Impact of Various Grass Species on Soil Bacteriobiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Plant Characteristics
2.3. Experimental Design
2.4. Determination of Bacterial Count and Activity of Soil Enzymes
2.5. Metagenomic Analysis
2.6. Bioinformatic Analysis
2.7. Statistical Analysis
3. Results
3.1. Grass Yield
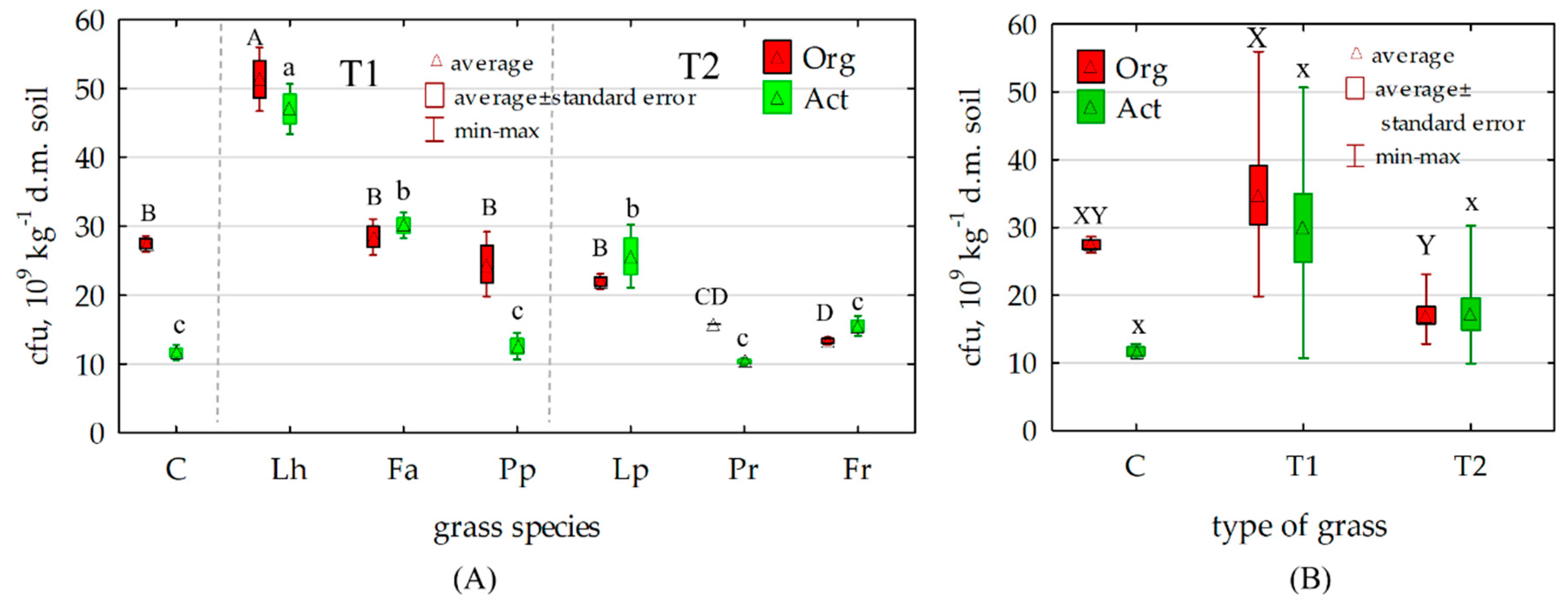
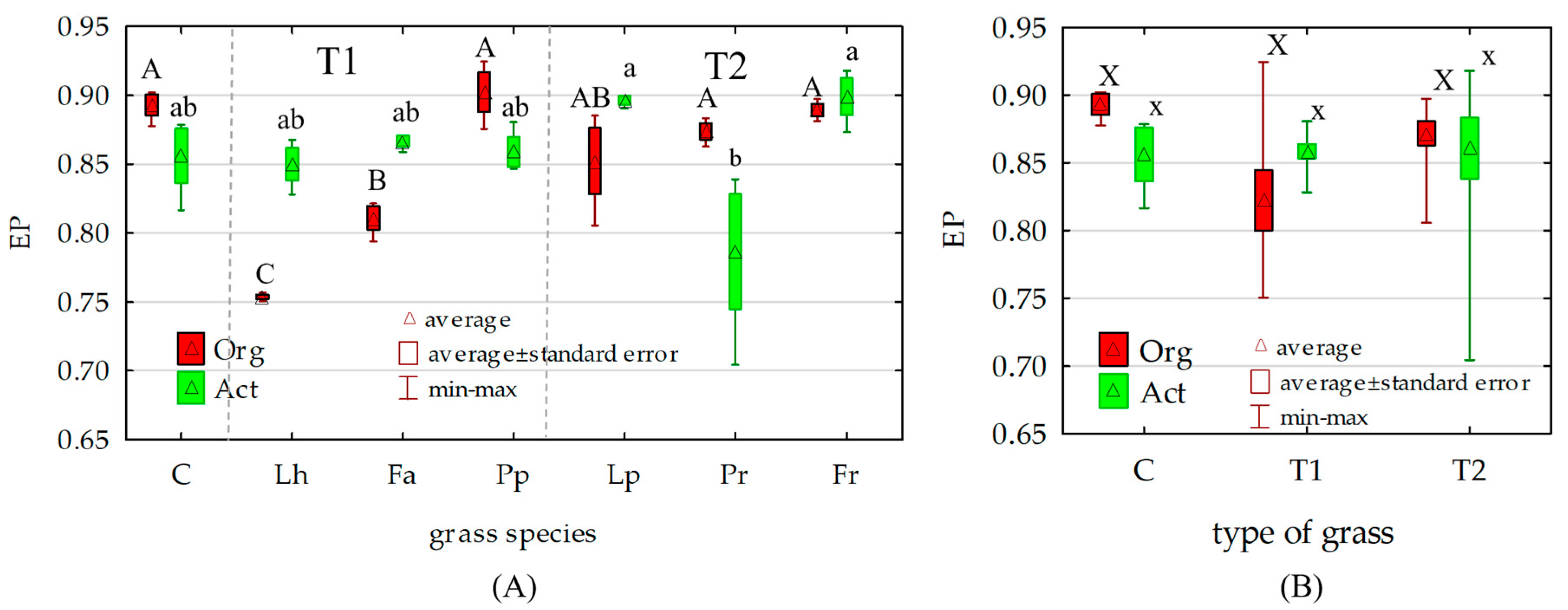
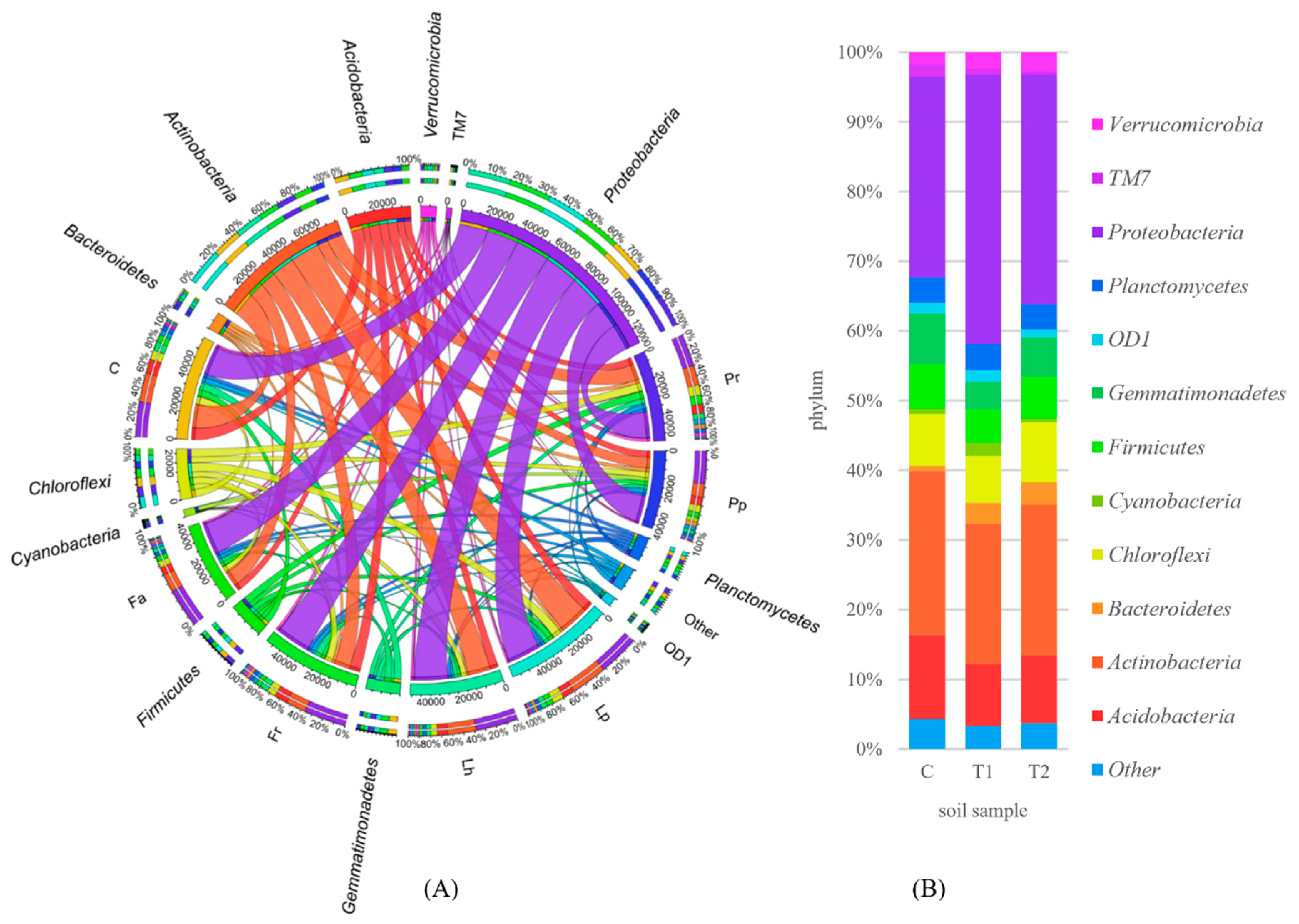
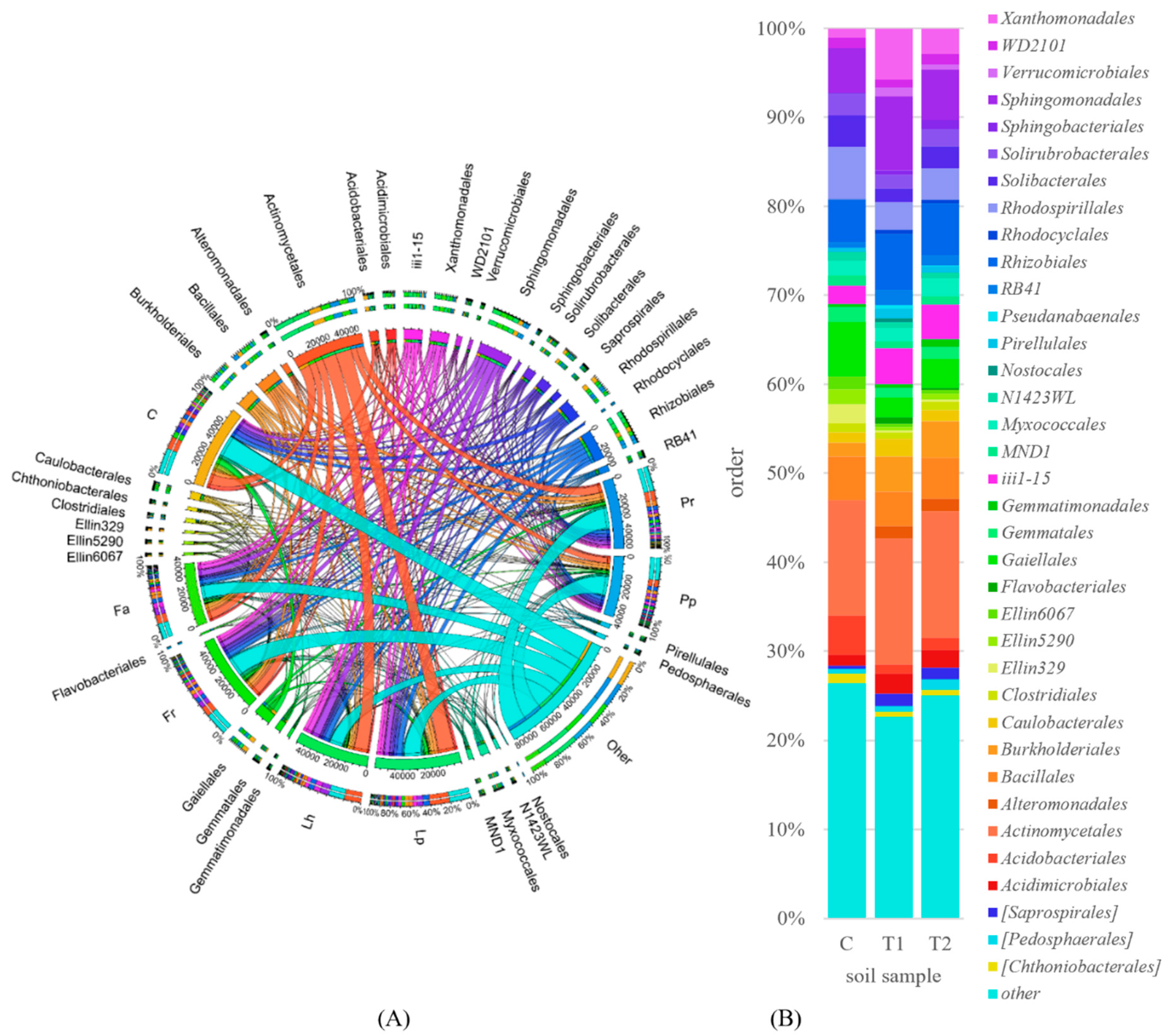
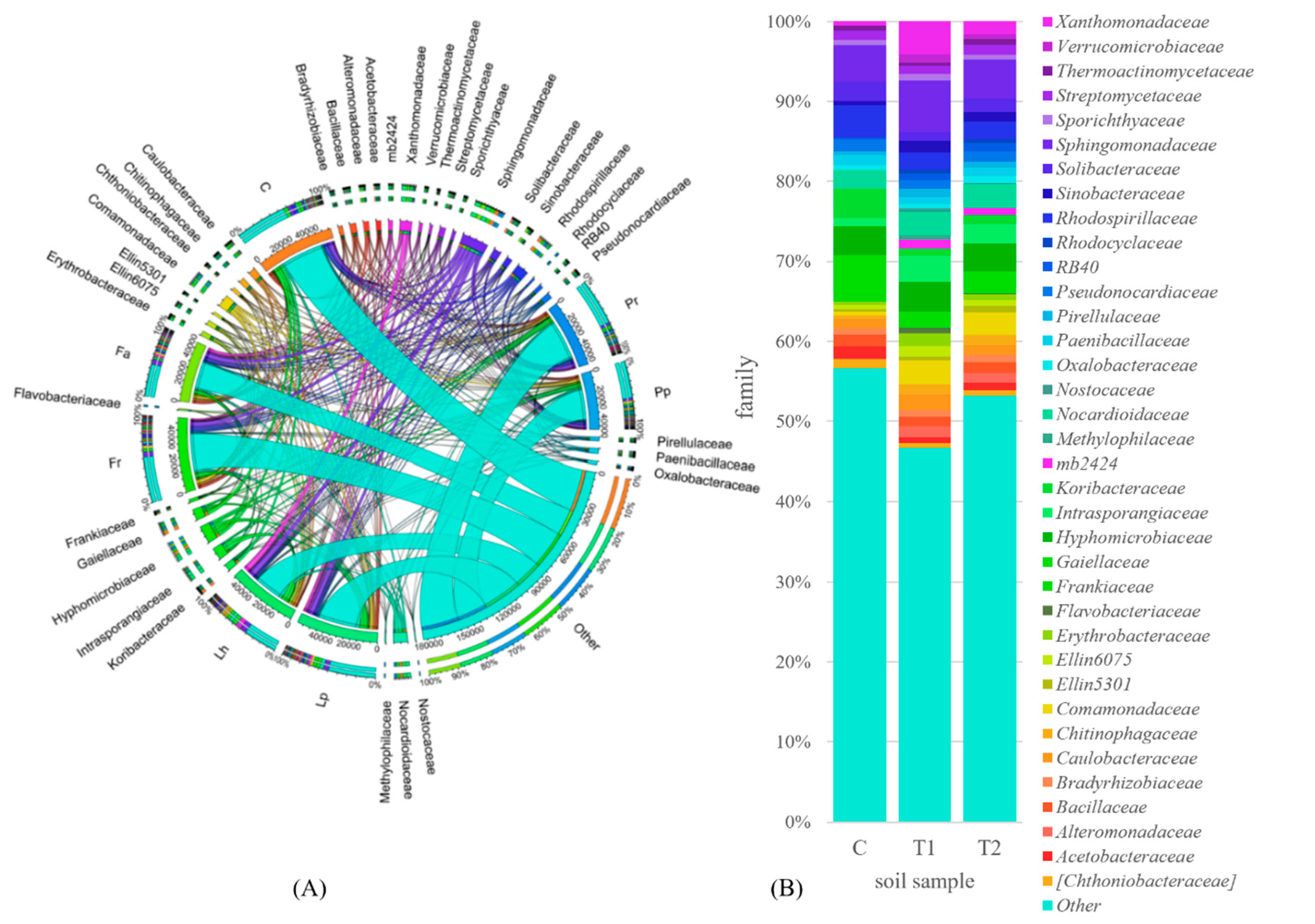
3.2. Counts and Diversity of Soil Bacteria
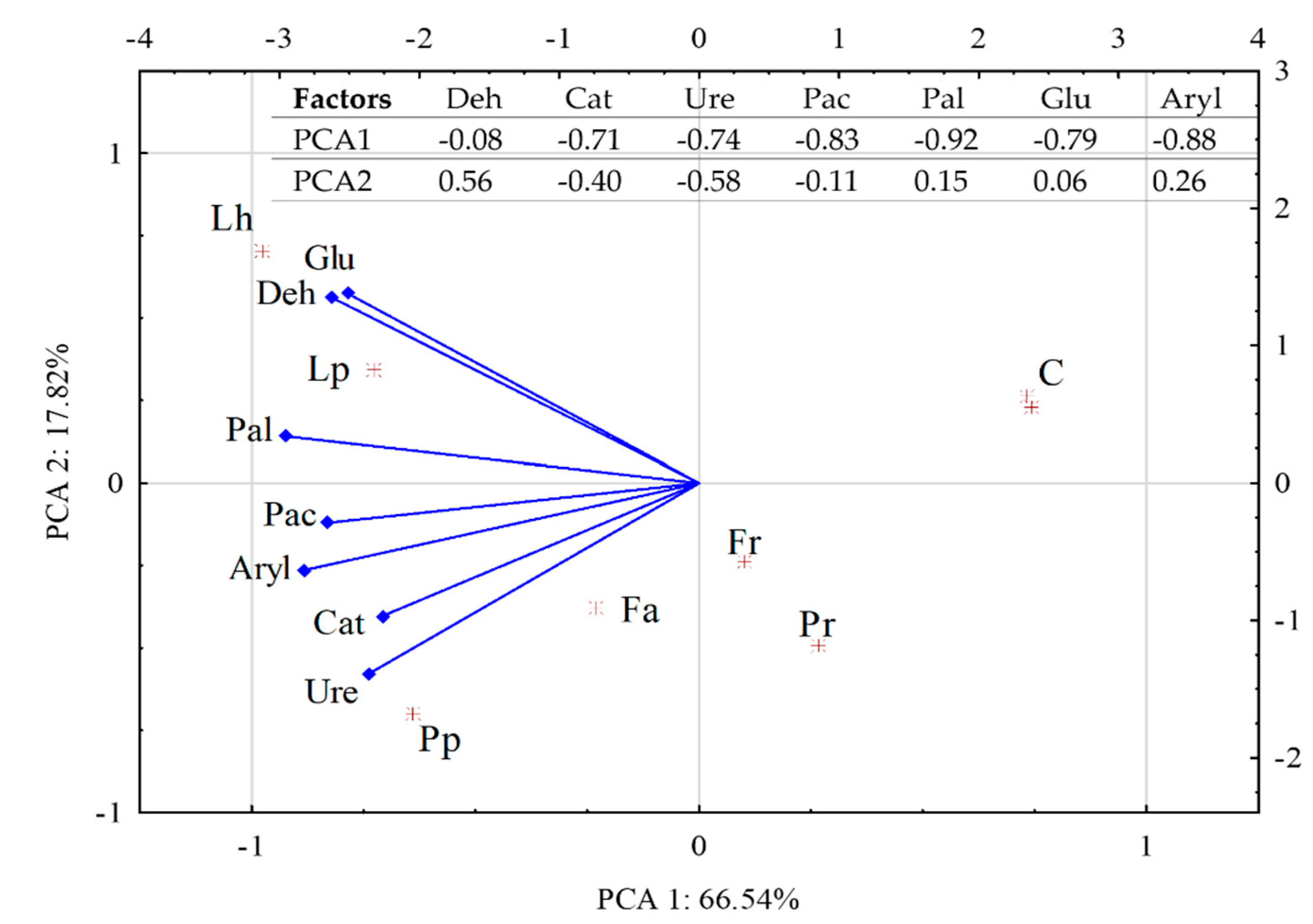
3.3. Activity of Soil Enzymes
4. Discussion
4.1. Grass Yield
4.2. Counts and Diversity of Soil Bacteria
4.3. Activity of Soil Enzymes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; Coleman-Derr, D. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.V.; Moitinho, M.R.; De Bortoli Teixeira, D.; André de Araújo Santos, G.; de Andrade Barbosa, M.; Bastos Pereira Milori, D.M.; Rigobelo, E.; Corá, J.E.; La Scala Júnior, N. Crop rotation and succession in a no-tillage system: Implications for CO2 emission and soil attributes. J. Environ. Manag. 