In Nitrate-Rich Soil, Fallopia x bohemica Modifies Functioning of N Cycle Compared to Native Monocultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species, Soils, and Plant Regeneration
2.2. Experimental Design
2.3. N Addition and Labeling
2.4. Soil and Plant Harvest and Processing
2.5. 15N Analysis
2.6. Plant Traits
2.7. Microbial Activity Measurements
2.7.1. Nitrification Enzyme Activity (NEA)
2.7.2. Denitrification Enzyme Activity (DEA)
2.7.3. Soil Nitrate and Ammonium Concentrations
2.8. Microbial Abundance Measurements
2.9. Statistical Analyses
3. Results
3.1. Comparison between Species for Growth and N Management in Plants
3.2. Impacts of F. x bohemica on Native Plants for both Soil Types (Low or High Nitrate Conditions)
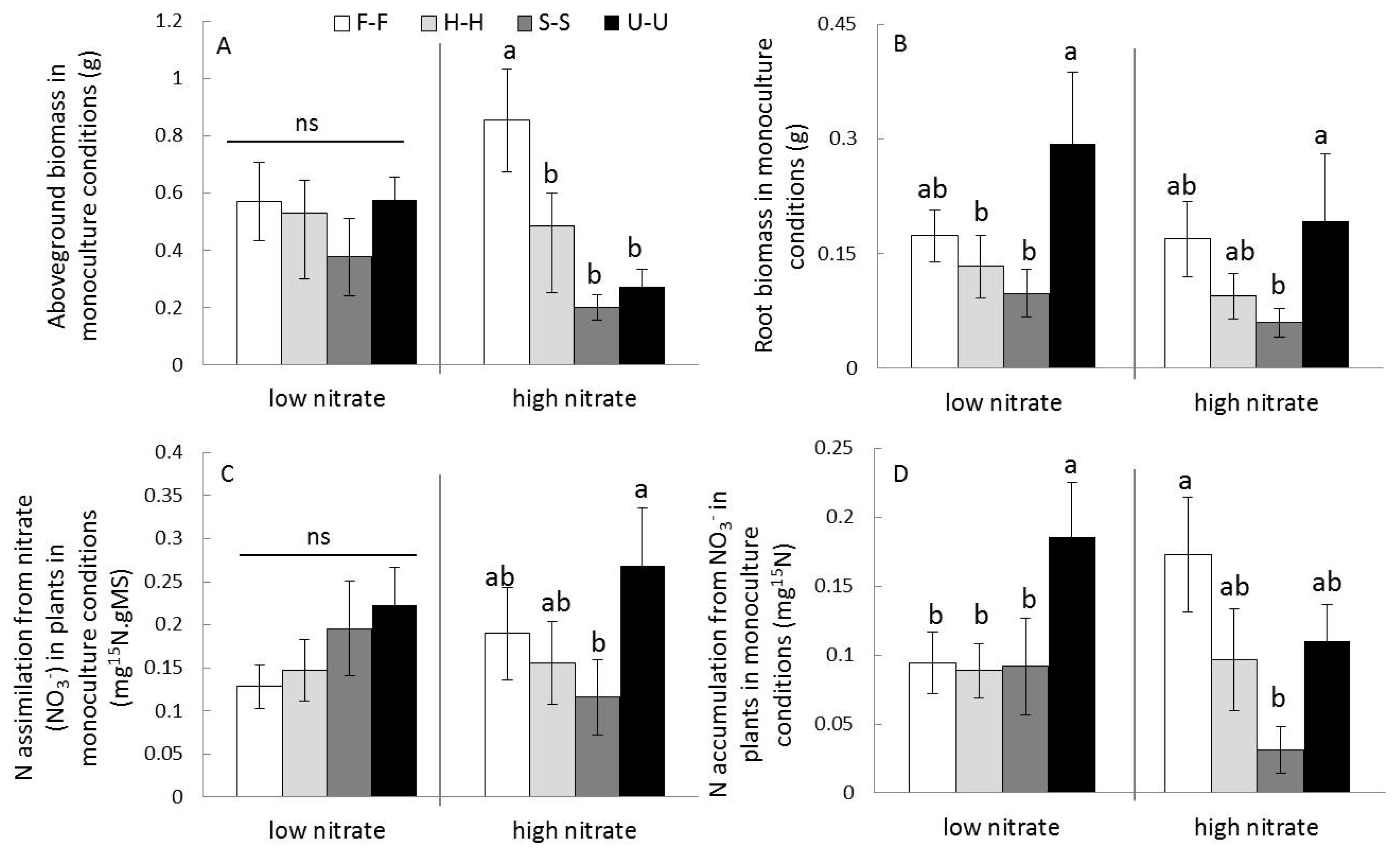
3.2.1. Impacts on Aboveground Traits and Root Biomass
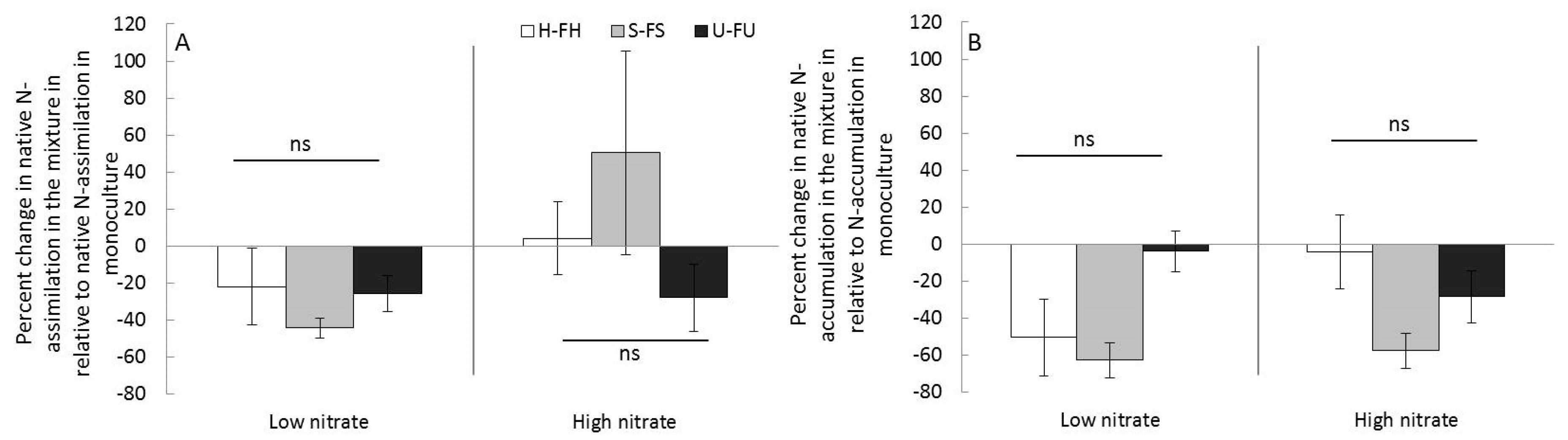
3.2.2. Impacts on Plant N Uptakes
3.3. Effects of Plant Type on Plant Biomass and Potential Rates of Nitrification and Denitrification in the Two Types of Soil
3.3.1. Effects on Plant Biomass
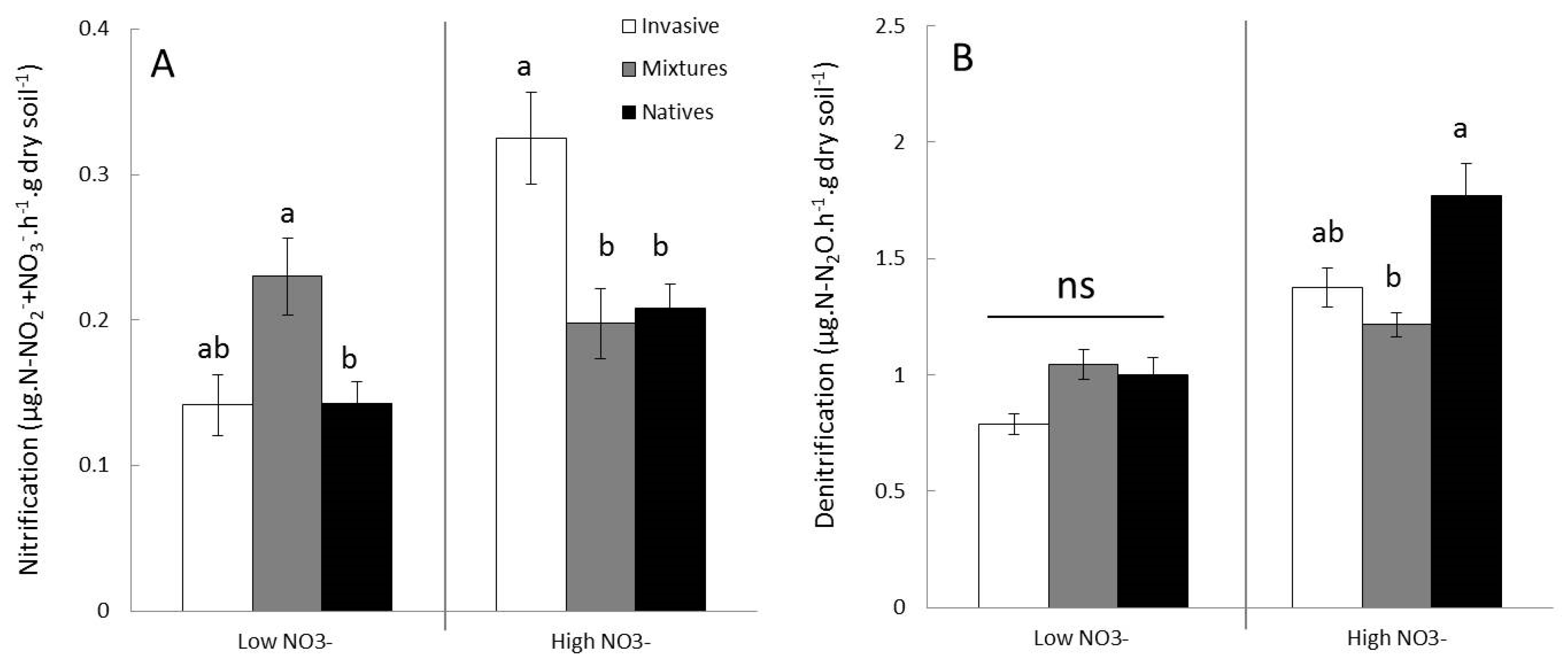
3.3.2. Effects on Nitrification and Denitrification
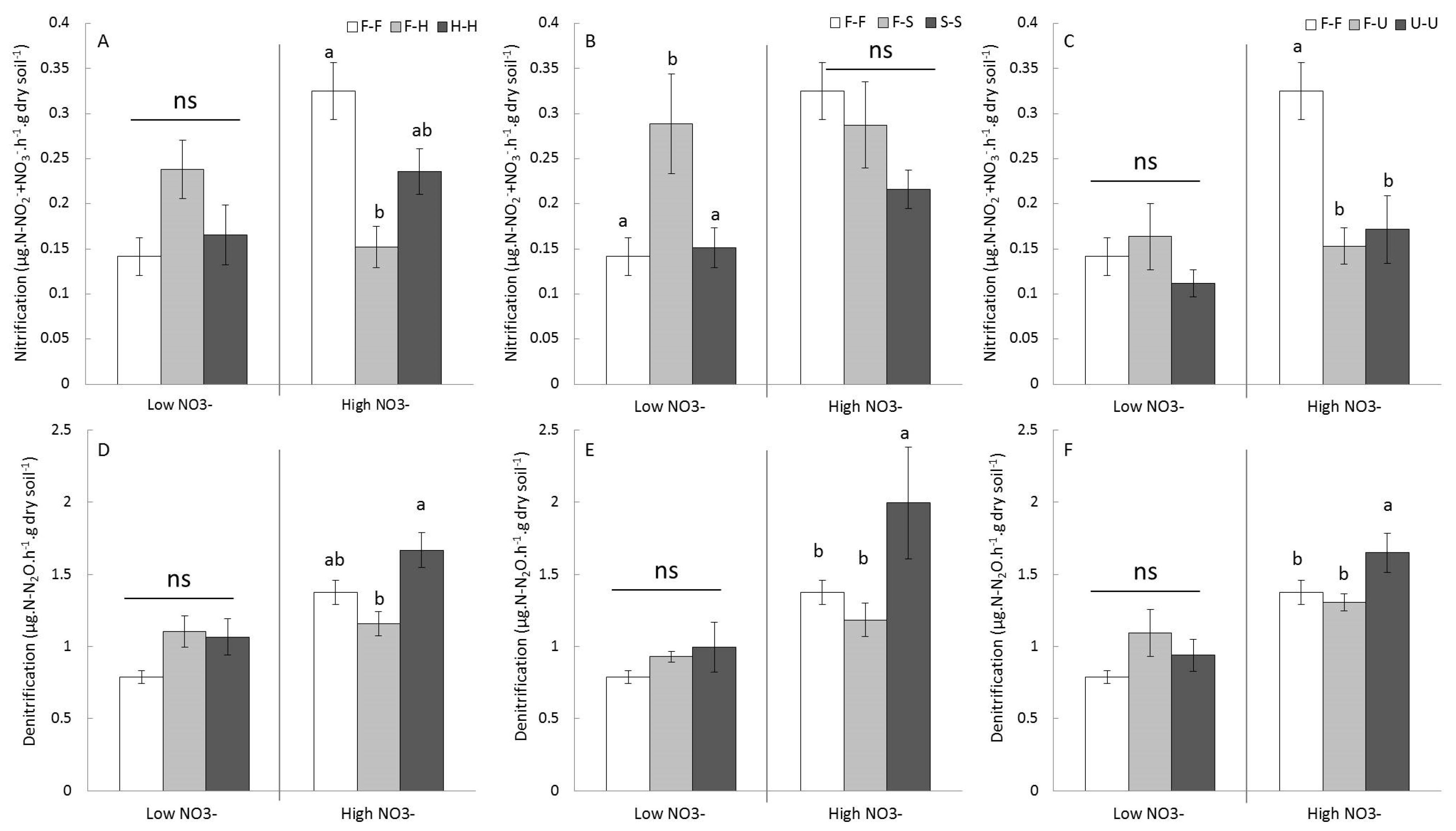
3.4. Effects of Competition Treatments on Soil Functioning (Nitrification and Denitrification) in Terms of Microbial Activities and Gene Abundances
4. Discussion
4.1. Impact of Knotweed on Competing Species: From Little Negative Effect to Facilitation
4.2. Impacts of knotweed on Soil N-Functions and Microbial Communities: The Surprising Case of NO3−Poor Soil
4.3. Impacts of Knotweed on Soil N-Functions and Microbial Communities: NO3−Rich Soil, Ideal Playground for Knotweed?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef]
