Same Diet, Different Strategies: Variability of Individual Feeding Habits across Three Populations of Ambrosi’s Cave Salamander (Hydromantes ambrosii)
Abstract
1. Introduction
2. Methods
2.1. Study Species and Data
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wake, D.B. The enigmatic history of the European, Asian and American plethodontid salamanders. Amphib. Reptil. 2013, 34, 323–336. [Google Scholar] [CrossRef]
- Lanza, B.; Pastorelli, C.; Laghi, P.; Cimmaruta, R. A review of systematics, taxonomy, genetics, biogeography and natural history of the genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae). Atti. Mus. Civ. Stor. Nat. Trieste 2006, 52, 5–135. [Google Scholar]
- Chiari, Y.; van der Meijden, A.; Mucedda, M.; Lourenço, J.M.; Hochkirch, A.; Veith, M. Phylogeography of Sardinian cave salamanders (genus Hydromantes) is mainly determined by geomorphology. PLoS ONE 2012, 7, e32332. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Lunghi, E.; Cimmaruta, R.; Manenti, R. Transgressive niche across a salamander hybrid zone revealed by microhabitat analyses. J. Biogeogr. 2019, 46, 1342–1354. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Lunghi, E.; Canedoli, C.; Padoa-Schioppa, E.; Pennati, R.; Manenti, R. Differences between microhabitat and broad-scale patterns of niche evolution in terrestrial salamanders. Sci. Rep. 2018, 8, 10575. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.C.; Jaeger, R.G.; Houck, L.D. (Eds.) The Biology of Plethodontid Salamanders; Springer Science+Business Media, LLC: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Spotila, J.R. Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 1972, 42, 95–125. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. (Eds.) The Biology of Caves and Other Subterranean Habitats, 2nd ed.; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Seasonal variation in microhabitat of salamanders: Environmental variation or shift of habitat selection? PeerJ 2015, 3, e1122. [Google Scholar] [CrossRef]
- Salvidio, S.; Palumbi, G.; Romano, A.; Costa, A. Safe caves and dangerous forests? Predation risk may contribute to salamander colonization of subterranean habitats. Sci. Nat. 2017, 104, 20. [Google Scholar] [CrossRef]
- Pastorelli, C.; Laghi, P. Predation of Speleomantes italicus (Amphibia: Caudata: Plethodontidae) by Meta menardi (Arachnida: Araneae: Metidae). In Atti del 6° Congresso Nazionale della Societas Herpetologica Italica (Roma, 27.IX-1.X.2006); Museo Regionale di Scienze Naturali: Torino, Italy, 2006; pp. 45–48. [Google Scholar]
- Manenti, R.; Lunghi, E.; Canedoli, C.; Bonaccorsi, M.; Ficetola, G.F. Parasitism of the leech, Batracobdella algira (Moquin-Tandon, 1846), on Sardinian cave salamanders (genus Hydromantes) (Caudata: Plethodontidae). Herpetozoa 2016, 29, 27–35. [Google Scholar]
- Lunghi, E.; Corti, C.; Manenti, R.; Barzaghi, B.; Buschettu, S.; Canedoli, C.; Cogoni, R.; De Falco, G.; Fais, F.; Manca, A.; et al. Comparative reproductive biology of European cave salamanders (genus Hydromantes): Nesting selection and multiple annual breeding. Salamandra 2018, 54, 101–108. [Google Scholar]
- Deban, S.M.; Dicke, U. Motor control of tongue movement during prey capture in Plethodontid salamanders. J. Exp. Biol. 1999, 202, 3699–3714. [Google Scholar] [PubMed]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Mulargia, M.; Cogoni, R.; Barzaghi, B.; Cornago, L.; Avitabile, D.; Veith, M.; Manenti, R.; et al. Field-recorded data on the diet of six species of European Hydromantes cave salamanders. Sci. Data 2018, 5, 180083. [Google Scholar] [CrossRef] [PubMed]