2019, 1, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2019, 25. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 2013, 17, 155–164. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G.; Valori, F. Effects of Root Exudates in Microbial Diversity and Activity in Rhizosphere Soils. In Molecular Mechanisms of Plant and Microbe Coexistence; Nautiyal, C.S., Dion, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 15, pp. 339–365. [Google Scholar] [CrossRef]
- Candan, N.; Cakmak, I.; Ozturk, L. Zinc-biofortified seeds improved seedling growth under zinc deficiency and drought stress in durum wheat. J. Plant Nutr. Soil Sci. 2018, 181, 388–395. [Google Scholar] [CrossRef]
- Dubey, R.K.; Tripathi, V.; Prabha, R.; Chaurasia, R.; Singh, D.P.; Rao, C.S.; El-Keblawy, A.; Abhilash, P.C. Belowground Microbial Communities: Key Players for Soil and Environmental Sustainability. In Unravelling the Soil Microbiome. Springer Briefs in Environmental Science; Springer: Cham, Switzerland, 2020; Volume 2, pp. 5–22. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef]
- Walker, T.S. Root Exudation and Rhizosphere Biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, Z.; Zhao, Y.; Gu, Y.; Wang, Y.; Yu, J.; Xu, H. The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci. Total Environ. 2019, 686, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, A.P.; Idnurm, A. Biotechnological potential of engineering pathogen effector proteins for use in plant disease management. Biotechnol. Adv. 2019, 37, 107387. [Google Scholar] [CrossRef] [PubMed]
- Ponge, J.F. Humus forms in terrestrial ecosystems: A framework to biodiversity. Soil Biol. Biochem. 2003, 35, 935–945. [Google Scholar] [CrossRef]
- Ponge, J.F. Plant–soil feedbacks mediated by humus forms: A review. Soil Biol. Biochem. 2013, 57, 1048–1060. [Google Scholar] [CrossRef]
