Low Diversity of Intertidal Canopy-Forming Macroalgae at Urbanized Areas along the North Portuguese Coast
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Sample Processing
2.3. Data Analyses
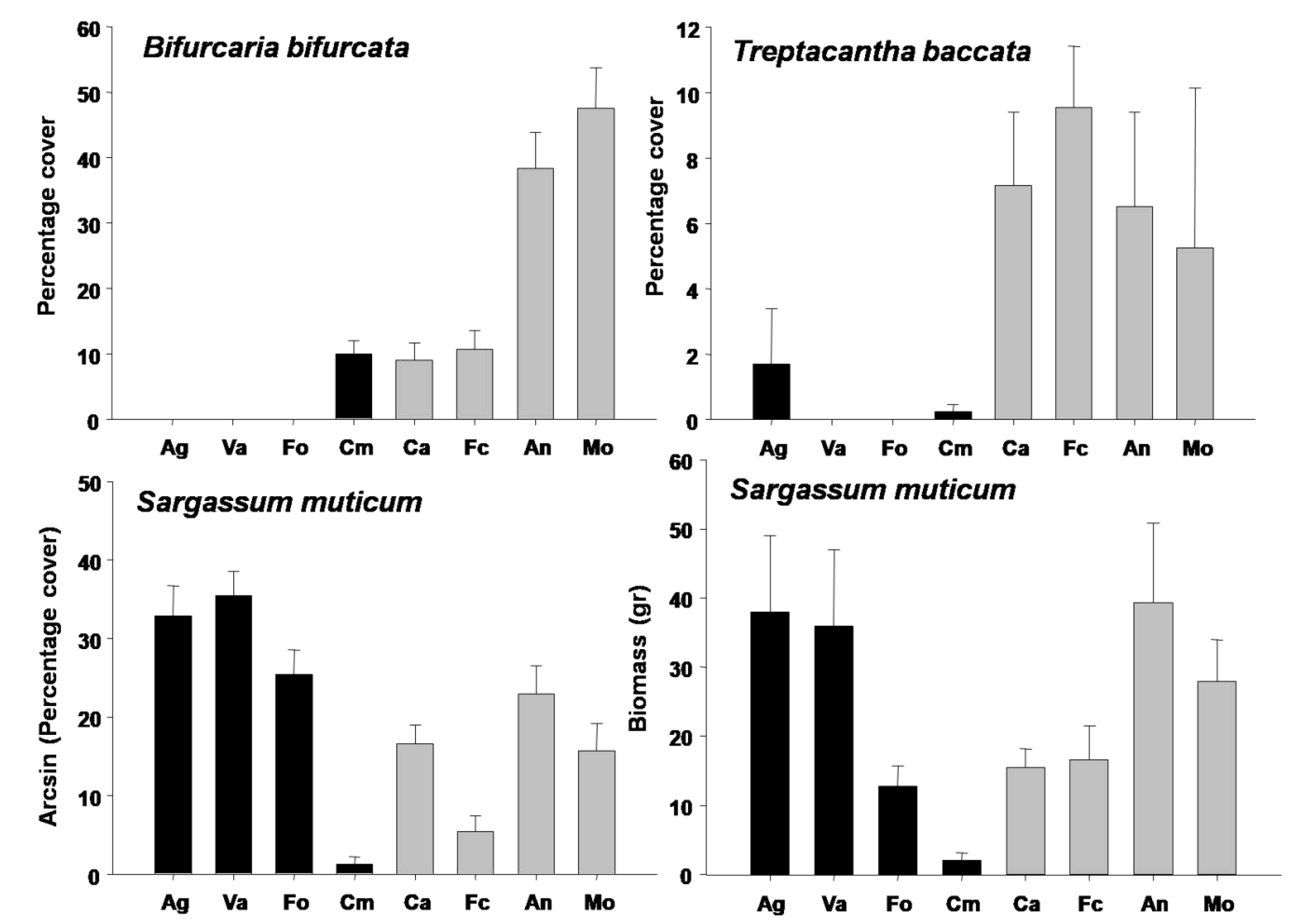
3. Results
3.1. Canopy-Forming Diversity
3.2. Canopy-Forming Assemblage Structure
3.3. Abundance and Biomass of Most Relevant Species
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duffy, M.E.; Benedetti-Cecchi, L.; Trinanes, J.; Muller-Karger, F.E.; Ambo-Rappe, R.; Boström, C.; Buschmann, A.H.; Byrnes, J.; Coles, R.G.; Creed, J.; et al. Toward a coordinated global observing system for seagrasses and marine macroalgae. Front. Mar. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Rise of turfs: A new battlefront for globally declining kelp forests. Bioscience 2018, 68, 64–76. [Google Scholar] [CrossRef]
- Airoldi, L.; Beck, M.W. Loss, status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol. Annu. Rev. 2007, 35, 345–405. [Google Scholar]
- Airoldi, L.; Balata, D.; Beck, M.W. The Gray Zone: Relationships between habitat loss and marine diversity and their applications in conservation. J. Exp. Mar. Biol. 2008, 366, 8–15. [Google Scholar] [CrossRef]
- Christie, H.; Andersen, G.S.; Bekkby, T.; Fagerli, C.W.; Gitmark, J.K.; Gundersen, H.; Rinde, E. Shifts between sugar kelp and turf algae in Norway: Regime shifts or fluctuations between different opportunistic seaweed species? Front. Mar. Sci. 2019, 6, 72. [Google Scholar] [CrossRef]
- Gorman, D.; Russell, B.D.; Connell, S.D. Land-to-sea connectivity: Linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. Ecol. Appl. 2009, 19, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Heery, E.C.; Loke, L.H.L.; Thurstan, R.H.; Kotze, D.J.; Swan, C. Towards an urban marine ecology: Characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 2019, 128, 1215–1242. [Google Scholar] [CrossRef]
- Tamburello, L.; Papa, L.; Guarnieri, G.; Basconi, L.; Zampardi, S.; Scipione, M.B.; Terlizzi, A.; Zupo, V.; Fraschetti, S. Are we ready for scaling up restoration actions? An insight from Mediterranean macroalgal canopies. PLoS ONE 2019, 14, e0224477. [Google Scholar] [CrossRef]
- Casado-Amezúa, P.; Araújo, R.; Bárbara, I.; Bermejo, R.; Borja, Á.; Díez, I.; Fernández, C.; Gorostiaga, J.M.; Guinda, X.; Hernández, I.; et al. Distributional shifts of canopy-forming seaweeds from the Atlantic coast of Southern Europe. Biodivers.Conserv. 2019, 28, 1151–1172. [Google Scholar] [CrossRef]
- Iveša l Djakovac, T.; Devescovi, M. Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 2016, 106, 162–173. [Google Scholar] [CrossRef]
- Sales, M.; Cebrian, E.; Tomas, F.; Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 2011, 92, 347–357. [Google Scholar] [CrossRef]
- Mangialajo, I.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef]
