Does Arbuscular Mycorrhiza Determine Soil Microbial Functionality in Nutrient-Limited Mediterranean Arid Ecosystems?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Roots and Soil Sampling
2.3. AMF Colonization Status
2.4. Quantification of AMF Spore Density
2.5. Soil Analysis: Physical and Chemical Properties
2.6. Microbiological and Biochemical Properties
2.7. Statistical Analyses
3. Results
3.1. Physical and Chemical Properties of Soils
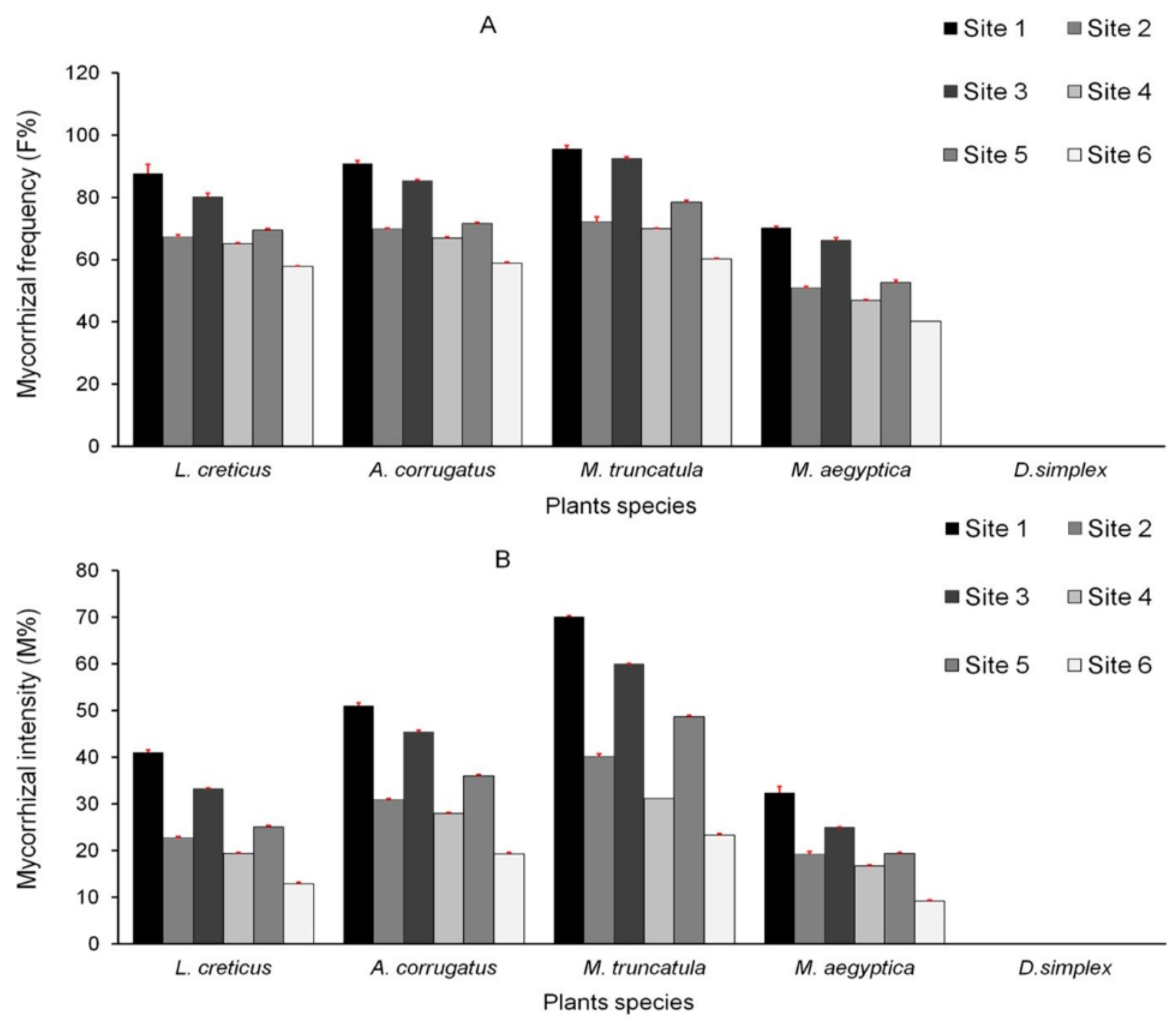
3.2. AMF Colonization of Plant Roots
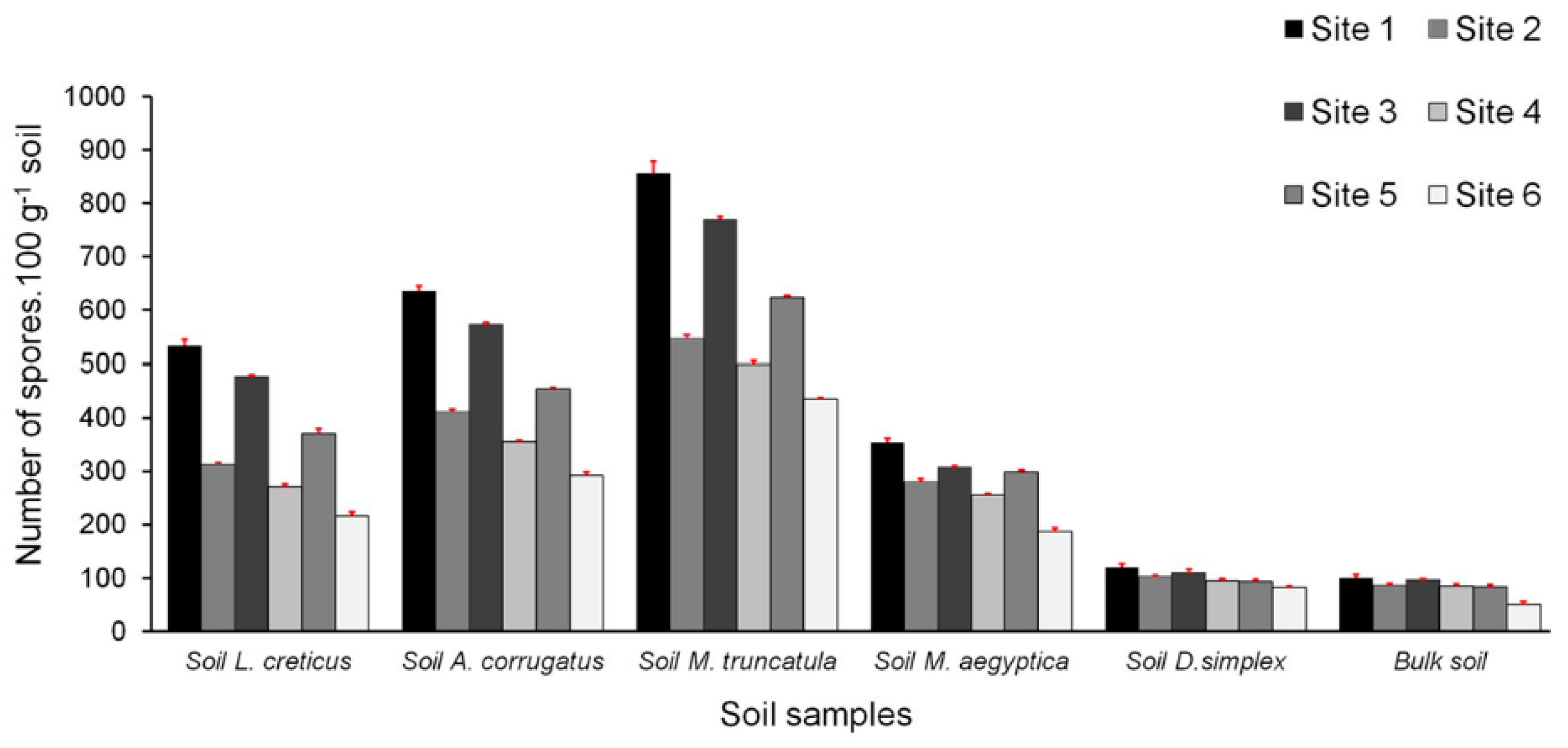
3.3. Densities of Spore Populations in the Studied Soils
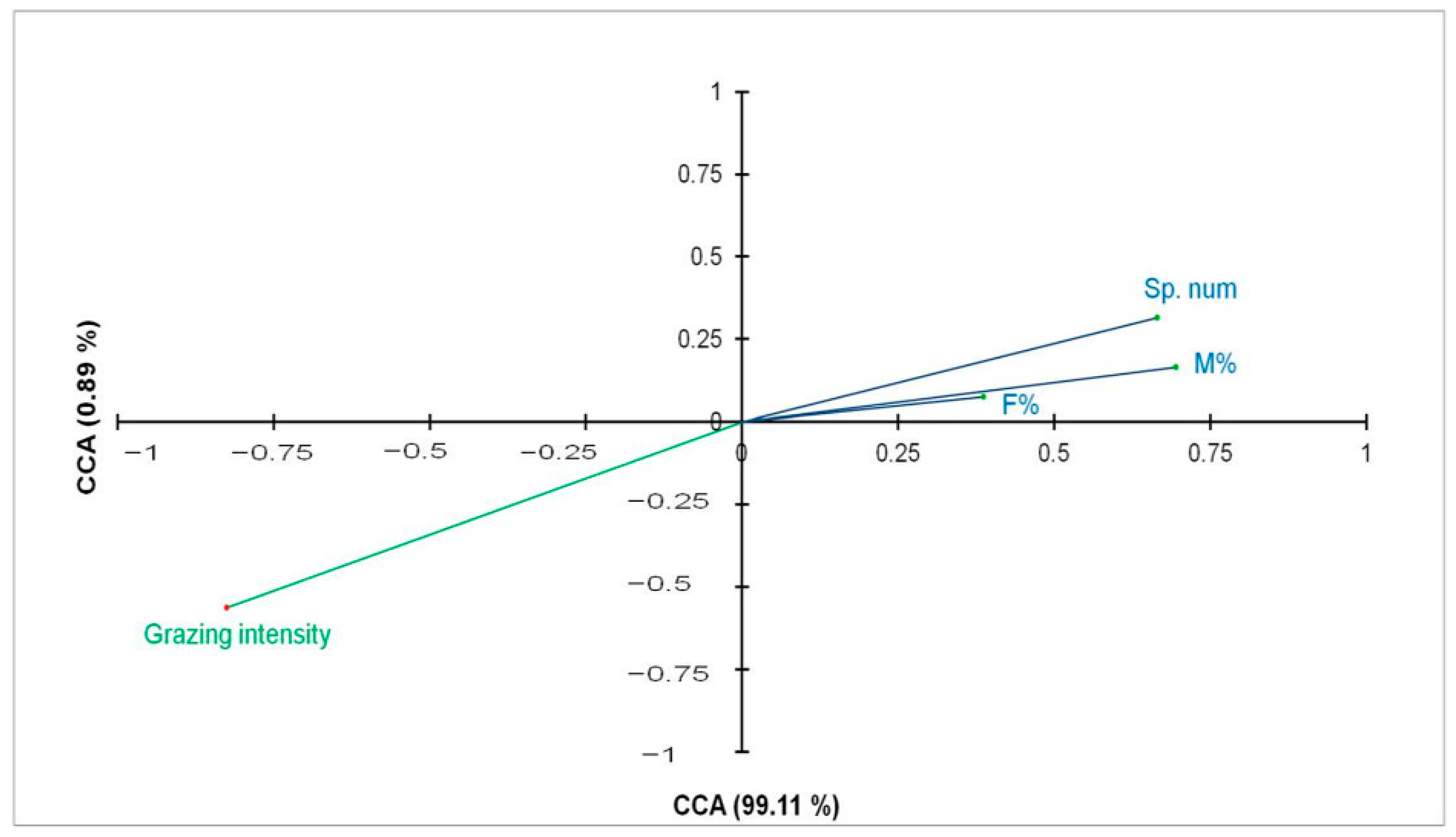
3.4. Effect of the Grazing Intensity on the Different AMF Parameters
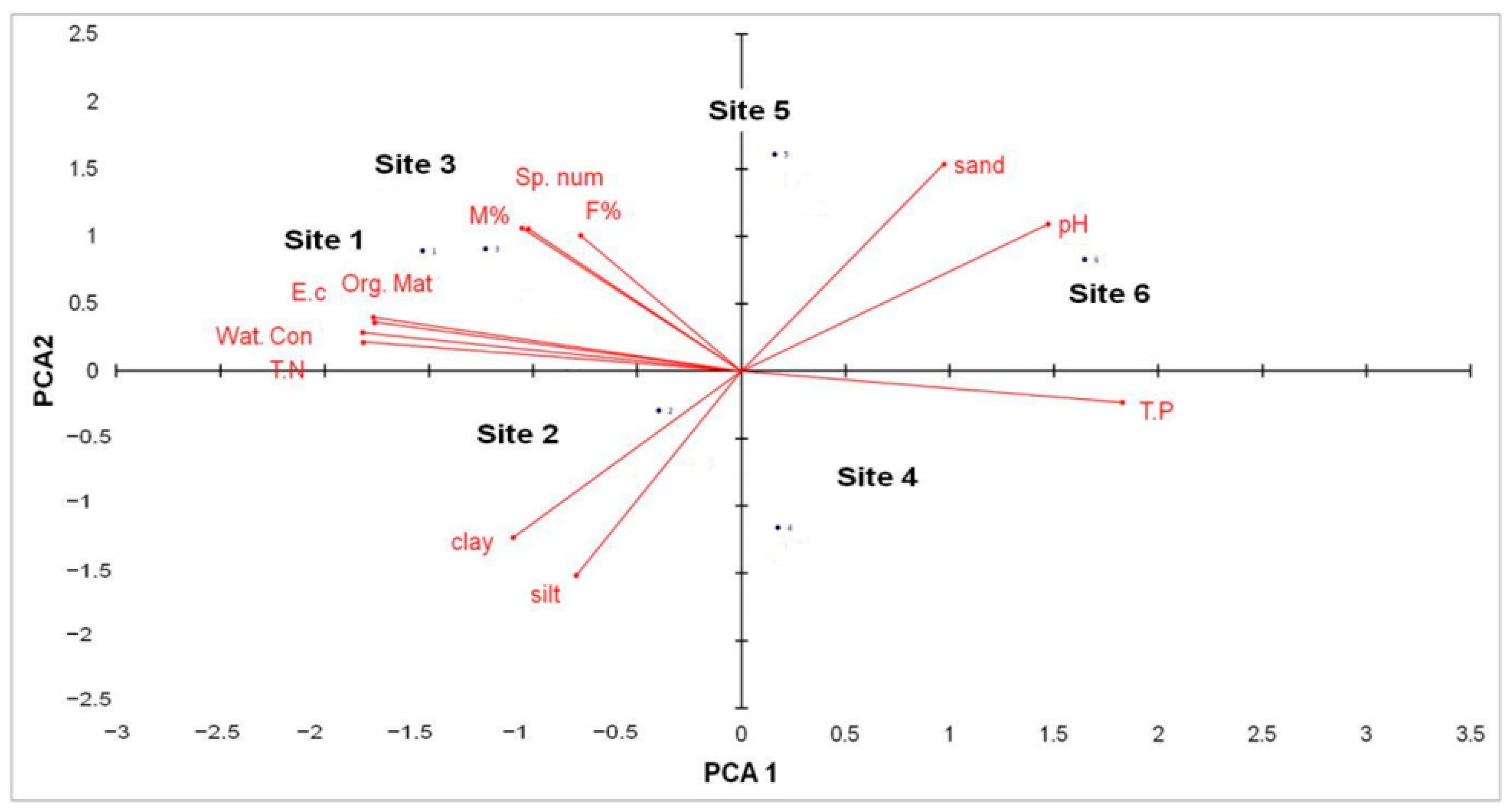
3.5. Effect of Soil Properties on the Different AMF Parameters
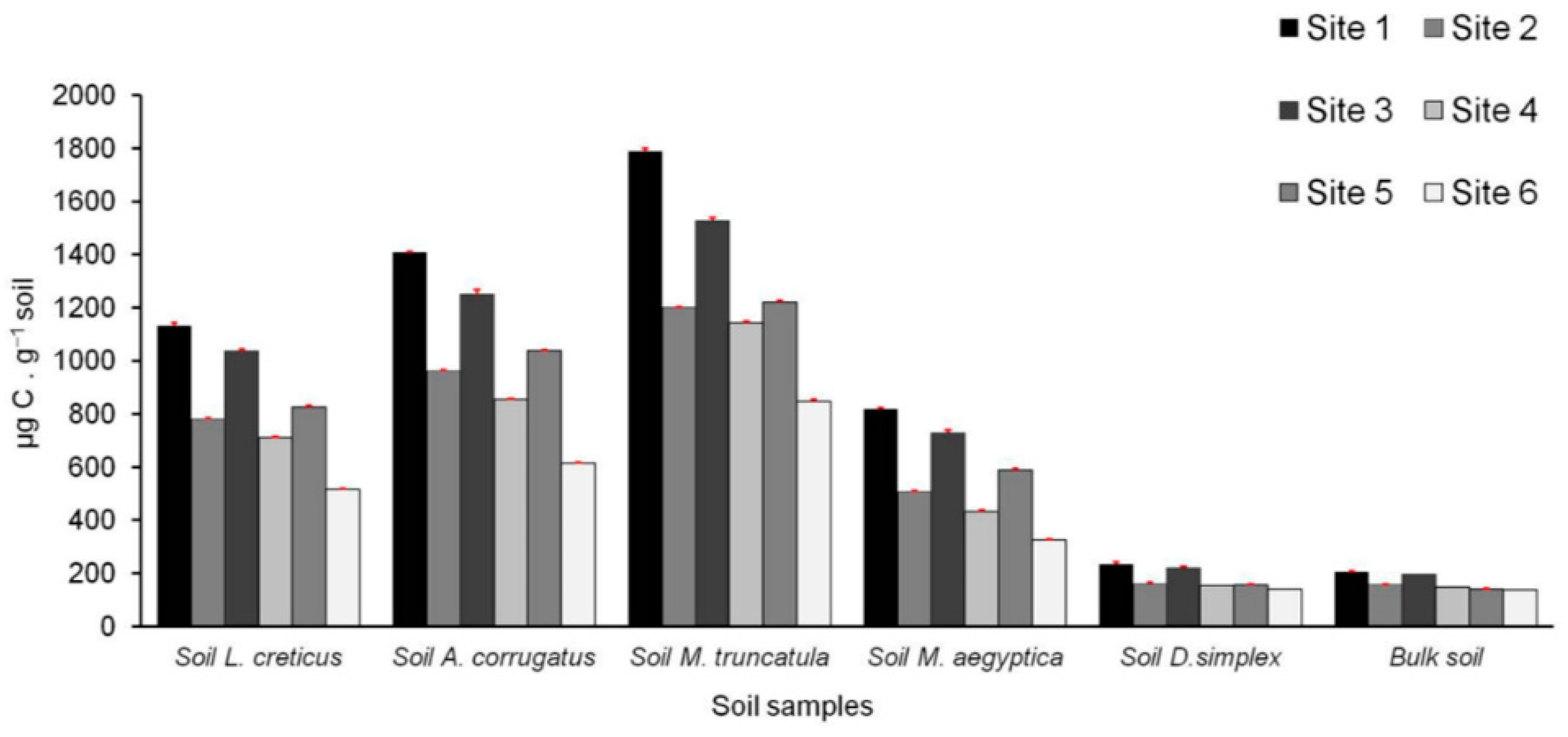
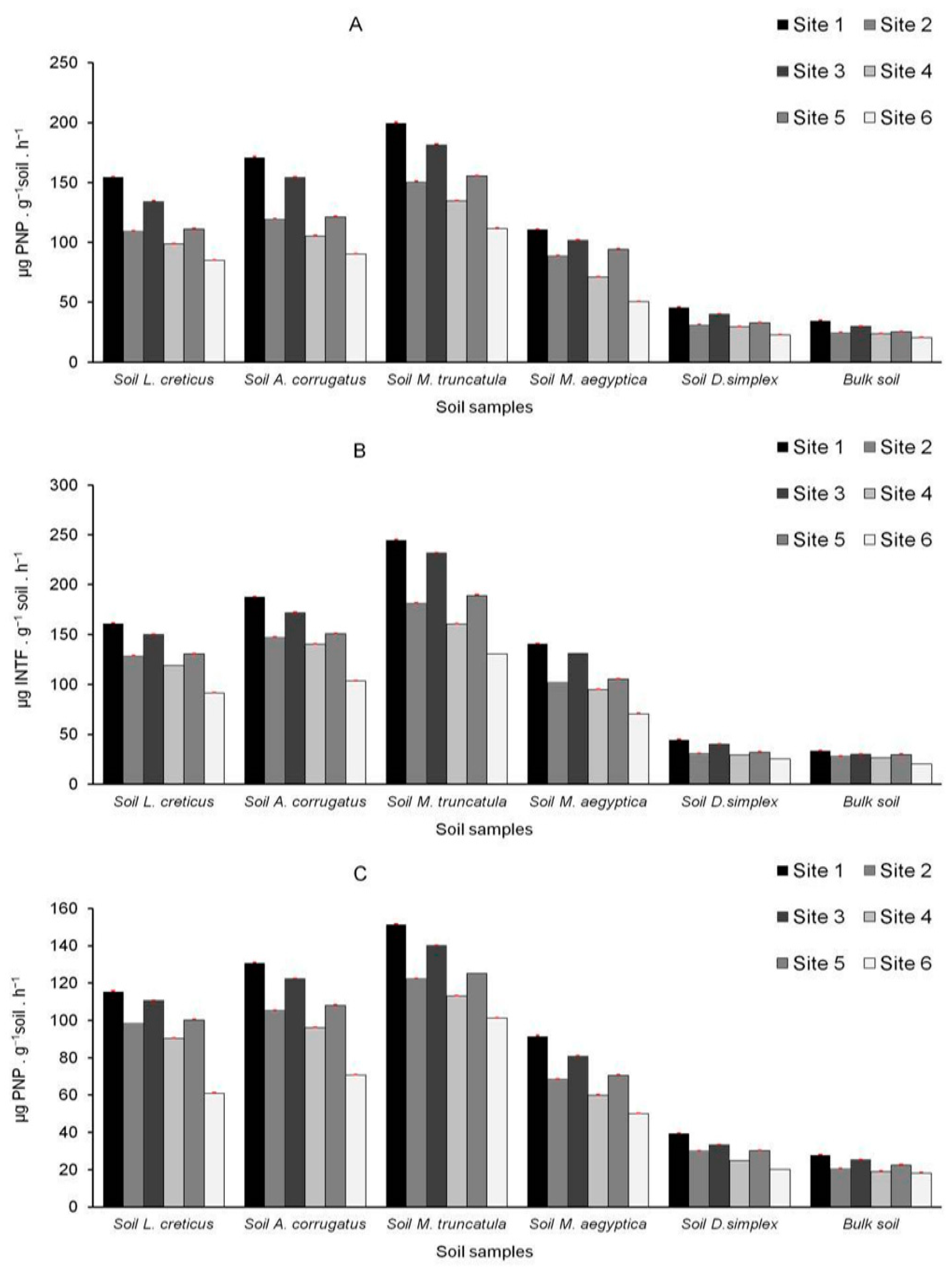
3.6. Microbiological and Biochemical Properties of the Soils and Impact of AMF on Soil Microbial Communities
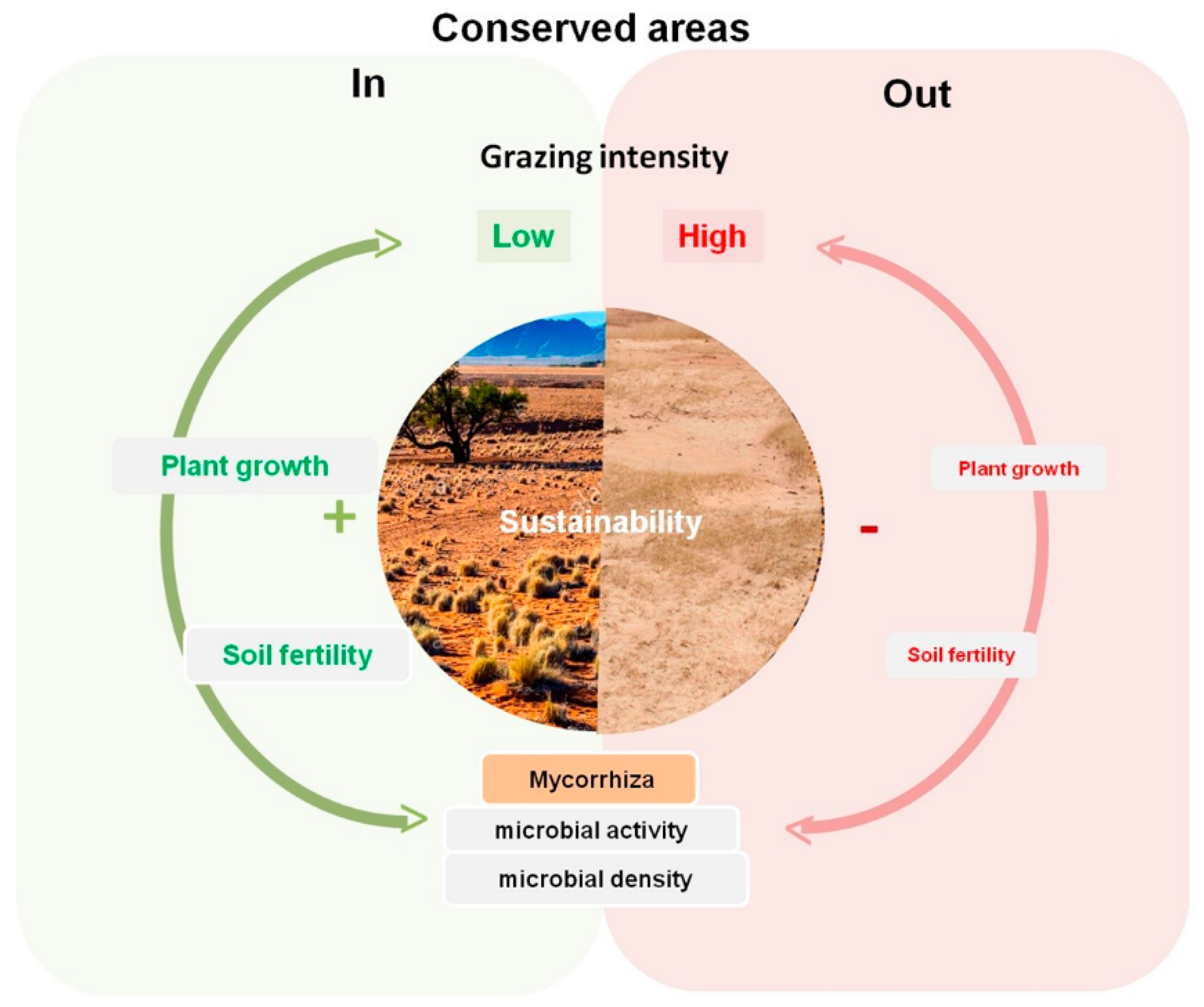
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez-García, L.; Miranda, J.; Pugnaire, F.I. Impacts of changing rainfall patterns on mycorrhizal status of a shrub from arid environments. Eur. J. Soil Biol. 2011, 50, 64–67. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Cruz, C.; Mahdhi, M.; Mars, M.; Caeiro, M.F. Arbuscular mycorrhizal fungi in soil, roots and rhizosphere of Medicago truncatula: Diversity and heterogeneity under semi-arid conditions. PeerJ 2019. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, M.; Philippe, D.L.; Mohamed, M. Molecular identification of arbuscular mycorrhizal fungal spores associated to the rhizosphere of Retama raetam in Tunisia. Soil Sci. Plant. Nutr. 2018, 64, 335–341. [Google Scholar] [CrossRef]
