems 2018, 3, 1–21. [Google Scholar] [CrossRef]
- Marais, C.; Wannenburgh, A.M. Restoration of water resources (natural capital) through the clearing of invasive alien plants from riparian areas in South Africa—Costs and water benefits. S. Afr. J. Bot. 2008, 74, 526–537. [Google Scholar] [CrossRef]
- Prins, N.; Holmes, P.M.; Richardson, D.M. A reference framework for the restoration of riparian vegetation in the Western Cape, South Africa, degraded by invasive Australian Acacias. S. Afr. J. Bot. 2004, 70, 767–776. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Wilson, J.R.; Wannenburgh, A.; Foxcroft, L.C. The Extent and Effectiveness of Alien Plant Control Projects in South Africa. In Biological Invasions in South Africa; Springer: Cham, Switzerland, 2020; pp. 597–628. ISBN 978-3-030-32394-3. [Google Scholar]
- Van Wilgen, B.W.; Measey, J.; Richardson, D.M.; Wilson, J.R.; Zengeya, T.A. Biological Invasions in South Africa; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-32393-6. [Google Scholar]
- Morris, T.L.; Esler, K.J.; Barger, N.N.; Jacobs, S.M.; Cramer, M.D. Ecophysiological traits associated with the competitive ability of invasive Australian Acacias. Divers. Distrib. 2011, 17, 898–910. [Google Scholar] [CrossRef]
- Tererai, F.; Gaertner, M.; Jacobs, S.M.; Richardson, D.M. Eucalyptus invasions in riparian forests: Effects on native vegetation community diversity, stand structure and composition. For. Ecol. Manag. 2013, 297, 84–93. [Google Scholar] [CrossRef]
- Crous, C.J.; Jacobs, S.M.; Esler, K.J. Drought-tolerance of an invasive alien tree, Acacia mearnsii and two native competitors in fynbos riparian ecotones. Biol. Invasions 2012, 14, 619–631. [Google Scholar] [CrossRef]
- Gaertner, M.; Breeyen, A.D.; Hui, C.; Richardson, D.M. Impacts of alien plant invasions on species richness in mediterranean-type ecosystems: A meta-analysis. Prog. Phys. Geogr. 2009, 33, 319–338. [Google Scholar] [CrossRef]
- Henderson, L. Invasive, naturalized and casual alien plants in southern Africa: A summary based on the Southern African Plant Invaders Atlas (SAPIA). Bothalia 2007, 37, 215–248. [Google Scholar] [CrossRef]
- Constantinides, M.; Fownes, J.H. Nitrogen Mineralization from Leaves and Litter of Tropical Plants: Relationship to Nitrogen, Lignin and Soluble Polyphenol Concentrations. Soil Biol. Biochem. 1994, 26, 49–55. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.S.; Song, M.W. Biomass and carbon storage of Eucalyptus and Acacia plantations in the Pearl River Delta, South China. For. Ecol. Manag. 2012, 277, 90–97. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J.; et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Lazzaro, L.; Giuliani, C.; Fabiani, A.; Agnelli, A.E.; Pastorelli, R.; Lagomarsino, A.; Benesperi, R.; Calamassi, R.; Foggi, B. Soil and plant changing after invasion: The case of Acacia dealbata in a Mediterranean ecosystem. Sci. Total Environ. 2014, 497–498, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Yelenik, S.G.; Stock, W.D.; Richardson, D.M. Functional group identity does not predict invader impacts: Differential effects of nitrogen-fixing exotic plants on ecosystem function. Biol. Invasions 2007, 9, 117–125. [Google Scholar] [CrossRef]
- Tye, D.R.C.; Drake, D.C. An exotic Australian Acacia fixes more N than a coexisting indigenous Acacia in a South African riparian zone. Plant Ecol. 2012, 213, 251–257. [Google Scholar] [CrossRef]