- Rubal, M.; Veiga, P.; Reis, P.A.; Bertocci, I.; Sousa-Pinto, I. Effects of subtle pollution at different levels of biological organisation on species-rich assemblages. Environ. Pollut. 2014, 191, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Dethier, M.N.; Graham, E.S.; Cohen, S.; Tear, L.M. Visual versus random-point percent cover estimations:“objective” is not always better. Mar. Ecol. Prog. Ser. 1993, 96, 93–100. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variances; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Clarke, K.R. Nonparametric multivariate analyses of changes in community structure. Austral. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Araújo, R.; Bárbara, I.; Tibaldo, M.; Berecibar, E.; Diaz Tapia, P.; Pereira, R.; Santos, R.; Sousa-Pinto, I. Checklist of benthic marine algae and cyanobacteria of northern Portugal. Bot. Mar. 2009, 52, 24–46. [Google Scholar] [CrossRef]
- Lluch, R.J.; Gómez Garreta, A.; Barceló, M.C.; Ribera, M.A. Mapas de distribución de algas marinas de la Península Ibérica e Islas Baleares. VII. Cystoseira, C. Agardh (Grupo, C. baccata) y Sargassum, C. Agardh (S. muticum y S. vulgare). Botanica Complutenses 1994, 19, 131–138. [Google Scholar]
- Veiga, P.; Rubal, M.; Sousa-Pinto, I. Structural complexity of macroalgae influences epifaunal assemblages associated with native and invasive species. Mar. Environ. Res. 2014, 101, 115–123. [Google Scholar] [CrossRef]
- Veiga, P.; Sousa-Pinto, I.; Rubal, M. Meiofaunal assemblages associated with native and non-indigenous macroalgae. Cont. Shelf Res. 2016, 123, 1–8. [Google Scholar] [CrossRef]
- Salvaterra, T.; Geen, D.S.; Crowe, T.P.; O’Gorman, E.J. Impacts of the invasive alga Sargassum muticum on ecosystem functioning and food web structure. Biol. Invasions 2013, 15, 2563–2576. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Verlaque, M.; Boudouresque, C.F.; Ruitton, S. The Sargassum conundrum: Highly rare, threatened or locally extinct in the NW Mediterranean and still lacking protection. Hydrobiologia 2016, 781, 3–23. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Cottalorda, J.M.; Hereu, B.; Susini, M.L.; Verlaque, M. Unexpected temporal stability of Cystoseira and Sargassum forests in Port-Cros, one of the oldest Mediterranean marine National Parks. Cryptogam. Algol. 2016, 37, 61–90. [Google Scholar] [CrossRef]
- Devescovi, M. Effects of bottom topography and anthropogenic pressure on northern Adriatic Cystoseira spp. (Phaeophyceae, Fucales). Aquat. Bot. 2015, 121, 26–32. [Google Scholar] [CrossRef]
- Incera, M.; Olabarria, C.; Cacabelos, E.; César, J.; Troncoso, J.S. Distribution of Sargassum muticum on the North West coast of Spain: Relationships with urbanization and community diversity. Cont. Shelf Res. 2011, 31, 488–495. [Google Scholar] [CrossRef]
