Morphological Disparity of the Humerus in Modern Birds
Abstract
1. Introduction
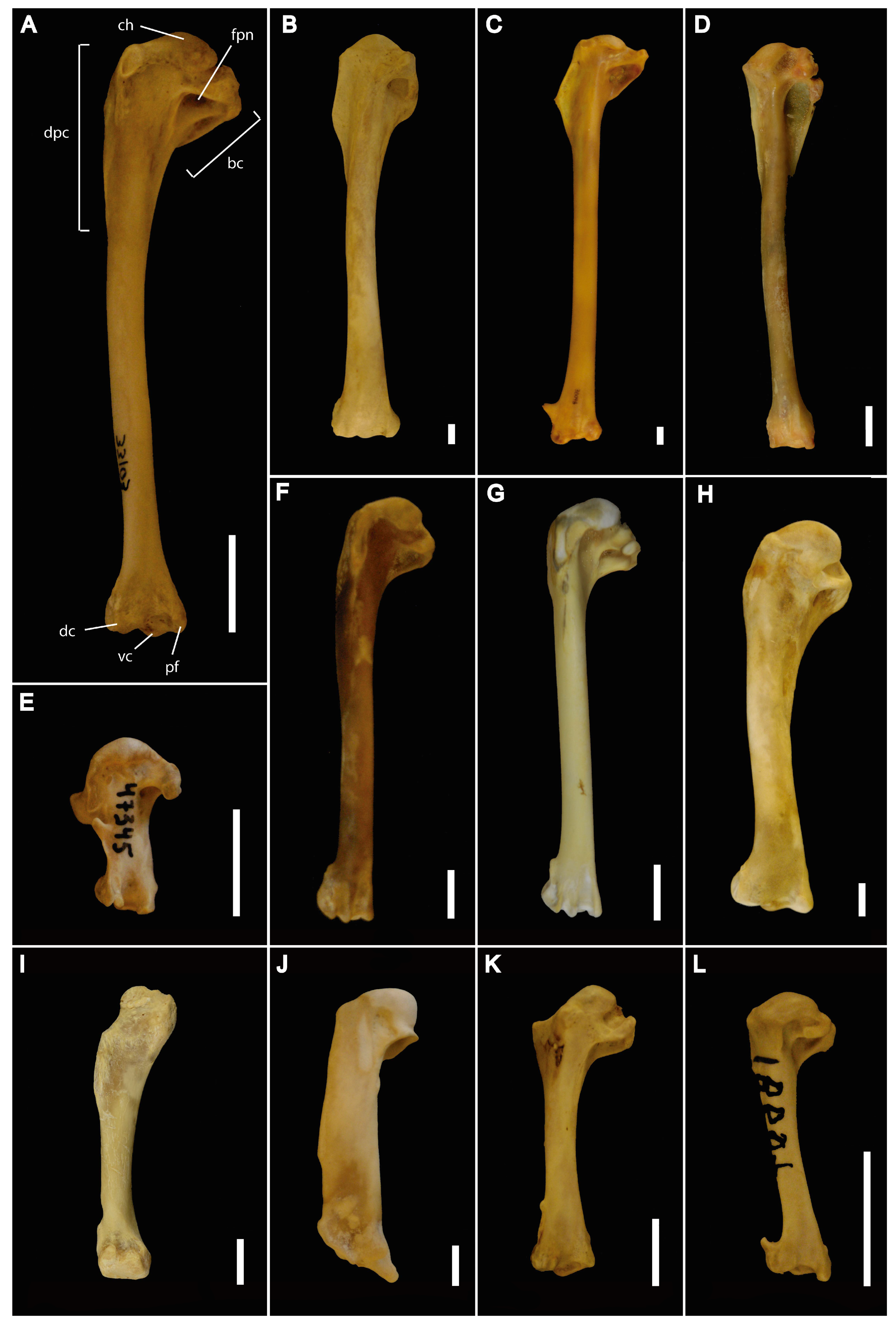
2. Material and Methods
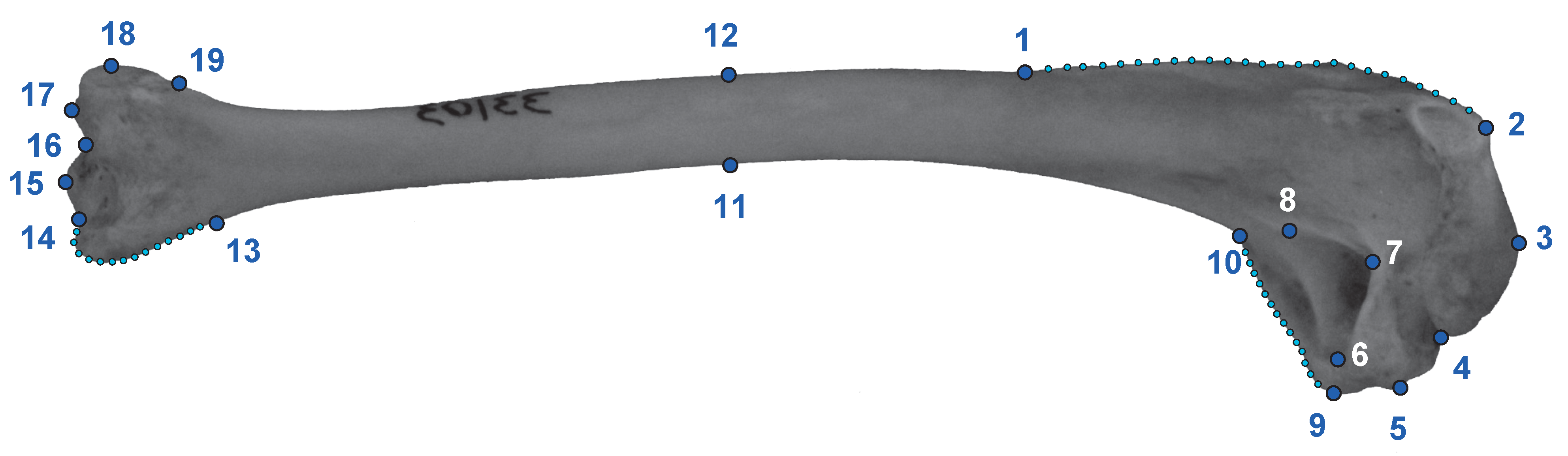
2.1. Data Acquisition
2.2. Analyses
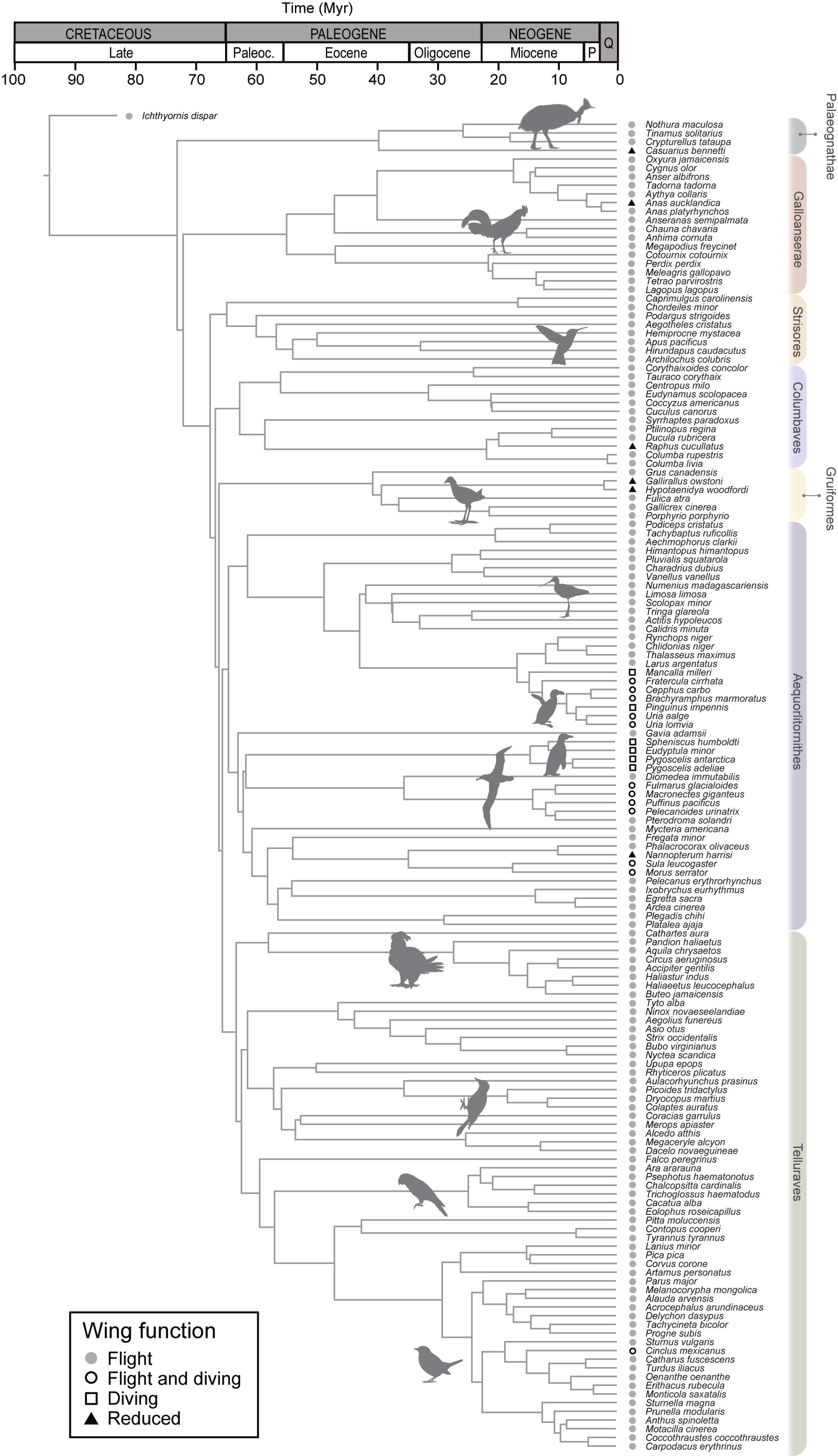
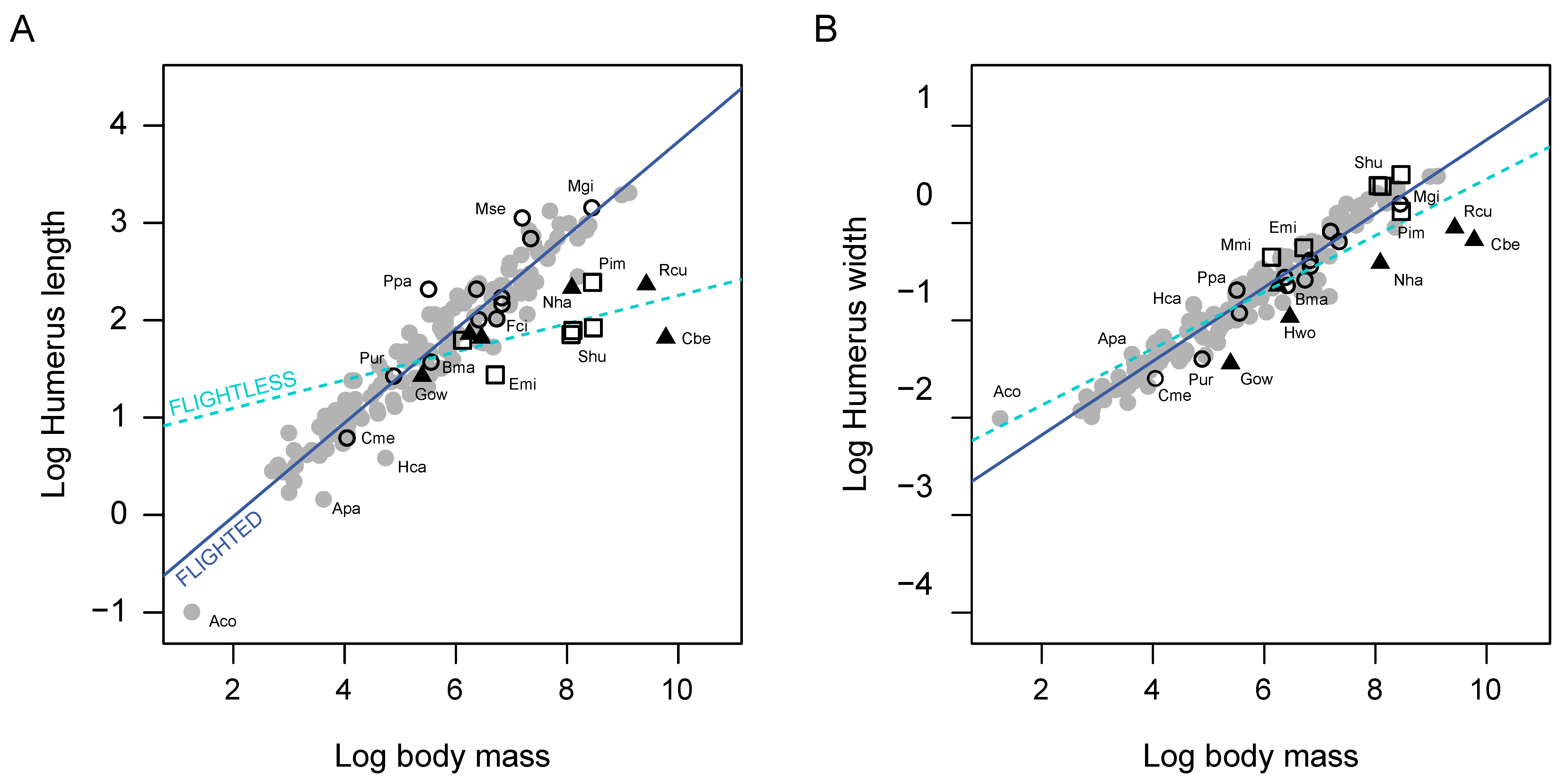
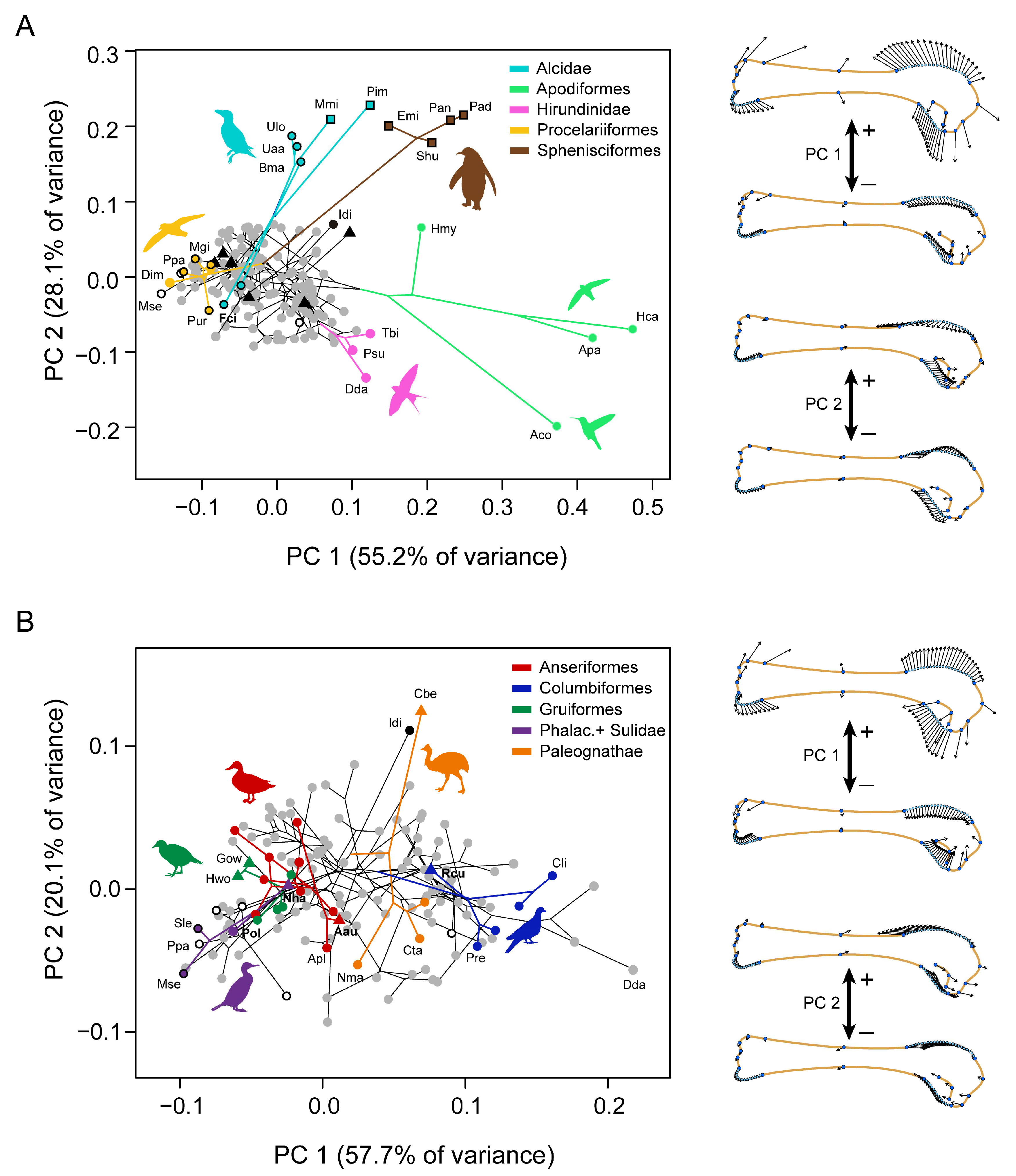
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barrowclough, G.F.; Cracraft, J.; Klicka, J.; Zink, R.M. How many kinds of birds are there and why does it matter? PLoS ONE 2016, 11, e0166307. [Google Scholar] [CrossRef] [PubMed]
- Norberg, U.L. Flight and scaling of flyers in nature. Flow Phenom. Nat. 2007, 1, 120–154. [Google Scholar]
- Butler, P.J. The physiological basis of bird flight. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150384. [Google Scholar] [CrossRef] [PubMed]
- Tobalske, B.W. Avian Flight. In Handbook of Bird Biology, 3rd ed.; Lovette, I.J., Fitzpatrick, J.W., Eds.; Wiley-Blackwell: The Cornell Lab of Ornithology: Ithaca, NY, USA, 2016; pp. 149–169. [Google Scholar]