- Harcombe, P.; Cameron, G.N.; Glumac, E.G. Above-ground net primary productivity in adjacent grassland and woodland on the coastal prairie of Texas, USA. J. Veg. Sci. 1993, 4, 521–530. [Google Scholar] [CrossRef]
- Lett, M.S.; Knapp, A.K.; Briggs, J.M.; Blair, J.M. Influence of shrub encroachment on aboveground net primary productivity and carbon and nitrogen pools in a mesic grassland. Can. J. Bot. 2004, 82, 1363–1370. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Polley, H.W. Aboveground productivity and root-shoot allocation differ between native and introduced grass species. Oecologia 2006, 150, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Kourtev, P.; Huang, W. Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol. Appl. 2001, 11, 1287–1300. [Google Scholar] [CrossRef]
- Lata, J.C.; Degrange, V.; Raynaud, X.; Maron, P.A.; Lensi, R.; Abbadie, L. Grass populations control nitrificationin savanna soils. Funct. Ecol. 2004, 18, 605–611. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Rondon, M.; Ito, O.; Ishikawa, T.; Rao, I.M.; Nakahara, K.; Lascano, C.; Berry, W.L. Biological nitrificationinhibition (BNI)-Is it a widespread phenomenon? Plant Soil 2007, 294, 5–18. [Google Scholar] [CrossRef]
- Dassonville, N.; Guillaumaud, N.; Piola, F.; Meerts, P.; Poly, F. Niche construction by the invasive Asian knotweeds (species complex Fallopia): Impact on activity, abundance and community structure of denitrifiers and nitrifiers. Biol. Invasions 2011, 13, 1115–1133. [Google Scholar] [CrossRef]
- Bardon, C.; Piola, F.; Bellvert, F.; Haichar, F.Z.; Comte, G.; Meiffren, G.; Pommier, T.; Puijalon, S.; Tsafack, N.; Poly, F. Evidence for biological denitrification inhibition (BDI) by plant secondary metabolites. New Phytol. 2014, 204, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Hortal, S.; Lozano, Y.M.; Bastida, F.; Armas, C.; Moreno, J.L.; Garcia, C.; Pugnaire, F.I. Plant-plant competition outcomes are modulated by plant effects on the soil bacterial community. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Castro-Díez, P.; Godoy, O.; Alonso, A.; Gallardo, A.; Saldaña, A. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol. Lett. 2014, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gallien, L.; Carboni, M. The community ecology of invasive species: Where are we and what’s next? Ecography 2017, 40, 335–352. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Gilbert, B.; Levine, J.M. Plant invasions and the niche. J. Ecol. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Skurski, T.C.; Rew, L.J.; Maxwell, B.D. Mechanisms underlying nonindigenous plant impacts: a review of recent experimental research. Invasive Plant Sci. Manag. 2014, 7, 432–444. [Google Scholar] [CrossRef]
- Bailey, J.P.; Bímová, K.; Mandák, B. Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l. Sets the stage for the “Battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203. [Google Scholar] [CrossRef]
- Parepa, M.; Fischer, M.; Krebs, C.; Bossdorf, O. Hybridization increases invasive knotweed success. Evol. Appl. 2014, 7, 413–420. [Google Scholar] [CrossRef]
- Nentwig, W.; Bacher, S.; Kumschick, S.; Pyšek, P.; Vilà, M. More than “100 worst” alien species in Europe. Biol. Invasions 2018, 20, 1611–1621. [Google Scholar] [CrossRef]
- Lavoie, C. The impact of invasive knotweed species (Reynoutria spp.) on the environment: Review and research perspectives. Biol. Invasions 2017, 19, 2319–2337. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Majewska, M.L.; Stanek, M.; Nobis, M.; Zubek, S. Differential influence of four invasive plant species on soil physicochemical properties in a pot experiment. J. Soils Sediments 2018, 18, 1409–1423. [Google Scholar] [CrossRef]