- Deban, S.M.; Dicke, U. Activation patterns of the tongue-projector muscle during feeding in the imperial cave salamander Hydromantes imperialis. J. Exp. Biol. 2004, 207, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Deban, S.M.; Richardson, J.C. Cold-Blooded snipers: Thermal independence of ballistic tongue projection in the salamander Hydromantes platycephalus. J. Exp. Zool. 2011, 315, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, L.; Caldera, F.; Bologna, M.A. Trophic niche of cave populations of Speleomantes italicus. J. Nat. Hist. 2006, 40, 1841–1850. [Google Scholar] [CrossRef]
- Salvidio, S.; Romano, A.; Oneto, F.; Ottonello, D.; Michelon, R. Different season, different strategies: Feeding ecology of two syntopic forest-dwelling salamanders. Acta Oecol. 2012, 43, 42–50. [Google Scholar]
- Salvidio, S. Diet and food utilization in the European plethodontid Speleomantes ambrosii. Vie et Milieu 1992, 42, 35–39. [Google Scholar]
- Lunghi, E.; Manenti, R.; Mulargia, M.; Veith, M.; Corti, C.; Ficetola, G.F. Environmental suitability models predict population density, performance and body condition for microendemic salamanders. Sci. Rep. 2018, 8, 7527. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Sendra, A.; Garay, P.; Reboleira, A.S.P.S. Energy and speleogenesis: Key determinants of terrestrial species richness in caves. Ecol. Evol. 2017, 7, 10207–10215. [Google Scholar] [CrossRef]
- Crump, M.L. Intra-population variability in energy parameters of the salamander Plethodon cinereus. Oecologia 1979, 38, 235–247. [Google Scholar] [CrossRef]
- Marvin, G.A.; Bryan, R.; Hardwick, J. Effect of chronic low body temperature on feeding and gut passage in a plethodontid salamander. J. Therm. Biol. 2017, 69, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Manenti, R.; Mancinelli, G.; Corti, C.; Ficetola, G.F. What shapes the trophic niche of European plethodontid salamanders? PLoS ONE 2018, 13, e0205672. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Yang, L.H.; Fordyce, J.A.; Davis, J.M.; Svanbäck, R. Measuring individual-level resource specialization. Ecology 2002, 83, 2936–2941. [Google Scholar] [CrossRef]
- Roughgarden, J. Evolution of niche width. Am. Nat. 1972, 106, 683–718. [Google Scholar] [CrossRef]
- Roughgarden, J. Niche width: Biogeographic patterns among Anolis lizard populations. Am. Nat. 1974, 108, 429–442. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araújo, C.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Layman, C.A.; Newsome, S.D.; Crawford, T.G. Individual-level niche specialization within populations: Emerging areas of study. Oecologia 2015, 178, 1–4. [Google Scholar] [CrossRef]
- Svanbäck, R.; Bolnick, D.I. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. B 2007, 274, 839–844. [Google Scholar] [CrossRef]
- Araújo, M.S.; Bolnick, D.I.; Martinelli, L.A.; Giaretta, A.A.; dos Reis, S.F. Individual-level diet variation in four species of Brazilian frogs. J. Anim. Ecol. 2009, 78, 848–856. [Google Scholar] [CrossRef]
- Terraube, J.; Guixé, D.; Arroyo, B. Diet composition and foraging success in generalist predators: Are specialist individuals better foragers? Basic Appl. Ecol. 2014, 15, 616–624. [Google Scholar] [CrossRef]
- Salvidio, S.; Oneto, F.; Ottonello, D.; Costa, A.; Romano, A. Trophic specialization at the individual level in a terrestrial generalist salamander. Can. J. Zool. 2015, 93, 79–83. [Google Scholar] [CrossRef]
- Salvidio, S.; Pasmans, F.; Bogaerts, S.; Martel, A.; van de Loo, M.; Romano, A. Consistency in trophic strategies between populations of the Sardinian endemic salamander Speleomantes imperialis. Anim. Biol. 2017, 67, 1–16. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Corti, C.; Ficetola, G.F.; Mancinelli, G. Inter-specific and inter-population variation in individual diet specialization: Do environmental factors have a role? Ecology 2020. [Google Scholar] [CrossRef]