- Pennycuick, C.J. Modelling the Flying Bird, 1st ed.; AP Theoretical Ecology Series; Academic Press: Oxford, UK, 2008; Volume 5, p. 496. [Google Scholar]
- Habib, M. The structural mechanics and evolution of aquaflying birds. Biol. J. Linn. Soc. 2010, 99, 687–698. [Google Scholar] [CrossRef]
- Smith, N.A.; Clarke, J.A. Osteological histology of the Pan-Alcidae (Aves, Charadriiformes): Correlates of wing-propelled diving and flightlessness. Anat. Rec. 2014, 297, 188–199. [Google Scholar] [CrossRef]
- De Margerie, E.; Sanchez, S.; Cubo, J.; Castanet, J. Torsional resistance as a principal component of the structural design of long bones: Comparative multivariate evidence in birds. Anat. Record Part A 2005, 282, 49–66. [Google Scholar]
- Nudds, R.L.; Dyke, G.J.; Rayner, J.M.V. Avian brachial index and wing kinematics: Putting movement back into bones. J. Zool. 2007, 272, 218–226. [Google Scholar] [CrossRef]
- Habib, M.B.; Ruff, C. The effects of locomotion on the structural characteristics of avian limb bones. Zool. J. Linn. Soc. 2008, 153, 601–624. [Google Scholar] [CrossRef]
- Simons, E.L.; Hieronymus, T.L.; O’Connor, P.M. Cross sectional geometry of the forelimb skeleton and flight mode in pelecaniform birds. J. Morphol. 2011, 272, 958–971. [Google Scholar] [CrossRef]
- Raikow, R.J. Locomotor system. In Form and Function in Birds; King, A.S., McLellan, J., Eds.; Academic Press: Cambridge, MA, USA, 1985; Volume 3, pp. 57–147. [Google Scholar]
- Baumel, J.J.; King, A.S.; Breazile, J.; Evans, H.; Vanden Berge, J.C. Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd ed.; Harvard Univ Nuttall Ornithological Club: Cambridge, MA, USA, 1993; p. 779. [Google Scholar]
- Bright, J.A.; Marugán-Lobón, J.; Cobb, S.N.; Rayfield, E.J. The shapes of bird beaks are highly controlled by nondietary factors. Proc. Natl. Acad. Sci. USA 2016, 113, 5352–5357. [Google Scholar] [CrossRef]