- Brundrett, M. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, J.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytologist. 2015, 204, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.M.; Azcón, R.; Azcón-Aguilar, C. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie van Leeuwenhoek. Inter. J. Gen. Mol. Microb. 2002, 81, 343–351. [Google Scholar]
- Entry, J.A.; Rygiewicz, P.T.; Watrud, L.S.; Donnelly, P.K. Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Adv. Environ. Res. 2002, 7, 123–138. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Van Aarle, I.M.; Cavagnaro, T.R.; Smith, S.E.; Smith, F.A.; Dickson, S. Metabolic activity of Glomus intraradices in Arumand Paris-type arbuscular mycorrhizal colonization. New Phytologist. 2005, 166, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Bago, B.; Vierheilig, H.; Piché, Y.; Ázcon-Aguilar, C. Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytol. 1996, 133, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Azcón-Aguilar, C.; Palenzuela, E.J.; Roldan, A.; Bautista, S.; Vallejo, R.; Barea, J.M. Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification-threatened Mediterranean shrub lands. Appl. Soil Ecol. 2003, 22, 29–37. [Google Scholar] [CrossRef]
- Barto, E.K.; Rilling, M.C. Does herbivory really suppress mycorrhiza? A meta-analysis. J. Ecol. 2010, 98, 745–753. [Google Scholar] [CrossRef]
- Mendoza, R.; Cabello, M.; Anchorena, J.; García, M.L. Soil parameters and host plants associated with arbuscular mycorrhizae in the grazed Magellanic steppe of Tierra del Fuego. Agric. Ecosyst. Environ. 2011, 140, 411–418. [Google Scholar] [CrossRef]