- Araújo, M.S.; Bolnick, D.L.; Layman, C.A. The ecological causes of individual specialisation. Ecol. Lett. 2011, 14, 948–958. [Google Scholar] [CrossRef]
- Araujo, M.S.; dos Reis, S.F.; Giaretta, A.A.; Machado, G.; Bolnick, D.I. Intrapopulation diet variation in four frogs (Leptodactylidae) of the Brazilian savannah. Copeia 2007, 2007, 855–865. [Google Scholar] [CrossRef]
- Pyke, G.H.; Pullian, H.R.; Charnov, E.L. Optimal foraging: A selective review of theory and tests. Quart. Rev. Biol. 1977, 52, 137–154. [Google Scholar] [CrossRef]
- Quiroga, M.F.; Bonansea, M.I.; Vaira, M. Population diet variation and individual specialization in the poison toad, Melanophryniscus rubriventris (Vellard, 1947). Amphib. Reptil. 2011, 32, 261–265. [Google Scholar] [CrossRef]
- Rosenblatt, A.E.; Nifong, J.C.; Heithaus, M.R.; Mazzotti, F.J.; Cherkiss, M.S.; Jeffery, B.M.; Elsey, R.M.; Decker, R.A.; Silliman, B.R.; Guillette, L.G.J.; et al. Factors affecting individual foraging specialization and temporal diet stability across the range of a large “generalist” apex predator. Oecologia 2015, 178, 5–16. [Google Scholar] [CrossRef]
- Lunghi, E.; Bruni, G. Long-term reliability of Visual Implant Elastomers in the Italian cave salamander (Hydromantes italicus). Salamandra 2018, 54, 283–286. [Google Scholar]
- Salvidio, S. Homing behaviour in Speleomantes strinatii (Amphibia Plethodontidae): A preliminary displacement experiment. North West. J. Zool. 2013, 9, 429–433. [Google Scholar]
- Smith, M.A.; Green, D.M. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations? Ecography 2005, 28, 110–128. [Google Scholar] [CrossRef]
- Lunghi, E.; Corti, C.; Manenti, R.; Ficetola, G.F. Consider species specialism when publishing datasets. Nat. Ecol. Evol. 2019, 3, 319. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Tinker, M.T. Timescales alter the inferred strength and temporal consistency of intraspecific diet specialization. Oecologia 2015, 178, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Barzaghi, B.; Melotto, A.; Muraro, M.; Lunghi, E.; Canedoli, C.; Lo Parrino, E.; Nanni, V.; Silva-Rocha, I.; Urso, A.; et al. N-mixture models reliably estimate the abundance of small vertebrates. Sci. Rep. 2018, 8, 10357. [Google Scholar] [CrossRef]
- Băncilă, R.I.; Hartel, T.; Plăiaşu, R.; Smets, J. Cogălniceanu, D. Comparing three body condition indices in amphibians: A case study of yellow-bellied toad Bombina variegata. Amphib. Reptil. 2010, 31, 558–562. [Google Scholar] [CrossRef]
- Labocha, M.K.; Schutz, H.; Hayes, J.P. Which body condition index is best? Oikos 2014, 123, 111–119. [Google Scholar] [CrossRef]
- Scott, D.E.; Casey, E.D.; Donovan, M.F.; Lynch, T.K. Amphibian lipid levels at metamorphosis correlate to post-metamorphic terrestrial survival. Oecologia 2007, 153, 521–532. [Google Scholar] [CrossRef]
- Roughgarden, J. Theory of Population Genetics and Evolutionary Ecology; Macmillan Publishing Company: New York, NY, USA, 1979; p. 612. [Google Scholar]
- Zaccarelli, N.; Bolnick, D.I.; Mancinelli, G. RInSp: An R package for the analysis of individual specialization in resource use. Methods Ecol. Evol. 2013, 4, 1018–1023. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 18 September 2019).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. Available online: http://cran.r-project.org/web/packages/vegan (accessed on 28 January 2020).
- Dray, S.; Dufour, A.B. ade4: Analysis of Ecological Data - Exploratory and Euclidean Methods in Environmental Sciences. Available online: https://CRAN.R-project.org/package=ade4 (accessed on 28 January 2020).