- Marugán-Lobón, J.; Watanabe, A.; Kawabe, S. Studying avian encephalization with geometric morphometrics. J. Anat. 2016, 229, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Cooney, C.R.; Bright, J.A.; Capp, E.J.; Chira, A.M.; Hughes, E.C.; Moody, C.J.; Nouri, L.O.; Varley, Z.K.; Thomas, G.H. Mega-evolutionary dynamics of the adaptive radiation of birds. Nature 2017, 542, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Felice, R.N.; Goswami, A. Developmental origins of mosaic evolution in the avian cranium. Proc. Natl. Acad. Sci. USA 2018, 115, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Navalón, G.; Bright, J.A.; Marugán-Lobón, J.; Rayfield, E.J. The evolutionary relationship among beak shape, mechanical advantage, and feeding ecology in modern birds. Evolution 2019, 73, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Navalón, G.; Marugán-Lobón, J.; Bright, J.A.; Cooney, C.R.; Rayfield, E.J. The consequences of craniofacial integration for the adaptive radiations of Darwin’s finches and Hawaiian honeycreepers. Nat. Ecol. Evol. 2020, 4, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Shatkovska, O.V.; Ghazali, M. Integration of skeletal traits in some passerines: Impact (or the lack thereof) of body mass, phylogeny, diet and habitat. J. Anat. 2020, 236, 274–287. [Google Scholar] [CrossRef]
- Close, R.A.; Rayfield, E.J. Functional morphometric analysis of the furcula in Mesozoic birds. PLoS ONE 2012, 7, e36664. [Google Scholar] [CrossRef]
- Wang, X.; Clarke, J.A. The evolution of avian wing shape and previously unrecognized trends in covert feathering. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151935. [Google Scholar] [CrossRef]
- Watanabe, J. Clade-specific evolutionary diversification along ontogenetic major axes in avian limb skeleton. Evolution 2018, 72, 2632–2652. [Google Scholar] [CrossRef]
- Nebreda, S.M.; Navalón, G.; Menéndez, I.; Sigurdsen, T.; Chiappe, L.M.; Marugán-Lobón, J. Disparity and macroevolutionary transformation of the maniraptoran manus. Bull. Am. Mus. Nat. Hist. in press.