- Legay, N.; Baxendale, C.; Grigulis, K.; Krainer, U.; Schloter, M.; Bardgett, R.D.; Arnoldi, C.; Bahn, M.; Dumont, M.; Poly, F.; et al. Contribution of above-and below-ground plant traits to the structure and func-tion of grassland soil microbial communities. Ann. Bot. 2014, 114, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Faivre, C.; Matejicek, A.; Strbik, F.; Philippot, L.; Mougel, C. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology 2015, 96, 2300–2310. [Google Scholar] [CrossRef]
- Thion, C.E.; Poirel, J.D.; Cornulier, T.; De Vries, F.T.; Bardgett, R.D.; Prosser, J.I. Plant nitrogen-use strategy as a driver of rhizosphere archaeal and bacterial ammonia oxidizer abundance. FEMS Microbiol. Ecol. 2016, 92, fiw09. [Google Scholar] [CrossRef] [PubMed]
- Czarnes, S.; Dexter, A.R.; Bartoli, F. Wetting and drying cycles in the maize rhizosphere under controlled conditions. Mechanics of the root-adhering soil. Plant Soil 2000, 221, 253–271. [Google Scholar] [CrossRef]
- Warembourg, F.R. N2 fixation in soil and plant systems. In Nitrogen Isotope Techniques; Knowles, R., Blackburn, T.H., Eds.; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Díaz, S.; Cabido, M. Plant functional types and ecosystem function in relation to global change. J. Veg. Sci. 1997, 8, 463–474. [Google Scholar] [CrossRef]
- Cantarel, A.A.M.; Dumont, M.; Lainé, P.; Lemauviel-Lavenant, S.; Personeni, E.; Pommier, T.; Kastl, E.M.; Diquelou, S.; Desclos-Theveniau, M.; Grassein, F.; et al. Using plant functional traits to explain plant-microorganisms relationships for N-resources acquisition. Ecology 2015, 96, 788–799. [Google Scholar] [CrossRef]
- Patra, A.K.; Abbadie, L.; Clays-Josserand, A.; Degrange, V.; Grayston, S.J.; Loiseau, P.; Louault, F.; Mahmood, S.; Nazaret, S.; Philippot, L.; et al. Effect of grazing on microbial functional groups involved in soil N dynamics. Ecol. Monogr. 2005, 75, 65–80. [Google Scholar] [CrossRef]