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef]
- Kirk, D.A.; Gosler, A.G. Body condition varies with migration and competition in migrant and resident south American vultures. Auk 1994, 111, 933–944. [Google Scholar] [CrossRef]
- Petren, K.; Case, T.J. Habitat structure determines competition intensity and invasion success in gecko lizards. Proc. Natl. Acad. Sci. USA 1998, 95, 11739–11744. [Google Scholar] [CrossRef] [PubMed]
- Baur, B.; Cremene, C.; Groza, G.; Schileyko, A.A.; Baur, A.; Erhardt, A. Intensified grazing affects endemic plant and gastropod diversity in alpine grasslands of the Southern Carpathian mountains (Romania). Biologia 2007, 62, 438–445. [Google Scholar] [CrossRef]
- Norbury, G.; Heyward, R.; Parkes, J. Skink and invertebrate abundance in relation to vegetation, rabbits and predators in a New Zealand dryland ecosystem. N. Z. J. Ecol. 2009, 33, 24–31. [Google Scholar]
- Tinker, M.T.; Bentall, G.; Estes, J.A. Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc. Natl. Acad. Sci. USA 2008, 105, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.S.; Costa-Pereira, R. Latitudinal gradients in intraspecific ecological diversity. Biol. Lett. 2013, 9, 20130778. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Lu, C.