- Navalón, G.; Meng, Q.; Marugán-Lobón, J.; Zhang, Y.; Wang, B.; Xing, H.; Liu, D.; Chiappe, L. Diversity and evolution of the Confuciusornithidae: Evidence from a new 131-million-year-old specimen from the Huajiying Formation in NE China. J. Asian Earth Sci. 2018, 152, 12–22. [Google Scholar] [CrossRef]
- Mayr, G. Avian Evolution: The Fossil Record of Birds and its Paleobiological Significance; John Wiley and Sons: Chichester, UK, 2017; p. 306. [Google Scholar]
- Olmos, M.; Casinos, A.; Cubo, J. Limb allometry in birds. Annales des Sciences Naturelles-Zoologie et Biologie Animale 1996, 17, 39–49. [Google Scholar]
- Cubo, J.; Casinos, A. Biomechanical significance of cross-sectional geometry of avian long bones. Eur. J. Morphol. 1998, 36, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nudds, R.L. Wing-bone length allometry in birds. J. Avian Biol. 2007, 38, 515–519. [Google Scholar] [CrossRef]
- Sullivan, T.N.; Meyers, M.A.; Arzt, E. Scaling of bird wings and feathers for efficient flight. Sci. Adv. 2019, 5, eaat4269. [Google Scholar] [CrossRef]
- Sievwright, H.; Macleod, N. Eigensurface analysis, ecology, and modelling of morphological adaptation in the falconiform humerus (Falconiformes: Aves). Zool. J. Linn. Soc. 2012, 165, 390–419. [Google Scholar] [CrossRef][Green Version]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix 2013, 24, 7. [Google Scholar]
- Prum, R.O.; Berv, J.S.; Dornburg, A.; Field, D.J.; Townsend, J.P.; Lemmon, E.M.; Lemmon, A.R. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 2015, 526, 569–573. [Google Scholar] [CrossRef]
- Marugán-Lobón, J.; Buscalioni, A.D. Geometric morphometrics in macroevolution: Morphological diversity of the skull in modern avian form in contrast to some theropods dinosaurs. In Morphometrics: Applications in Biology and Paleontology; Elewa, A., Ed.; Springer: New York, NY, USA, 2004; pp. 157–171. [Google Scholar]
- Hume, J.P.; Steel, L. Fight club: A unique weapon in the wing of the solitaire, Pezophaps solitaria (Aves: Columbidae), an extinct flightless bird from Rodrigues, Mascarene Islands. Biol. J. Linn. Soc. 2013, 110, 32–44. [Google Scholar] [CrossRef]
- Clarke, J.A. Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Apatornis (Avialae: Ornithurae). Bull. Am. Mus. Nat. Hist. 2004, 286, 1–179. [Google Scholar] [CrossRef]
- Porras-Múzquiz, H.G.; Chatterjee, S.; Lehman, T.M. The carinate bird Ichthyornis from the Upper Cretaceous of Mexico. Cretac. Res. 2014, 51, 148–152. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.5. 2011. Available online: http://mesquiteproject.org (accessed on 1 April 2020).
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Del Hoyo, J.; Elliott, A.; Sargatal, J.; Christie, D.A.; Kirwan, G. (Eds.) Handbook of the Birds of the World Alive; Lynx Edicions: Barcelona, Spain, 2020; Available online: http://www.hbw.com/ (accessed on 1 April 2020).
- Myers, P.; Espinosa, R.; Parr, C.S.; Jones, T.; Hammond, G.S.; Dewey, T.A. The Animal Diversity Web (online). 2020. Available online: https://animaldiversity.org (accessed on 1 April 2020).
- Serrano, F.J.; Palmqvist, P.; Sanz, J.L. Multivariate analysis of neognath skeletal measurements: Implications for body mass estimation in Mesozoic birds. Zool. J. Linn. Soc. 2015, 173, 929–955. [Google Scholar] [CrossRef]
- Smith, N.A. Evolution of body mass in the Pan-Alcidae (Aves, Charadriiformes): The effects of combining neontological and paleontological data. Paleobiology 2016, 42, 8–26. [Google Scholar] [CrossRef]
- Howard, H. A review of the extinct avian genus, Mancalla. Los Angel. Cty. Mus. Contrib. Sci. 1970, 203, 1–12. [Google Scholar]
- van Heteren, A.H.; van Dierendonck, R.C.; van Egmond, M.A.; Sjang, L.; Kreuning, J. Neither slim nor fat: Estimating the mass of the dodo (Raphus cucullatus, Aves, Columbiformes) based on the largest sample of dodo bones to date. PeerJ 2017, 5, e4110. [Google Scholar] [CrossRef]
- Rohlf, F.J. TPS Dig v.2.25; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2016; Available online: http://life.bio.sunysb.edu/morph/ (accessed on 1 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://cran.R-project.org (accessed on 1 April 2020).
- Adams, D.C.; Collyer, M.; Kaliontzopoulou, A.; Sherratt, E. Geomorph: Software for Geometric Morphometric Analyses. 2016. Available online: https://cran.r-project.org/web/packages/geomorph/index.html (accessed on 1 April 2020).