- Simaika, J.P.; Jacobs, S.; Railoun, Z.; Wiener, K. Towards the Development of a Tool to Quantify and Monitor Stream Restoration Success Following Removal of Riparian Invasive Alien Plants; Water Research Commission: Pretoria, South Africa, 2018; ISBN 978-0-6392-0038-5. [Google Scholar]
- Werner, C.; Peperkorn, R.; Máguas, C.; Beyschlag, W. Competitive balance between the alien invasive Acacia longifolia and native Mediterranean species. In Plant Invasions: Human Perception, Ecological Impacts and Management; Tokarska-Guzik, B., Brock, J., Brundu, G., Child, L., Daehler, C., Pysek, P., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 261–275. ISBN 978-3-8236-1528-6. [Google Scholar]
- Yelenik, S.G.; Richardson, W.D.; Stock, D.M. Ecosystem Level Impacts of Invasive Acacia saligna in the South African Fynbos. Restor. Ecol. 2004, 12, 44–51. [Google Scholar] [CrossRef]
- Badalamenti, E.; Gristina, L.; Laudicina, V.A.; Novara, A.; Pasta, S.; La Mantia, T. The impact of Carpobrotus cfr. acinaciformis (L.) L. Bolus on soil nutrients, microbial communities structure and native plant communities in Mediterranean ecosystems. Plant Soil 2016, 409, 19–34. [Google Scholar] [CrossRef]
- Tererai, F.; Gaertner, M.; Jacobs, S.M.; Richardson, D.M. Resilience of Invaded Riparian Landscapes: The Potential Role of Soil-Stored Seed Banks. Environ. Manag. 2015, 55, 86–99. [Google Scholar] [CrossRef]
- Kerr, T.F.; Ruwanza, S. Does Eucalyptus grandis invasion and removal affect soils and vegetation in the Eastern Cape Province, South Africa? Austral. Ecol. 2016, 41, 328–338. [Google Scholar] [CrossRef]
- Nsikani, M.M.; Novoa, A.; van Wilgen, B.W.; Keet, J.H.; Gaertner, M. Acacia saligna’s soil legacy effects persist up to 10 years after clearing: Implications for ecological restoration. Austral Ecol. 2017, 42, 880–889. [Google Scholar] [CrossRef]
- Witkowski, W.T.F.; Mitchell, D.T. Variations in Soil Phosphorus in the Fynbos Biome, South Africa. J. Ecol. 1987, 75, 1159–1171. [Google Scholar] [CrossRef]
- Witkowski, W.T.F. Effects of Invasive Alien Acacias on Nutrient Cycling in the Coastal Lowlands of the Cape Fynbos. J. Appl. Ecol. 1991, 28, 1–15. [Google Scholar] [CrossRef]
- Ruwanza, S.; Gaertner, M.; Richardson, D.M.; Esler, K.J. Soil water repellency in riparian systems invaded by Eucalyptus camaldulensis: A restoration perspective from the Western Cape Province, South Africa. Geoderma 2013, 200–201, 9–17. [Google Scholar] [CrossRef]
- Jacobs, S.; Cogill, L.; Jacobs, K.; Juba, R.; Maubane, T.; Slabbert, E.; Smart, R. Determining the Biomass and Nutrient Content of Invasive Acacia mearnsii and Eucalyptus camaldulensis Trees in Fynbos Riparian Zones; Water Research Commission: Pretoria, South Africa, 2017; ISBN 978-1-4312-0890-6. [Google Scholar]
- Ruwanza, S.; Gaertner, M.; Esler, K.J.; Richardson, D.M. Allelopathic effects of invasive Eucalyptus camaldulensis on germination and early growth of four native species in the Western Cape, South Africa. South. For. 2015, 77, 91–105. [Google Scholar] [CrossRef]
- Richardson, D.M.; Allsopp, N.; D’Antonio, C.M.; Milton, S.J.; Rejmánek, M. Plant invasions—The role of mutualisms. Biol. Rev. 2000, 75, 65–93. [Google Scholar] [CrossRef]
- Ravichandran, K.R.; Thangavelu, M. Role and influence of soil microbial communities on plant invasion. Ecol. Quest. 2017, 27, 9–23. [Google Scholar] [CrossRef][Green Version]