-W.; Chu, J.-H.; Grismer, L.L.; Hung, C.-M.; Lin, S.-M. Historical demography of four gecko species specializing in boulder cave habitat – its implications in the evolutionary dead end hypothesis and conservation. Mol. Ecol. 2019, 28, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Salvidio, S.; Pastorino, M.V. Spatial segregation in the European plethodontid Speleomantes strinatii in relation to age and sex. Amphib. Reptil. 2002, 23, 505–510. [Google Scholar]
- Ficetola, G.F.; Pennati, R.; Manenti, R. Spatial segregation among age classes in cave salamanders: Habitat selection or social interactions? Pop. Ecol. 2013, 55, 217–226. [Google Scholar] [CrossRef]
- Cejuela Tanalgo, K.; Tabora, J.A.G.; Hughes, A.C. Bat cave vulnerability index (BCVI): A holistic rapid assessment tool to identify priorities for effective cave conservation in the tropics. Ecol. Indic. 2018, 89, 852–860. [Google Scholar] [CrossRef]
- Whitten, T. Applying ecology for cave management in China and neighbouring countries. J. Appl. Ecol. 2009, 46, 520–523. [Google Scholar] [CrossRef]
- Cloyed, C.S.; Eason, P.K. Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. R. Soc. Open Sci. 2017, 4, 170060. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pereira, R.; Rudolf, V.H.W.; Souza, F.L.; Araújo, M.S. Drivers of individual niche variation in coexisting species. J. Anim. Ecol. 2018, 87, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
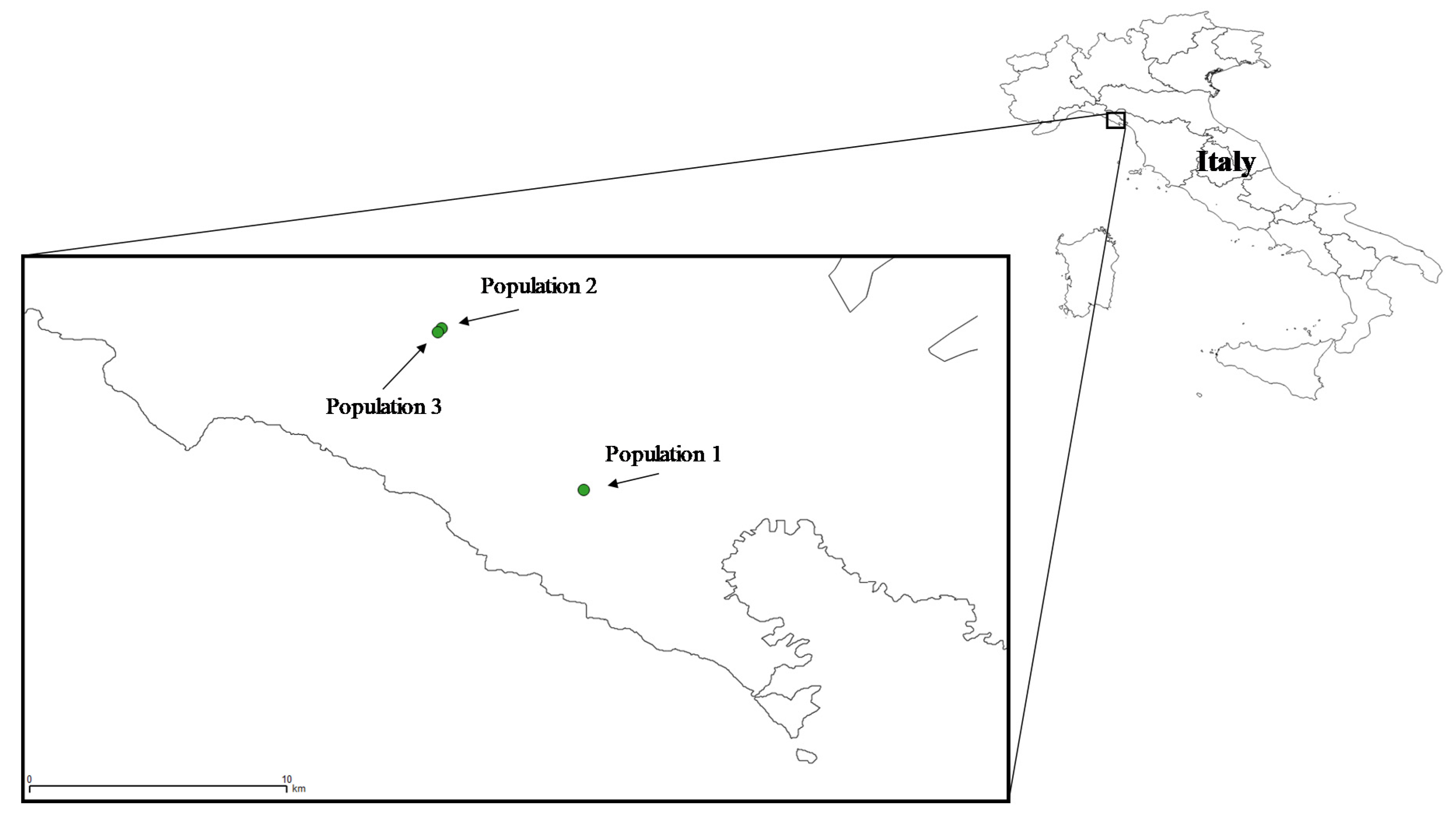
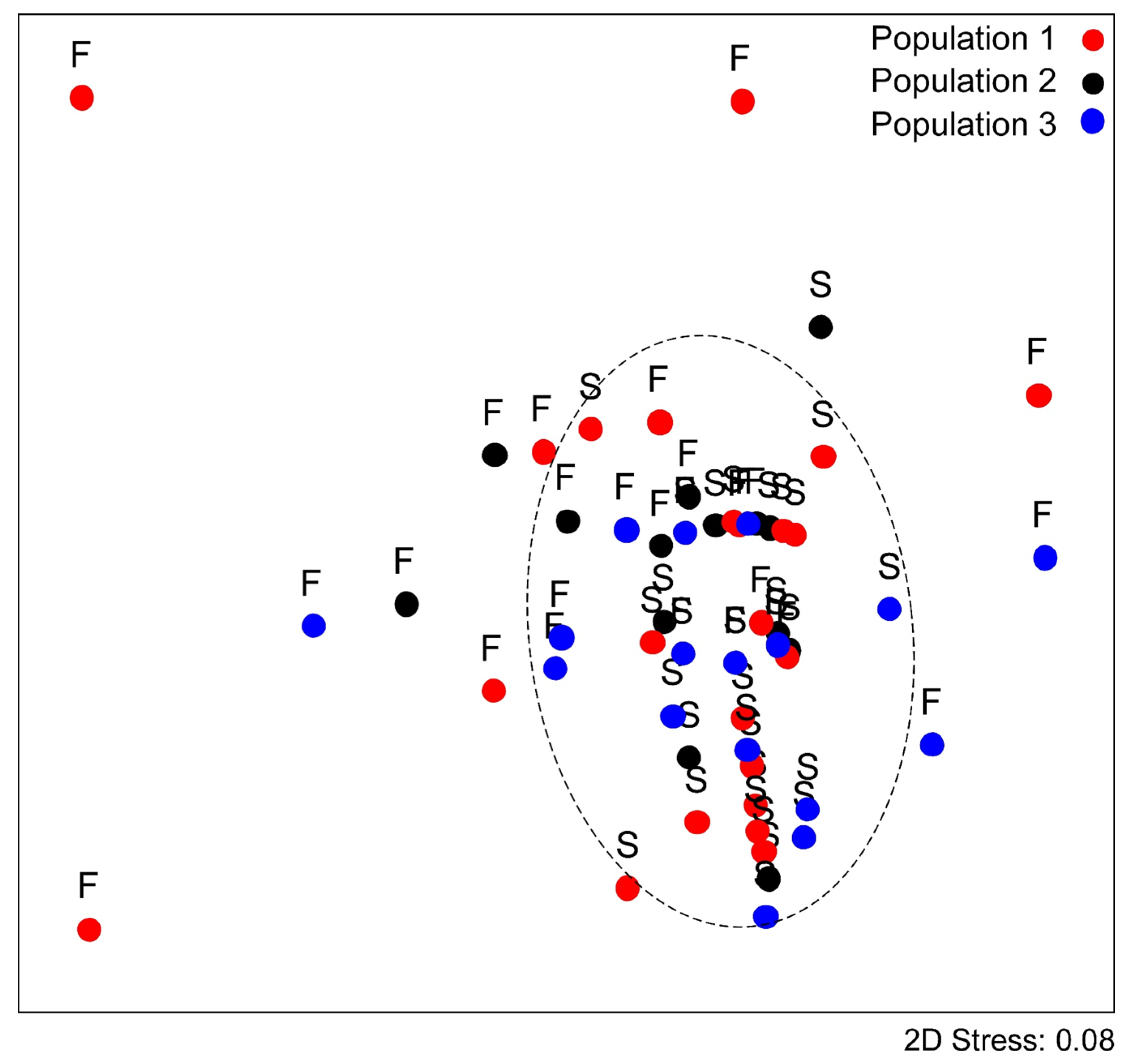
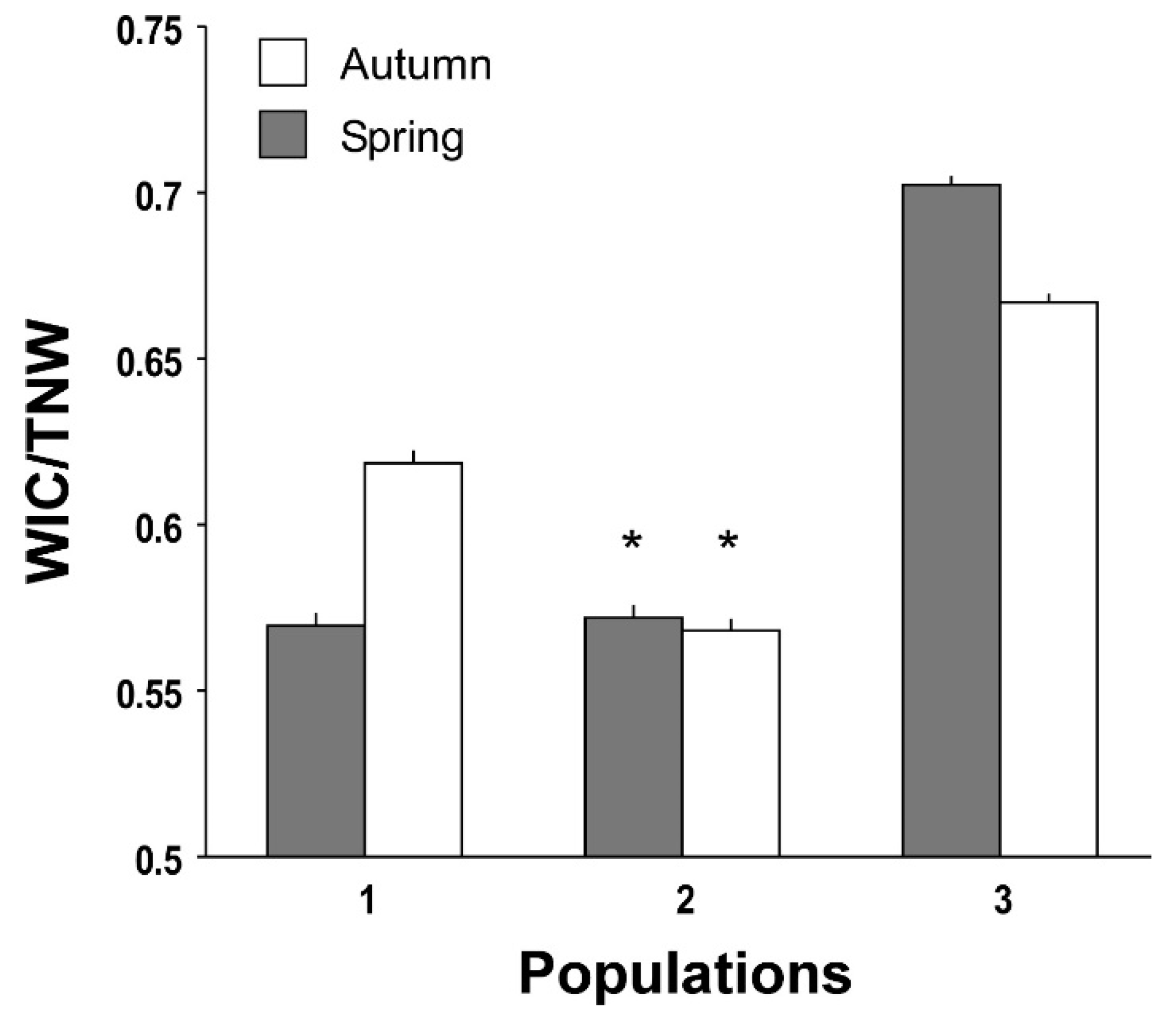
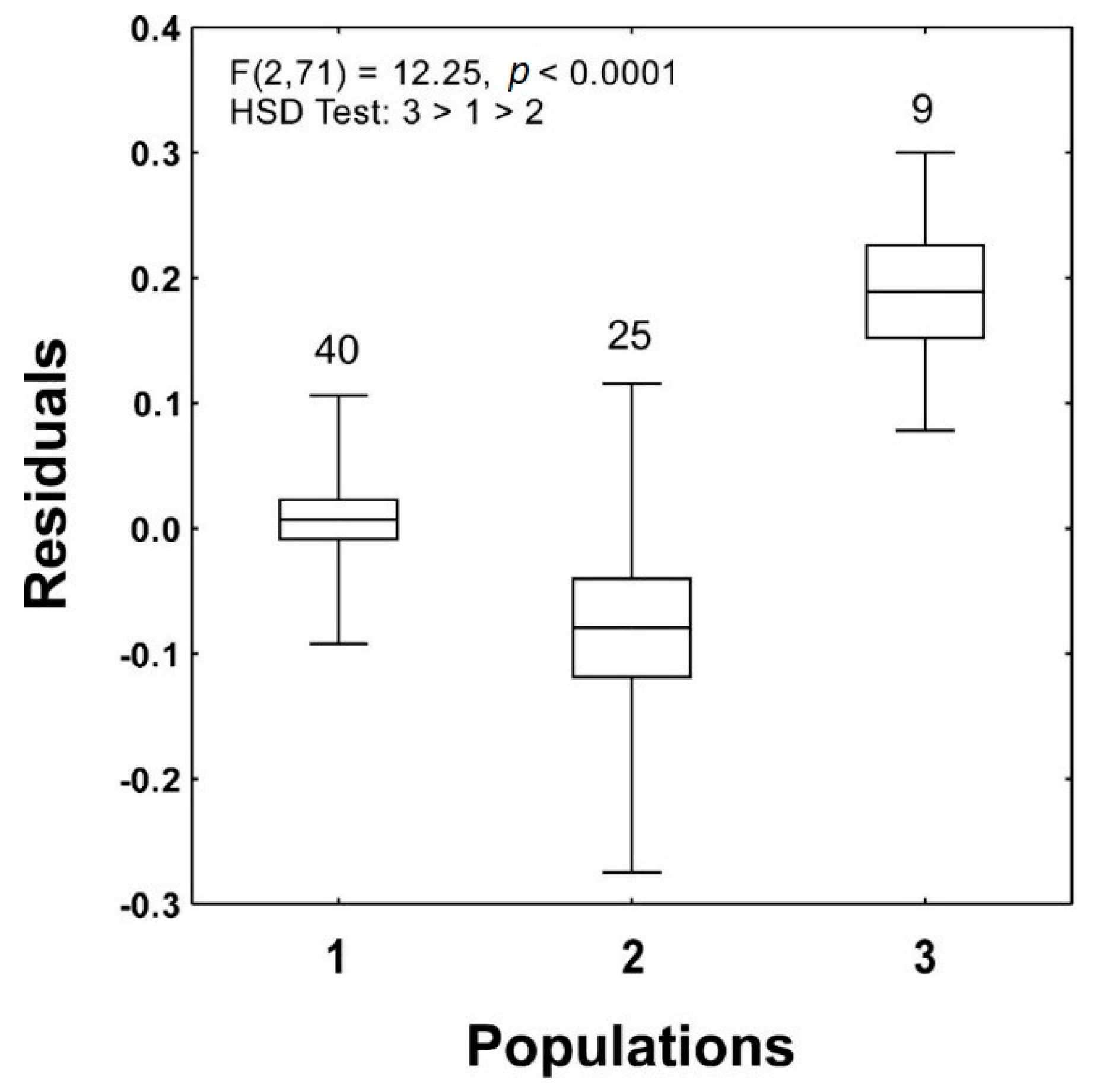
| Population 1 | Population 2 | Population 3 | F2,3 | HSD | |
|---|---|---|---|---|---|
| Longitude | 9.77 | 9.72 | 9.72 | ||
| Latitude | 44.12 | 44.18 | 44.17 | ||
| Elevation | 331 | 206 | 260 | ||
| Arboreal vegetation | 55.3 (3.6) | 53.2 (3.5) | 45.75 (1.85) | 2.63 | 1 = 2 = 3 |
| Non-arboreal vegetation | 38 (0.4) | 34.1 (2.6) | 47.1 (1.4) | 14.58 * | 1 = 2 < 3 |
| Factor | All Salamanders | Adult Only | ||
|---|---|---|---|---|
| Pseudo-F | p(MC) | Pseudo-F | p(MC) | |
| (1) SVL | 0.91 | 0.454 | 0.54 | 0.781 |
| (2) Population | 1.23 | 0.246 | 0.99 | 0.444 |
| (3) Age/Sex | 1.5 | 0.175 | 1.5 | 0.164 |
| (4) Season | 16.03 | 0.001 | 14.07 | 0.001 |
| (1) × (2) | 1.14 | 0.328 | 1.16 | 0.28 |
| (1) × (3) | 1.64 | 0.124 | 0.29 | 0.976 |
| (1) × (4) | 0.77 | 0.570 | 1.14 | 0.308 |
| (2) × (3) | 0.44 | 0.981 | 0.70 | 0.755 |