- Simonin, M.; Richaume, A.; Guyonnet, J.P.; Dubost, A.; Martins, J.M.F.; Pommier, T. Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers. Sci. Rep. 2016, 6, 33643. [Google Scholar] [CrossRef]
- Baudoin, E.; Philippot, L.; Chèneby, D.; Chapuis-Lardy, L.; Fromin, N.; Bru, D.; Rabary, B.; Brauman, A. Direct seeding mulch-based cropping increases both the activity and abundance of denitrifier communities in a tropical soil. Soil Biol. Biochem. 2009, 41, 1703–1709. [Google Scholar] [CrossRef]
- Parepa, M.; Kahmen, A.; Werner, R.A.; Fischer, M.; Bossdorf, O. Invasive knotweed has greater nitrogen-use efficiency than native plants: Evidence from a 15N pulse-chasing experiment. Oecologia 2019, 191, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Bottollier-Curtet, M.; Planty-Tabacchi, A.M.; Tabacchi, E. Competition between young exotic invasive and native dominant plant species: Implications for invasions within riparian areas. J. Veg. Sci. 2013, 24, 1033–1042. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology. A Functional Approach to Common British Species; Springer: Dordrecht, The Netherlands, 1989; p. 742. [Google Scholar] [CrossRef]
- Bímová, K.; Mandák, B.; Kasparová, I. How does Reynoutria invasion fit the various theories of invasibility? J. Veg. Sci. 2004, 15, 495–504. [Google Scholar] [CrossRef]
- Chmura, D.; Tokarska-Guzik, B.; Nowak, T.; Woźniak, G.; Bzdęga, K.; Koszela, K.; Gancarek, M. The influence of invasive Fallopia taxa on resident plant species in two river valleys (southern Poland). Acta Soc. Bot. Pol. 2015, 84, 23–33. [Google Scholar] [CrossRef]
- Rosnitschek-Schimmel, I. Effect of ammonium and nitrate supply on dry matter production and nitrogen distribution in Urtica dioica. Z. Pflanzenphysiol. 1982, 108, 329–341. [Google Scholar] [CrossRef]