- Rousk, J.E.; Bääth, P.C.; Brookes, C.L.; Lauber, C.; Lozupone, J.G.; Caporaso, R.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 10, 1340–1351. [Google Scholar] [CrossRef]
- Rillig, M.; Muller, L.; Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.T.; Xiang, X.J.; Sun, R.B.; Yang, T.; He, D.; Zhang, K.P.; Ni, Y.Y.; Zhu, Y.G.; Adams, J.M.; et al. Spatial scale affects the relative role of stochasticity versus determinism in soil bacterial communities in wheat fields across the North China Plain. Microbiome 2018, 6, 27. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Yuan, M.; Adams, J.M.; Pan, X.; Yang, Y.; Chu, H. A biogeographic map of soil bacterial communities in wheats field of the North China Plain. Soil Ecol. Lett. 2019, 1, 50–58. [Google Scholar] [CrossRef]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econom. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Soil moisture as a factor affecting the microbiological and biochemical activity of soil. Plant Soil Environ. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Impact of temperature on the biological properties of soil. Int. Agrophys. 2016, 30, 1–8. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2017, 54, 11–19. [Google Scholar] [CrossRef]
- VCU 2019, Value for Cultivation and Use (VCU) BSPB Plant Breeding Matters. Available online: http://plantbreedingmatters.com/evaluation.php (accessed on 3 February 2020).
- Raman, J.K.; Alves, C.M.; Gnansounou, E. A review on moringa tree and vetiver grass—Potential biorefinery feedstocks. Bioresour. Technol. 2018, 249, 1044–1051. [Google Scholar] [CrossRef]
- Peeters, A. Importance, evolution, environmental impact and future challenges of grasslands and grassland-based systems in Europe. Grassl. Sci. 2009, 55, 113–125. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). 2019. Available online: https://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites-3 (accessed on 20 February 2020).
- European Commission. 2019. Available online: www.ec.europa.eu/environment/soil/index_en.htm (accessed on 20 February 2020).