- Raiesi, F.; Asadi, E. Soil microbial activity and litter turnover in native grazed and ungrazed rangelands in a semiarid ecosystem. Biol. Fertil. Soil 2006, 43, 76–82. [Google Scholar] [CrossRef]
- Liao, J.D.; Boutton, T.W. Soil microbial biomass response to woody plant invasion of grassland. Soil Biol. Biochem. 2008, 40, 1207–1216. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedure for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- AFNOR. Recueil de normes françaises, qualité des sols, méthodes d’analyses, 1st ed.; Association française de normalisation (Afnor): Paris, France, 1987. [Google Scholar]
- Naanaa, W.; Susini, J. Méthodes d’analyse physique et chimique des sols. ES 252, Direction des Sols; Ministère de l’Agriculture: Tunis, Tunisie, 1988. [Google Scholar]
- Amato, M.; Ladd, J.N. Assay for microbial biomass based on ninhydrin-reactive nitrogen in extracts of fumigated soils. Soil Biol. Biochem. 1988, 20, 107–114. [Google Scholar] [CrossRef]
- Caravaca, F.; Alguacil, M.; Torres, P.; Roldán, A. Plant type mediates rhizospheric microbial activities and soil aggregation in a semiarid Mediterranean salt marsh. Geoderma 2005, 12, 338–375. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plan. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Laidlaw, A.S.; Christie, P.; Hammond, M.E. The specificity of arbuscular mycorrhizal fungi in perennial ryegrass, white clover pasture. Agric. Ecosyst. Environ. 2000, 77, 211–278. [Google Scholar] [CrossRef]