- Carlton, J.T. Pattern, process, and prediction in marine invasion ecology. Biol. Conserv. 1996, 78, 97–106. [Google Scholar] [CrossRef]



| Source of Variation | df | S | H | ||||
|---|---|---|---|---|---|---|---|
| MS | F | p | MS | F | p | ||
| Urbanization = Ur | 1 | 39.0 | 205.7 | 0.000 | 9.48 | 175.6 | 0.000 |
| Shore(Ur) = Sh(Ur) | 6 | 0.18 | 0.9 | 0.49 | 0.05 | 1.23 | 0.29 |
| Residual | 152 | 0.21 | 0.04 | ||||
| Total | 156 | ||||||
| Transform | none | none | |||||
| Cochran’s Test | C = 0.3312 | s | C = 0.2832 | s | |||
| Source of Variation | df | MS | Pseudo-F | p | Unique Permutations |
|---|---|---|---|---|---|
| Urbanization = Ur | 1 | 64289 | 2.73 | 0.04 | 35 |
| Shore(Ur) = Sh(St) | 6 | 23527 | 14.03 | 0.001 | 999 |
| Residual | 152 | 1677 | |||
| Total | 159 |
| Species | Average Abundance | δi | δi% | δi/SD(δi) | |
|---|---|---|---|---|---|
| Urban | Non-Urban | ||||
| Bifurcariabifurcata | 2.49 | 26.41 | 33.01 | 42.48 | 1.26 |
| Sargassummuticum | 22.10 | 13.10 | 29.85 | 38.41 | 1.33 |
| Treptacanthabaccata | 0.49 | 7.11 | 13.66 | 7.77 | 17.57 |
| Source of Variation | df | S. muticum | B. bifurcata | T. baccata | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS | F | p | MS | F | p | MS | F | p | ||
| Urbanization = Ur | 1 | 2979.8 | 1.01 | 0.35 | 22,393.4 | 7.46 | 0.03 | 1755.6 | 44.68 | 0.001 |
| Shore(Ur) = Sh(Ur) | 6 | 2952.8 | 17.16 | 0.000 | 3000.4 | 20.14 | 0.000 | 39.3 | 0.36 | 0.9 |
| Residual | 152 | 172.0 | 149 | 109.4 | ||||||
| Total | 156 | |||||||||
| Transform | ArcSin (%) | none | none | |||||||
| Cochran’s Test | C = 0.2075 | n.s. | C = 0.2487 | n.s. | C = 0.5454 | s. | ||||
| Source of Variation | df | S. muticum (Biomass) | ||
|---|---|---|---|---|
| MS | F | p | ||
| Urbanization = Ur | 1 | 141.0 | 0.06 | 0.81 |
| Shore(Ur) = Sh(Ur) | 6 | 2177.2 | 3.82 | 0.002 |
| Residual | 72 | 569.9 | ||
| Total | 79 | |||
| Transform | none | |||
| Cochran’s Test | C = 0.2919 | ns | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.R.; Torres, C.A.; Veiga, P. Low Diversity of Intertidal Canopy-Forming Macroalgae at Urbanized Areas along the North Portuguese Coast. Diversity 2020, 12, 211. https://doi.org/10.3390/d12060211
García MR, Torres CA, Veiga P. Low Diversity of Intertidal Canopy-Forming Macroalgae at Urbanized Areas along the North Portuguese Coast. Diversity. 2020; 12(6):211. https://doi.org/10.3390/d12060211
Chicago/Turabian StyleGarcía, Marcos Rubal, Catarina A. Torres, and Puri Veiga. 2020. "Low Diversity of Intertidal Canopy-Forming Macroalgae at Urbanized Areas along the North Portuguese Coast" Diversity 12, no. 6: 211. https://doi.org/10.3390/d12060211
APA StyleGarcía, M. R., Torres, C. A., & Veiga, P. (2020). Low Diversity of Intertidal Canopy-Forming Macroalgae at Urbanized Areas along the North Portuguese Coast. Diversity, 12(6), 211. https://doi.org/10.3390/d12060211