- Le Roux, J.J.; Clusella-Trullas, S.; Mokotjomela, T.M.; Mairal, M.; Richardson, D.M.; Skein, L.; Wilson, J.R.; Weyl, O.L.F.; Geerts, S. Biotic Interactions as Mediators of Biological Invasions: Insights from South Africa. In Biological Invasions in South Africa; Springer: Cham, Switzerland, 2020; ISBN 9783030323943. [Google Scholar]
- Jacobsen, A.L.; Roets, F.; Jacobs, S.M.; Esler, K.J.; Pratt, R. Dieback and mortality of South African fynbos shrubs is likely driven by a novel pathogen and pathogen-induced hydraulic failure. Austral Ecol. 2012, 37, 227–235. [Google Scholar] [CrossRef]
- Stępkowski, T.; Moulin, L.; Krzyżańska, A.; McInnes, A.; Law, I.J.; Howieson, J. European Origin of Bradyrhizobium Populations Infecting Lupins and Serradella in Soils of Western Australia and South Africa. Appl. Environ. Microbiol. 2005, 71, 501–519. [Google Scholar] [CrossRef]
- Warrington, S.; Ellis, A.; Novoa, A.; Wandrag, E.M.; Hulme, P.E.; Duncan, R.P.; Valentine, A.; Le Roux, J.J. Cointroductions of Australian acacias and their rhizobial mutualists in the Southern Hemisphere. J. Biogeogr. 2019, 46, 1519–1531. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Le Roux, J.J.; Crisóstomo, J.A.; Ndlovu, J. Jack-of-all-trades and master of many? How does associated rhizobial diversity influence the colonization success of Australian Acacia species? Divers. Distrib. 2011, 17, 946–957. [Google Scholar] [CrossRef]
- Jairus, T.; Mpumba, R.; Chinoya, S.; Tedersoo, L. Invasion potential and host shifts of Australian and African ectomycorrhizal fungi in mixed eucalypt plantations. New Phytol. 2011, 192, 179–187. [Google Scholar] [CrossRef]
- Hurley, B.P.; Slippers, B.; Wingfield, M.J. A comparison of control results for the alien invasive woodwasp, Sirex noctilio, in the southern hemisphere. Agric. For. Entomol. 2007, 9, 159–171. [Google Scholar] [CrossRef]
- Wood, A.R.; Morris, M.J. Impact of the gall-forming rust fungus Uromycladium tepperianum on the invasive tree Acacia saligna in South Africa: 15 years of monitoring. Biol. Control 2007, 41, 68–77. [Google Scholar] [CrossRef]
- Smart, R. The Effect of Fire Scars on Microbial Diversity of Fynbos Soil; Stellenbosch University: Stellenbosch, South Africa, 2017. [Google Scholar]
- Nsikani, M.M.; van Wilgen, B.W.; Bacher, S.; Gaertner, M. Re-establishment of Protea repens after clearing invasive Acacia saligna: Consequences of soil legacy effects and a native nitrophilic weedy species. S. Afr. J. Bot. 2018, 116, 103–109. [Google Scholar] [CrossRef]
- Nsikani, M.M.; Gaertner, M.; Kritzinger-Klopper, S.; Ngubane, N.P.; Esler, K.J. Secondary invasion after clearing invasive Acacia saligna in the South African fynbos. S. Afr. J. Bot. 2019, 125, 280–289. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, K.; Conradie, T.; Jacobs, S. Microbial Communities in the Fynbos Region of South Africa: What Happens during Woody Alien Plant Invasions. Diversity 2020, 12, 254. https://doi.org/10.3390/d12060254
Jacobs K, Conradie T, Jacobs S. Microbial Communities in the Fynbos Region of South Africa: What Happens during Woody Alien Plant Invasions. Diversity. 2020; 12(6):254. https://doi.org/10.3390/d12060254
Chicago/Turabian StyleJacobs, Karin, Tersia Conradie, and Shayne Jacobs. 2020. "Microbial Communities in the Fynbos Region of South Africa: What Happens during Woody Alien Plant Invasions" Diversity 12, no. 6: 254. https://doi.org/10.3390/d12060254
APA StyleJacobs, K., Conradie, T., & Jacobs, S. (2020). Microbial Communities in the Fynbos Region of South Africa: What Happens during Woody Alien Plant Invasions. Diversity, 12(6), 254. https://doi.org/10.3390/d12060254