| (2) × (3) | 0.67 | 0.788 | 0.75 | 0.702 |
| (3) × (4) | 1.25 | 0.257 | 1.92 | 0.077 |
| (1) × (2) × (3) | 1.25 | 0.258 | 0.95 | 0.469 |
| (1) × (2) × (4) | 1.65 | 0.067 | 1.45 | 0.142 |
| (1) × (3) × (4) | 0.48 | 0.846 | 2.02 | 0.045 |
| (2) × (3) × (4) | nd | - | 1.82 | 0.19 |
| (1) × (2) × (3) × (4) | nd | - | nd | - |
| Season | Population 1 | Population 2 | Population 3 | |
|---|---|---|---|---|
| Fall | [3 F, 6 J] | [12 F, 4 M] | [4 F, 4 M, 2 J] | |
| WIC | 1.394 (1.38–1.408) | 1.482 (1.471–1.493) | 1.729 (1.718–1.741) | |
| BIC | 0.859 (0.853–0.866) | 1.126 (1.118–1.135) | 0.864 (0.858–0.87) | |
| TNW | 2.253 (2.24–2.267) | 2.608 (2.598–2.618) | 2.594 (2.581–2.606) | |
| IS | 0.442 † | 0.340 † | 0.427 † | |
| Spring | [22 F, 14 M, 6 J] | [17 M, 15 M, 1 J] | [9 F, 5 M] | |
| WIC | 0.684 (0.681–0.686) | 0.603 (0.6–0.606) | 0.799 (0.795–0.802) | |
| BIC | 0.517 (0.509–0.524) | 0.451 (0.444–0.458) | 0.338 (0.334–0.343) | |
| TNW | 1.201 (1.192–1.209) | 1.054 (1.046–1.062) | 1.137 (1.129–1.145) | |
| IS | 0.728 † | 0.699 † | 0.738 † | |
| Density | 0.422 (144.5) | 0.144 (137.2) | 0.106 (67.1) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Zhao, Y.; Manenti, R.; Corti, C.; Ficetola, G.F.; Mancinelli, G. Same Diet, Different