- Duponnois, R.; Founoune, H.; Masse, D.; Pontanier, R. Inoculation of Acacia holosericea with ectomycorrhizal fungi in a semi-arid site in Senegal: Growth response and influences on the mycorrhizal soil infectivity after 2 years plantation. For. Ecol. Manag. 2005, 207, 351–362. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T.; Rohr, J.R.; Aldrich-Wolfe, L.; Morton, J.B. Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J. Ecol. 2007, 95, 95–105. [Google Scholar] [CrossRef]
- Muchovej, R.M. Importance of Mycorrhizae for Agricultural Crops, SS-AGR-170; Agronomy Department, Florida Cooperative Extension Service; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2004. [Google Scholar]
- Strullu, D.G. Les mycorhizes des arbres et des plantes cultivées; Techniques et Documentation Lavoisier: Paris, France, 1991. [Google Scholar]
- Bagayogo, M. Site Specific Effects of Cereals/Legume Rotations in West Africa: Soil Mineral Nitrogen, Mycorrhizae and Nematodes; Verlag Graner: Stuttgard, Germany, 1999. [Google Scholar]
- Henriques, R.P.; Hay, J.D. The plant communities of a foredune in southeastern Brazil. Can. J. Bot. 1998, 76, 1323–1330. [Google Scholar]
- Mahesh, V.; Selvaraj, T. Occurrence and distribution VA mycorrhizal fungi in the soils polluted with Tannery Effluent. Adv. Biotech. 2008, 8, 34–36. [Google Scholar]
- Dickson, S.; Smith, S.E.; Smith, F.A. Characterization of two arbuscular mycorrhiza fungi in symbiosis with Allium porum: Colonization, plant growth and phosphate uptake. New Phytol. 1999, 144, 163–172. [Google Scholar] [CrossRef]
- Bouamri, B.; Dalpé, Y.; Serrhini, M.N.; Bennani, A. Arbuscular Mycorrhizal fungi species associated with rhizosphere of Phoenix dactylifera L. in Morocco. Afr. J. Boitech. 2006, 5, 510–516. [Google Scholar]