- Zhang, X.; Xu, S.; Li, C.; Zhao, L.; Feng, H.; Yue, G.; Ren, Z.; Cheng, G. The soil carbon/nitrogen ratio and moisture affect microbial community structures in alkaline permafrost-affected soils with different vegetation types on the Tibetan plateau. Res. Microbiol. 2014, 165, 128–139. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Bioaugmentation of soil contaminated with diesel oil. J. Elem. 2018, 23, 1161–1178. [Google Scholar] [CrossRef]
- Chikere, C.B.; Okpokwasili, G.C.; Chikere, B.O. Monitoring of microbial hydrocarbon remediation in the soil. 3 Biotech. 2011, 1, 3. [Google Scholar] [CrossRef]
- Lipińska, A.; Wyszkowska, J.; Kucharski, J. Diversity of organotrophic bacteria, activity of dehydrogenases and urease as well as seed germination and root growth Lepidium sativum, Sorghum saccharatum and Sinapis alba under the influence of polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. Int. 2015, 22, 18519–18530. [Google Scholar] [CrossRef] [PubMed]
- Telesiński, A.; Krzyśko-Łupicka, T.; Cybulska, K.; Wróbel, J. Response of soil phosphatase activities to contamination with two types of tar oil. Environ. Sci. Pollut. Res. 2018, 25, 28642–28653. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biochemical and microbiological activity of soil contaminated with o-cresol and biostimulated with Perna canaliculus mussel meal. Environ. Monit Assess. 2018, 190, 602. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, J.; Wieczorek, K.; Wyszkowska, J. Changes in the enzymatic activity in sandy loam soil exposed to zinc pressure. J. Elem. 2011, 16, 577–589. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Borowik, A.; Baćmaga, M.; Kucharski, J.; Tomkiel, M. Implication of zinc excess on soil health. J. Environ. Sci. Health B 2016, 51, 261–270. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biological activity of soil contaminated with cobalt, tin and molybdenum. Environ. Monit. Assess. 2016, 188, 398. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Waraczewska, Z.; Budka, A.; Ratajczak, K. An assessment of the influence of selected herbicides on the microbial parameters of soil in maize (Zea mays) cultivation. Appl. Ecol. Env. Res. 2018, 16, 4735–4752. [Google Scholar] [CrossRef]
- Tomkiel, M.; Baćmaga, M.; Borowik, A.; Kucharski, J.; Wyszkowska, J. Effect of a mixture of flufenacet and isoxaflutole on population numbers of soil-dwelling microorganisms, enzymatic activity of soil, and maize yield. J. Environ. Sci. Health B 2019, 1, 11. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Response of Avena sativa L. and the soil microbiota to the contamination of soil with shell diesel oil. Plant Soil Environ. 2018, 64, 102–107. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, Y.; Liu, X.; Feng, Z.; Zhu, H.; Yao, Q. Soil bacterial function associated with stylo (Legume) and bahiagrass (grass) is affected more strongly by soil chemical property than by bacterial community composition. Front. Microbiol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Oszust, K. Functional diversity of fungal communities in soil contaminated with diesel oil. Front. Microbiol. 2017, 8, 1862. [Google Scholar] [CrossRef]
- Tshikantwa, T.S.; Ullah, M.W.; He, F.; Yang, G. Current trends and potential applications of microbial interactions for human welfare. Front. Microbiol. 2018, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp. 2013, e50961. [Google Scholar] [CrossRef] [PubMed]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organicand conventional vegetable farming in west java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, W.; Zhou, L.; Liang, J.; Jiang, T. Interactive effect of dissolved organic matter and phenanthrene on soil enzymatic activities. J. Environ. Sci. 2010, 22, 607–614. [Google Scholar] [CrossRef]
- Knight, T.R.; Dick, R.P. Differentiating microbial and stabilized β-glucosidase activity relative to soil quality. Soil Biol. Biochem. 2004, 36, 2089–2096. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Wyszkowski, M. Activity of soil dehydrogenases, urease and acid and alkaline phosphatase in soil polluted with petroleum. J. Toxicol. Environ. Health Part A 2010, 73, 1202–1210. [Google Scholar] [CrossRef]
- Vong, P.C.; Piutti, S.; Slezack-Deschaumes, S.; Beniziri, E.; Guckert, A. Sulphur immobilization and arylsulphatase activity in two calcareous arable and fallow soils as affected by glucose additions. Geoderma 2008, 148, 79–84. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Olszewski, J.; Kucharski, J. Soil bacterial community and soil enzyme activity depending on the cultivation of Triticum aestivum, Brassica napus, and Pisum sativum ssp. arvense. Diversity 2019, 11, 246. [Google Scholar] [CrossRef]
- Bunt, J.S.; Rovira, A.D. Microbiological studies of some subantarctic soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Parkinson, D.; Gray, F.R.G.; Williams, S.T. Methods for Studying the Ecology of Soil Microorganism; IBP Handbook 19; Blackwell Scientific Publication: Oxford, UK, 1971. [Google Scholar]
- De Leij, F.A.A.M.; Whipps, J.M.; Lynch, J.M. The use of colony development for the charactization of bacterial communities in soil and on roots. Microb. Ecol. 1994, 27, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Öhlinger, R. Dehydrogenase Activity with the Substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin, Germany, 1996; pp. 241–243. [Google Scholar]