- Chmura, D.; Krywult, M.; Kozak, J.L. Nitrate reductase activity (NRA) in the invasive alien Fallopia japonica: Seasonal variation, differences among habitats types, and comparison with native species. Acta Soc. Bot. Pol. 2016, 85, 3514. [Google Scholar] [CrossRef]
- Siemens, T.J.; Blossey, B. An evaluation of mechanisms preventing growth and survival of two native species in invasive bohemian knotweed (Fallopia x bohemica, Polygonaceae). Am. J. Bot. 2007, 94, 776–783. [Google Scholar] [CrossRef]
- Barney, J.N.; Tharayil, N.; DiTommaso, A.; Bhowmik, P.C. The biology of invasive alien plants in Canada. 5. Polygonum cuspidatum Sieb. & Zucc. [= Fallopia japonica (Houtt.) Ronse Decr.]. Can. J. Plant Sci. 2006, 86, 887–905. [Google Scholar]
- Hirose, T.; Tateno, M. Soil nitrogen patterns induced by colonization of Polygonum cuspidatum on Mt. Fuji. Oecologia 1984, 61, 218–223. [Google Scholar] [CrossRef]
- Tateno, M.; Hirose, T. Nitrification and nitrogen accumulation in the early stages of primary succession on Mt. Fuji. Ecol. Res. 1987, 2, 113–120. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhu, Y.G.; Xia, Y.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea: Important players in paddy rhizosphere soil? Environ. Microbiol. 2008, 10, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Könneke, M.; Schubert, D.M.; Brown, P.C.; Hügler, M.; Standfest, S.; Schwander, T.; von Borzyskowski, L.S.; Erb, T.J.; Stahl, D.A.; Berg, I.A. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl. Acad. Sci. USA 2014, 111, 8239–8244. [Google Scholar] [CrossRef]
- Hou, S.P.; Ai, C.; Zhou, W.; Liang, G.Q.; He, P. Structure and assembly cues for rhizospheric nirK- and nirS-type denitrifier communities in long-term fertilized soils. Soil Biol. Biochem. 2018, 119, 32–40. [Google Scholar] [CrossRef]