- Liu, W.; Zhang, Y.; Jiang, S.; Deng, Y.; Christic, P.; Murray, P.J.; Li, X.; Zhang, J. Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, A.; Zhao, Z. Seasonality of arbuscular mycorrhizal symbiosis and dark septate endophytes in grassland site in Southwest China. Microbial. Ecol. 2005, 54, 367–373. [Google Scholar]
- Houngnandan, P.; Yemadje, R.G.; Kane, A.; Boeckx, P.; Van Cleemput, O. Les glomales indigènes de la forêt claire à Isoberliniadoka (Craib et Stapf) à Wari-Maro au centre du Bénin. Tropicultura 2009, 27, 83–87. [Google Scholar]
- Sghir, F.; Chliyeh, M.; Kachkouch, W.; Khouader, M.; OuazzaniTouhami, A.; Benkirane, R.; Douira, A. Mycorrhizal status of Olea europaea ssp. Oleaster in Morocco. J. Appl. Biosci. 2013, 61, 4478–4489. [Google Scholar] [CrossRef]
- Al-Areqi, A.N.; Chliyeh, M.; Sghir, F.; Ouazzani, A.; Benkirane, R.T.; Douira, A. Diversity of Arbuscular mycorrhizal fungi in the rhizosphere of Coffea Arabica in the Republic of Yemen. J. Appl. Biosci. 2013, 64, 4888–4901. [Google Scholar] [CrossRef][Green Version]
- Gemma, J.N.; Koske, R.E.; Carreiro, M. Seasonal variation in spore abundance and dormancy of Gigasporagigantea in Mycorrhizal inoculum potential of a dune soil. Mycologia 1989, 80, 211–216. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Andersen, K.; Morton, J.B. Arbuscular mycorrhizal communities in tropical forests are affected by host tree species and environment. Oecologia 2003, 135, 268–297. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.J.; Hamad, S.R.; Malkawi, H.I. Population of arbuscular mycorrhizal fungi in semi-arid environment of Jordan as influenced by biotic and abiotic factors. J. Arid Environ. 2003, 53, 409–417. [Google Scholar] [CrossRef]