- Johnson, J.I.; Temple, K.L. Some variables affecting the measurement of catalase activity in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–216. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic London: London, UK, 1988; pp. 316–365. [Google Scholar]
- De Santis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System), Version 13.1; Dell Inc.: Tulsa, OK, USA, 2016. [Google Scholar]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Krzywinski, M.I.; Schein, J.E.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Broadbent, A.A.D.; Stevens, C.J.; Ostle, N.J.; Orwin, K.H. Biogeographic differences in soil biota promote invasive grass response to nutrient addition relative to co-occurring species despite lack of belowground enemy release. Oecologia 2018, 186, 611–620. [Google Scholar] [CrossRef]
- Shukla, P.; Chaurasia, P.; Younis, K.; Qadri, O.S.; Faridi, S.A.; Srivastava, G. Nanotechnology in sustainable agriculture: Studies from seed priming to post-harvest management. Nanotechnol. Environ. Eng. 2019, 4, 1. [Google Scholar] [CrossRef]
- Deru, J.; Schilder, H.; Van der Schoot, J.R.; Van Eekeren, N. No Trade-off between Root Biomass and Aboveground Production in Lolium perenne. In Breeding in a World of Scarcity; Roldán-Ruiz, I., Baert, J., Reheul, D., Eds.; Springer: Cham, Switzerland, 2016; Volume 43, pp. 289–292. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, C.T.; Steinberg, C.; Alabouvette, C.; Moenne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Saleh, D.; Jarry, J.; Rani, M.; Aliferis, K.; Seguin, P.; Jabaji, S.H. Diversity, distribution and multi-functional attributes of bacterial communities associated with the rhizosphere and endosphere of timothy (Phleum pratense L.). J. Appl. Microbiol. 2019, 127, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.A.; Foster, B.L.; Gao, C. temporal dynamics in rhizosphere bacterial communities of three perennial grassland species. Agronomy 2016, 6, 17. [Google Scholar] [CrossRef]
- Singh, B.K.; Munro, S.; Potts, J.M.; Millard, P. Influence of grass species and soil type on rhizosphere microbial structure in grassland soils. Appl. Soil Ecol. 2007, 36, 147–155. [Google Scholar] [CrossRef]
- Chen, K.; Chang, Y.; Chiou, W. Remediation of diesel-contaminated soil using in situ chemical oxidation (ISCO) and the effects of common oxidants on the indigenous microbial community: A comparison study. J. Chem. Technol. Biotechnol. 2016, 91, 1877–1888. [Google Scholar] [CrossRef]
- Marschner, P.; Neumann, G.; Kania, A.; Weiskopf, L.; Lieberei, R. Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant Soil 2002, 246, 167–174. [Google Scholar] [CrossRef]
- Kielak, A.; Pijl, A.S.; van Veen, J.A.; Kowalchuk, G.A. Differences in vegetation composition and plant species identity lead to only minor changes in soil-borne microbial communities in a former arable field. FEMS Microbiol. Ecol. 2008, 63, 372–382. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Ghuneim, L.-A.J.; Jones, D.L.; Golyshin, P.N.; Golyshina, O.V. Nano-sized and filterable bacteria and archaea: Biodiversity and function. Front. Microbiol. 2018, 9, 1971. [Google Scholar] [CrossRef]
- Chu, H.; Sun, H.; Tripathi, B.M.; Adams, J.M.; Huang, R.; Zhang, Y.; Shi, Y. Bacterial community dissimilarity between the surface and subsurface soils equals horizontal differences over several kilometers in the western Tibetan Plateau. Environ. Microbiol. 2016, 18, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, J.; Mohammadipanah, F. Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria. J. Ind. Microbiol. Biotechnol. 2015, 42, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of soil pollution with diesel oil and BP petroleum with ACTIVE Technology for soil health. Int. J. Environ. Res. Public Health 2019, 16, 2474. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Ratajczak, K.; Waraczewska, Z.; Budka, A.; Głuchowska, K. The influence of biostimulants and foliar fertilisers on the process of biological nitrogen fixation and the level of soil biochemical activity in soybean (Glycine Maxl.) cultivation. Appl. Ecol. Environ. Res. 2019, 17, 12649–12666. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 2014, 214—215, 10–18. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of soil microbial and enzyme activities are linked to soil C, N and P stoichiometry in afforested ecosystems. For. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Yan, J.; Li, H.; He, J. Effects of regenerating vegetation on soil enzyme activity and microbial structure in reclaimed soils on a surface coal mine site. Appl. Soil Ecol. 2015, 87, 56–62. [Google Scholar] [CrossRef]
- Raiesi, F.; Beheshti, A. Microbiological indicators of soil quality and degradation following conversion of native forests to continuous croplands. Ecol. Indic. 2015, 50, 173–185. [Google Scholar] [CrossRef]
- García-Gaytán, V.; Hernández-Mendoza, F.; Coria-Téllez, A.; García-Morales, S.; Sánchez-Rodríguez, E.; Rojas-Abarca, L.; Daneshvar, H. Fertigation: Nutrition, Stimulation and Bioprotection of the Root in High Performance. Plants 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]