- Dryden, I.L.; Mardia, K. Statistical Analysis of Shape; Wiley: Chichester, UK, 1998; p. 347. [Google Scholar]
- Gunz, P.; Mitteroecker, P. Semilandmarks: A method for quantifying curves and surfaces. Hystrix 2013, 24, 103–109. [Google Scholar]
- Collyer, M.L.; Sekora, D.J.; Adams, D.C. A method for analysis of phenotypic change for phenotypes described by high-dimensional data. Heredity 2015, 115, 357–365. [Google Scholar] [CrossRef]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Maddison, W.P. Squared-change parsimony reconstructions of ancestral states for continuous-valued characters on a phylogenetic tree. Syst. Biol. 1991, 40, 304–314. [Google Scholar] [CrossRef]
- Kulemeyer, C.; Asbahr, K.; Gunz, P.; Frahnert, S.; Bairlein, F. Functional morphology and integration of corvid skulls–a 3D geometric morphometric approach. Front. Zool. 2009, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Cubo, J.; Casinos, A. Scaling of skeletal element mass in birds. Belg. J. Zool. 1994, 124, 127–137. [Google Scholar]
- Baier, D.B.; Gatesy, S.M.; Jenkins, F.A. A critical ligamentous mechanism in the evolution of avian flight. Nature 2007, 445, 307–310. [Google Scholar] [CrossRef]
- Tobalske, B.W.; Warrick, D.R.; Jackson, B.E.; Dial, K.P. Morphological and Behavioral Correlates of Flapping Flight. In Living Dinosaurs: The Evolutionary History of Modern Birds; Dyke, G., Kaiser, G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 257–281. [Google Scholar]
- Serrano, F.J.; Chiappe, L.M. Aerodynamic modelling of a Cretaceous bird reveals thermal soaring capabilities during early avian evolution. J. R. Soc. Interface 2017, 14, 20170182. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; Goslow, G.E. Analysis of vertebrate structure, 5th ed.; John Wiley & Sons: New York, NY, USA, 2001; p. 656. [Google Scholar]
- Alexander, R.M. Principles of Animal Locomotion; Princeton University Press: Princeton, NJ, USA, 2003; p. 384. [Google Scholar]
- Chai, P.; Millard, D. Flight and size constraints: Hovering performance of large hummingbirds under maximal loading. J. Exp. Biol. 1997, 200, 2757–2763. [Google Scholar]
- Videler, J.J. Avian Flight. In Oxford Ornithology Series; Birkhead, T.R., Ed.; Oxford University Press: Oxford, UK, 2006; p. 288. [Google Scholar]
- Karkhu, A.A. Morphological divergence within the order Apodiformes as revealed by the structure of the humerus. Natur. Hist. Mus. Los Angel. Co. Sci. Ser. 1992, 36, 379–384. [Google Scholar]
- Savile, D.B.O. Adaptive evolution in the avian wing. Evolution 1957, 11, 212–224. [Google Scholar] [CrossRef]
- Rayner, J.M. Form and function in avian flight. In Current Ornithology; Springer: Berlin/Heidelberg, Germany, 1988; pp. 1–66. [Google Scholar]
- Turner, A. Swallows and Martins (Hirundinidae). In Handbook of the Birds of the World Alive; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2020; Available online: https://www.hbw.com/node/52303 (accessed on 28 February 2020).