- Cruz, R.E.; Garcia, M.U. Nitrogen fixation and mycorrhizae in acacias on degraded grasslands. In Tropical Acacias in East Asia and the Pacific; Kamiset, A., Taylor, D.A., Eds.; Winrock Intenational Institute for Agriculture Research: Bangkok, Thailand, 1992; pp. 59–71. [Google Scholar]
- Böhme, L.; Langer, U.; Böhme, F. Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agric. Ecosyst. Environ. 2005, 109, 141–152. [Google Scholar] [CrossRef]
- Flieβbach, A.; Oberholzer, H.R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–274. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? Microbial. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin and soil quality. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Nannipieri, P.; Kandeler, E.; Ruggiero, P. Enzyme activities and microbiological and biochemical processes in soil. In Enzymes in the Environment; Activity, Ecology and Applications; Burns, R.G., Dick, R.P., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 1–33. [Google Scholar]
| Conserved Areas | Site | Vegetation Type | Grazing Intensity | |
|---|---|---|---|---|
| Bou-Hedma | inside | 1 | Acacia spp. and spontaneous herbaceous plants | Light |
| outside | 2 | Cultivated and spontaneous herbaceous plants | Intensive | |
| Zarat | inside | 3 | Acacia spp. and spontaneous herbaceous plants | Light |
| outside | 4 | Cultivated and spontaneous herbaceous plants | Intensive | |
| Oued Dkouk | inside | 5 | Spontaneous herbaceous plants | Light |
| outside | 6 | Spontaneous herbaceous plants | Intensive | |
| Parameters | Bou-Hedma | Zarat | Oued Dkouk | |||
|---|---|---|---|---|---|---|
| Inside | Outside | Inside | Outside | Inside | Outside | |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | |
| Clay (%) | 11.0 ± 0.2 b | 12.8 ± 0.1 a | 9.2 ± 0.2 d | 10.2 ± 0.1 c | 6.5 ± 0.2 f | 7.0 ± 0.1 e |
| Silt (%) | 23.5 ± 1.1 d | 26.1 ± 0.1 b | 25.1 ± 0.2 c | 34.7 ± 0.3 a | 16.4 ± 0.7 e | 15.3 ± 0.5 f |
| Sand (%) | 65.8 ± 0.2 c | 60.0 ± 1.2 e | 64.7 ± 1.1 d | 55.0 ± 1.4 f | 77.0 ± 1.1 b | 78.6 ± 1.1 a |
| pH | 8.1 ± 0.1 b | 8.1 ± 0.1 b | 8.0 ± 0.1 b | 8.1 ± 0.1 b | 8.4 ± 0.1 a | 8.4 ± 0.1 a |
| E.c (s·m−1) | 2.5 ± 0.3 a | 1.8 ± 0.2 c | 2.4 ± 0.3 a | 1.6 ± 0.2 d | 2.0 ± 0.1 b | 1.3 ± 0.1 e |
| T.N (ppm) | 191.0 ± 23 a | 150.0 ± 10 d | 174.0 ± 15 b | 130.0 ± 10 e | 160.0 ± 10 c | 90.0 ± 5 f |
| T.P (ppm) | 7.0 ± 0.1 f | 10.6 ± 0.2 c | 8.2 ± 0.2 e | 12.4 ± 0.2 b | 10.0 ± 0.2 d | 15.3 ± 0.6 a |
| Org. Mat (%) | 2.6 ± 0.2 a | 1.4 ± 0.1 d | 2.0 ± 0.1 b | 1.2 ± 0.1 e | 1.6 ± 0.3 c | 0.8 ± 0.1 f |
| Wa. Cont (%) | 3.3 ± 0.1 a | 2.3 ± 0.1 d | 2.9 ± 0.2 b | 2.0 ± 0.1 e | 2.5 ± 0.1 c | 1.4 ± 0.3 f |
| Factor | Grazing Intensity | |
|---|---|---|
| F-Ratio | p-Value | |
| Mycorrhizal frequency (F%) | 3.52 | 0.07 ns |
| Mycorrhizal intensity (M%) | 11.26 | 0.001 *** |
| AMF spore density | 10.55 | 0.002 ** |
| Conserved Areas | Title | Cmic | Phosphatase | Deydrogenase | β-Glucosidade | |
|---|---|---|---|---|---|---|
| Bou Hedma | Site 1 | F% | 0.93 *** | 0.97 *** | 0.95 *** | 0.96 *** |
| M% | 0.99 *** | 0.99 *** | 0.99 *** | 0.98 *** | ||
| Sp. num | 0.93 *** | 0.97 *** | 0.95 *** | 0.96 *** | ||
| Site 2 | F% | 0.93 *** | 0.97 *** | 0.96 *** | 0.96 *** | |
| M% | 0.98 *** | 0.98 *** | 0.99 *** | 0.99 *** | ||
| Sp. num | 0.98 *** | 0.96 *** | 0.98 *** | 0.98 *** | ||
| Zarat | Site 3 | F% | 0.95 *** | 0.97 *** | 0.96 *** | 0.97 *** |
| M% | 0.99 *** | 0.98 *** | 0.99 *** | 0.98 *** | ||