| pHKCl | HAC | EBC | CEC | BS % | Content | Available Forms | Interchangeable Forms | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol (+) kg−1 d.m. of Soil | Ntotal | Ctotal | P | K | Mg | K | Ca | Na | Mg | ||||
| g kg−1 d.m. of Soil | mg kg−1 d.m. of Soil | ||||||||||||
| 6.70 | 11.40 | 49.00 | 60.40 | 81.10 | 0.62 | 9.30 | 93.68 | 141.10 | 42.00 | 156.00 | 623.50 | 40.00 | 59.50 |
| Grasses Kind | Common Name | Botanical Name | Abbreviation | Variety | Photosynthesis Kind |
|---|---|---|---|---|---|
| Fodder | Hybrid ryegrass | Lolium x hybridum Hausskn | Lh | Gala | C3 |
| Tall fescue | Festuca arundinacea | Fa | Rahela | C3 | |
| Timothy | Phleum pratense | Pp | Kaba | C3 | |
| Lawn | Perennial ryegrass | Lolium perenne | Lp | Bajka | C3 |
| Smooth-stalked meadowgrass | Poa pratensis | Pr | Sójka | C3 | |
| Red rescue | Festuca rubra | Fr | Dark | C3 |
| Tested Feature | Medium/Formula/Substrat | References |
|---|---|---|
| Medium | ||
| Organotrophic bacteria (Org) | peptone 1.0 g, yeast extract 1.0 g, (NH4)2SO4 0.5 g, CaCl2, K2HPO4 0.4 g, MgCl2 0,2 g, MgSO4 7H2O 0.5 g, salt Mo 0.03 g, FeCl2 0.01 g, agar 20.0 g, soil extract 250 cm3, distilled water 750 cm3, pH 6.6–7.0 | [59] [58] |
| Actinobacteria (Act) | soluble starch 10.0 g; casein 0.3 g; KNO3 2.0 g; NaCl 2.0 g; K2HPO4 2.0 g; MgSO4⋅7H2O 0.05 g; CaCO3 0.02 g; FeSO4 0.01 g; agar 20.0 g; H2O 1 dm3; 50 cm3 aqueous solution of nystatin 0.05%; 50 cm3 aqueous solution of actidione 0.05%; pH 7.0 | [60] [58] |
| Formula | ||
| Colony development index (CD) | CD = [N1/1 + N2/2 + N3/3 +…+ N10/10] × 100, where: N1, N2, N3,..., N10—the sum of ratios of the number of colonies of microorganisms identified in particular days (1, 2, 3,..., 10) to the total number of colonies identified throughout the study period | [61] |
| Ecophysiological diversity index (EP) | EP = −Σ(pi·log10 pi), where: pi—the ratio of the number of colonies of microorganisms identified in particular days to the total number of colonies identified throughout the study period. | |
| Substrat | ||
| Dehydrogenases (Deh) | C19H15ClN4 | [62] |
| Catalase (Cat) | H2O2 | [63] |
| Urease (Ure) | CON2H4 | [64] |
| β-glucosidase (Glu) | C12H15NO8 | |
| Acid phosphatase (Pac) | O2NC6H4OP(O)(ONa)2 6H2O | |
| Alkaline phosphatase (Pal) | ||
| Aryosulphatase (Aryl) | NO2C6H4OSO2OK | |
| Taxon | C | Lh | Fa | Pp | Lp | Pr | Fr | C | T1 | T2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Shannon-Wiener index | ||||||||||
| phylum | 2.05 ab | 1.86 d | 1.92 cd | 2.10 ab | 1.90 d | 2.12 a | 2.01 ac | 2.05 x | 1.96 x | 2.01 x |
| class | 2.81 bc | 2.67 c | 2.80 bc | 2.97 a | 2.82 abc | 2.96 ab | 2.90 ab | 2.81 x | 2.81 x | 2.89 x |
| order | 2.77 b | 2.75 b | 2.90 a | 2.91 a | 2.81 ab | 2.79 ab | 2.93 a | 2.77 x | 2.86 x | 2.84 x |
| family | 2.00 d | 2.49 a | 2.43 ab | 2.23 c | 2.34 bc | 2.04 d | 2.26 c | 2.00 z | 2.38 x | 2.21 y |
| genus | 0.76 c | 0.97 a | 0.86 b | 0.87 b | 0.87 b | 0.74 c | 0.78 c | 0.76 z | 0.90 x | 0.80 y |
| Simpson index | ||||||||||
| phylum | 0.84 ab | 0.78 c | 0.78 c | 0.83 ab | 0.80 b | 0.85 a | 0.82 ab | 0.84 x | 0.80 x | 0.82 x |
| class | 0.92 ab | 0.89 b | 0.92 ab | 0.93 a | 0.92 ab | 0.94 a | 0.93 a | 0.92 x | 0.91 x | 0.93 x |
| order | 0.94 ab | 0.91 b | 0.94 ab | 0.96 a | 0.92 b | 0.95 a | 0.95 a | 0.94 x | 0.94 x | 0.94 x |
| family | 0.67 ef | 0.82 a | 0.78 ab | 0.70 de | 0.75 bc | 0.66 f | 0.72 cd | 0.67 y | 0.77 x | 0.71 y |
| genus | 0.28 cd | 0.36 a | 0.32 b | 0.30 c | 0.31 b | 0.26 d | 0.28 cd | 0.28 y | 0.33 x | 0.28 y |
| Grass Species | Deh μmol TFF | Cat mol O2 | Ure mmol N-NH4 | Pac | Pal | Glu | Aryl |
|---|---|---|---|---|---|---|---|
| mmol PNP | |||||||
| C | 1.38 e | 0.09 e | 0.30 b | 0.79 d | 0.16 c | 0.30 b | 0.08 d |
| Lh | 10.29 a | 0.18 b | 0.47 ab | 1.20 ab | 0.40 a | 0.35 a | 0.15 a |
| Fa | 2.38 d | 0.14 c | 0.60 ab | 1.18 ab | 0.32 b | 0.30 b | 0.14 ab |
| Pp | 3.25 c | 0.19 ab | 0.88 a | 1.08 bc | 0.33 b | 0.31 b | 0.16 a |
| Lp | 6.87 b | 0.15 c | 0.68 ab | 1.30 a | 0.35 ab | 0.34 a | 0.11 bc |
| Pr | 0.65 f | 0.21 a | 0.48 ab | 0.98 c | 0.17 c | 0.30 b | 0.09 cd |
| Fr | 1.74 de | 0.11 d | 0.63 ab | 1.27 a | 0.14 c | 0.31 b | 0.11 cd |
| C | 1.38 z | 0.09 z | 0.30 y | 0.79 y | 0.16 z | 0.30 y | 0.08 y |
| T1 | 5.31 x | 0.17 x | 0.65 x | 1.15 x | 0.35 x | 0.32 x | 0.15 x |
| T2 | 3.09 y | 0.16 y | 0.60 x | 1.18 x | 0.22 y | 0.32 x | 0.10 y |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowik, A.; Wyszkowska, J.; Kucharski, J. Impact of Various Grass Species on Soil Bacteriobiome. Diversity 2020, 12, 212. https://doi.org/10.3390/d12060212
Borowik A, Wyszkowska J, Kucharski J. Impact of Various Grass Species on Soil Bacteriobiome. Diversity. 2020; 12(6):212. https://doi.org/10.3390/d12060212
Chicago/Turabian StyleBorowik, Agata, Jadwiga Wyszkowska, and Jan Kucharski. 2020. "Impact of Various Grass Species on Soil Bacteriobiome" Diversity 12, no. 6: 212. https://doi.org/10.3390/d12060212
APA StyleBorowik, A., Wyszkowska, J., & Kucharski, J. (2020). Impact of Various Grass Species on Soil Bacteriobiome. Diversity, 12(6), 212. https://doi.org/10.3390/d12060212