Strategies: Variability of Individual Feeding Habits across Three Populations of Ambrosi’s Cave Salamander (Hydromantes ambrosii). Diversity 2020, 12, 180. https://doi.org/10.3390/d12050180
Lunghi E, Cianferoni F, Ceccolini F, Zhao Y, Manenti R, Corti C, Ficetola GF, Mancinelli G. Same Diet, Different Strategies: Variability of Individual Feeding Habits across Three Populations of Ambrosi’s Cave Salamander (Hydromantes ambrosii). Diversity. 2020; 12(5):180. https://doi.org/10.3390/d12050180
Chicago/Turabian StyleLunghi, Enrico, Fabio Cianferoni, Filippo Ceccolini, Yahui Zhao, Raoul Manenti, Claudia Corti, Gentile Francesco Ficetola, and Giorgio Mancinelli. 2020. "Same Diet, Different Strategies: Variability of Individual Feeding Habits across Three Populations of Ambrosi’s Cave Salamander (Hydromantes ambrosii)" Diversity 12, no. 5: 180. https://doi.org/10.3390/d12050180
APA StyleLunghi, E., Cianferoni, F., Ceccolini, F., Zhao, Y., Manenti, R., Corti, C., Ficetola, G. F., & Mancinelli, G. (2020). Same Diet, Different Strategies: Variability of Individual Feeding Habits across Three Populations of Ambrosi’s Cave Salamander (Hydromantes ambrosii). Diversity, 12(5), 180. https://doi.org/10.3390/d12050180