| Sp. num | 0.95 *** | 0.97 *** | 0.96 *** | 0.97 *** | ||
| Site 4 | F% | 0.92 *** | 0.97 *** | 0.97 *** | 0.96 *** | |
| M% | 0.96 *** | 0.97 *** | 0.99 *** | 0.98 *** | ||
| Sp. num | 0.97 *** | 0.96 *** | 0.97 *** | 0.95 *** | ||
| Oued Dkouk | Site 5 | F% | 0.96 *** | 0.97 *** | 0.97 *** | 0.97 *** |
| M% | 0.99 *** | 0.99 *** | 0.98 *** | 0.97 *** | ||
| Sp. num | 0.98 *** | 0.97 *** | 0.99 *** | 0.98 *** | ||
| Site 6 | F% | 0.90 * | 0.94 *** | 0.95 *** | 0.91 * | |
| M% | 0.98 *** | 0.98 *** | 0.99 *** | 0.98 *** | ||
| Sp. num | 0.98 *** | 0.99 *** | 0.97 *** | 0.95 *** | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoudi, N.; Dias, T.; Mahdhi, M.; Cruz, C.; Mars, M.; Caeiro, M.F. Does Arbuscular Mycorrhiza Determine Soil Microbial Functionality in Nutrient-Limited Mediterranean Arid Ecosystems? Diversity 2020, 12, 234. https://doi.org/10.3390/d12060234
Mahmoudi N, Dias T, Mahdhi M, Cruz C, Mars M, Caeiro MF. Does Arbuscular Mycorrhiza Determine Soil Microbial Functionality in Nutrient-Limited Mediterranean Arid Ecosystems? Diversity. 2020; 12(6):234. https://doi.org/10.3390/d12060234
Chicago/Turabian StyleMahmoudi, Neji, Teresa Dias, Mosbah Mahdhi, Cristina Cruz, Mohamed Mars, and Maria F. Caeiro. 2020. "Does Arbuscular Mycorrhiza Determine Soil Microbial Functionality in Nutrient-Limited Mediterranean Arid Ecosystems?" Diversity 12, no. 6: 234. https://doi.org/10.3390/d12060234
APA StyleMahmoudi, N., Dias, T., Mahdhi, M., Cruz, C., Mars, M., & Caeiro, M. F. (2020). Does Arbuscular Mycorrhiza Determine Soil Microbial Functionality in Nutrient-Limited Mediterranean Arid Ecosystems? Diversity, 12(6), 234. https://doi.org/10.3390/d12060234