- Johansson, L.C.; Aldrin, B.S.W. Kinematics of diving Atlantic puffins (Fratercula arctica L.): Evidence for an active upstroke. J. Exp. Biol. 2002, 205, 371–378. [Google Scholar]
- Mayr, G. Tertiary plotopterids (Aves, Plotopteridae) and a novel hypothesis on the phylogenetic relationships of penguins (Spheniscidae). J. Zool. Syst. 2005, 43, 67–71. [Google Scholar] [CrossRef]
- Raikow, R.J.; Bicanovsky, L.; Bledsoe, A.H. Forelimb joint mobility and the evolution of wing-propelled diving in birds. Auk 1988, 105, 446–451. [Google Scholar] [CrossRef]
- Louw, G.J. Functional anatomy of the penguin flipper. J. S. Afr. Vet. Assoc. 1992, 63, 113–120. [Google Scholar]
- Lovvorn, J.R.; Liggins, G.A. Interactions of body shape, body size and stroke-acceleration patterns in costs of underwater swimming by birds. Funct. Ecol. 2002, 16, 106–112. [Google Scholar] [CrossRef]
- Livezey, B.C. Morphometrics of flightlessness in the Alcidae. Auk 1988, 105, 681–698. [Google Scholar] [CrossRef]
- Smith, N.A. Taxonomic revision and phylogenetic analysis of the flightless Mancallinae (Aves, Pan-Alcidae). ZooKeys 2011, 91, 1–116. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.H.; Ricklefs, R.E.; Gaston, A.J.; Hatch, S.A.; Speakman, J.R.; Davoren, G.K. High flight costs, but low dive costs, in auks support the biomechanical hypothesis for flightlessness in penguins. Proc. Natl. Acad. Sci. USA 2013, 110, 9380–9384. [Google Scholar] [CrossRef] [PubMed]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Wood, J.R.; Scofield, R.P.; Llamas, B.; Cooper, A. Ancient mitochondrial genome reveals unsuspected taxonomic affinity of the extinct Chatham duck (Pachyanas chathamica) and resolves divergence times for New Zealand and sub-Antarctic brown teals. Mol. Phylogenet. Evol. 2014, 70, 420–428. [Google Scholar] [CrossRef]
- Livezey, B.C. Flightlessness in the Galápagos cormorant (Compsohalieus [Nannopterum] harrisi): Heterochrony, giantism and specialization. Zool. J. Linn. Soc. 1992, 105, 155–224. [Google Scholar] [CrossRef]
- Wilson, R.P.; Hustler, K.; Ryan, P.G.; Burger, A.E.; Noldeke, E.C. Diving birds in cold water: Do Archimedes and Boyle determine energetic costs? Am. Nat. 1992, 140, 179–200. [Google Scholar] [CrossRef]
- Halsey, L.G.; Blackburn, T.M.; Butler, P.J. A comparative analysis of the diving behaviour of birds and mammals. Funct. Ecol. 2006, 20, 889–899. [Google Scholar] [CrossRef]
- Wilson, R.P.; Vargas, F.H.; Steinfurth, A.; Riordan, P.; Ropert-Coudert, Y.; Macdonald, D.W. What grounds some birds for life? Movement and diving in the sexually dimorphic Galapagos cormorant. Ecol. Monogr. 2008, 78, 633–652. [Google Scholar] [CrossRef]

| Variable | Linear Model | adj R2 | p-value | slope |
| CS | all birds | 0.811 | <0.001 | 0.423 |
| flighted | 0.907 | <0.001 | 0.484 | |
| flightless | 0.329 | 0.03 | 0.138 | |
| HL | all birds | 0.824 | <0.001 | 0.423 |
| flighted | 0.915 | <0.001 | 0.482 | |
| flightless | 0.340 | 0.027 | 0.145 | |
| HW | all birds | 0.906 | <0.001 | 0.365 |
| flighted | 0.937 | <0.001 | 0.379 | |
| flightless | 0.436 | 0.012 | 0.291 | |
| Variable | PGLS | adj R2 | p-value | |
| CS | Mb | 0.370 | <0.001 | |
| flighted-flightless | 0.099 | <0.001 | ||
| Mb: flighted-flightless | 0.259 | <0.001 | ||
| HL | Mb | 0.387 | <0.001 | |
| flighted-flightless | 0.092 | <0.001 | ||
| Mb: flighted-flightless | 0.260 | <0.001 | ||
| HW | Mb | 0.495 | <0.001 | |
| flighted-flightless | 0.016 | 0.014 | ||
| Mb: flighted-flightless | 0.148 | <0.001 |
| Linear Model | adj R2 | p-value |
| CS | 0.256 | <0.001 |
| Mb | 0.121 | <0.001 |
| PGLS | adj R2 | p-value |
| CS | 0.058 | <0.001 |
| flighted-flightless | 0.056 | <0.001 |
| CS: flighted-flightless | 0.012 | 0.108 |
| Mb | 0.022 | 0.016 |
| flighted-flightless | 0.057 | <0.001 |
| Mb: flighted-flightless | 0.014 | 0.074 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, F.J.; Costa-Pérez, M.; Navalón, G.; Martín-Serra, A. Morphological Disparity of the Humerus in Modern Birds. Diversity 2020, 12, 173. https://doi.org/10.3390/d12050173
Serrano FJ, Costa-Pérez M, Navalón G, Martín-Serra A. Morphological Disparity of the Humerus in Modern Birds. Diversity. 2020; 12(5):173. https://doi.org/10.3390/d12050173
Chicago/Turabian StyleSerrano, Francisco J., Mireia Costa-Pérez, Guillermo Navalón, and Alberto Martín-Serra. 2020. "Morphological Disparity of the Humerus in Modern Birds" Diversity 12, no. 5: 173. https://doi.org/10.3390/d12050173
APA StyleSerrano, F. J., Costa-Pérez, M., Navalón, G., & Martín-Serra, A. (2020). Morphological Disparity of the Humerus in Modern Birds. Diversity, 12(5), 173. https://doi.org/10.3390/d12050173