- Bardon, C.; Piola, F.; Haichar, F.Z.; Meiffren, G.; Comte, G.; Missery, B.; Balby, M.; Poly, F. Identification of B-type procyanidins in Fallopia spp. involved in biological denitrification inhibition (BDI). Environ. Microbiol. 2016, 18, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Bardon, C.; Poly, F.; Haichar, F.Z.; Le Roux, X.; Simon, L.; Meiffren, G.; Comte, G.; Rouifed, S.; Piola, F. Biological denitrification inhibition (BDI) with procyanidins induces modification of root traits, growth and N status in Fallopia x bohemica. Soil Biol. Biochem. 2017, 107, 41–49. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Bardon, C.; Misery, B.; Piola, F.; Poly, F.; Le Roux, X. Control of soil N cycle processes by Pteridium aquilinum and Erica cinerea in heathlands along a pH gradient. Ecosphere 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Carey, C.J.; Beman, J.M.; Eviner, V.T.; Malmstrom, C.M.; Hart, S.C. Soil microbial community structure is unaltered by plant invasion, vegetation clipping, and nitrogen fertilization in experimental semi-arid grasslands. Front. Microbiol. 2015, 6, 466. [Google Scholar] [CrossRef]

| Plant Treatments | Non-Parametic Variance Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Invasive | Mixture | Natives | p-Value | I vs. M | I vs. N | M vs. N | |
| (a) low nitrate | |||||||
| Aboveground biomass | 1.08 ± 0.11 | 1.05 ± 0.08 | 0.96 ± 0.08 | ns | - | - | - |
| Root biomass | 0.34 ± 0.02 | 0.35 ± 0.04 | 0.35 ± 0.06 | ns | - | - | - |
| (b) high nitrate | |||||||
| Aboveground biomass | 1.71 ± 0.26 | 1.39 ± 0.08 | 0.64 ± 0.09 | <0.0001 | - | 0.0003 | 0.0001 |
| Root biomass | 0.34 ± 0.04 | 0.31 ± 0.02 | 0.23 ± 0.05 | 0.0212 | - | - | 0.0101 |
| Plant Conditions | Variance Analysis | ||||
|---|---|---|---|---|---|
| (a) Low nitrate | FF | FH | HH | p-value | |
| Ammonia-oxidizing bacteria (106 copy per g of dry soil) | 4.2 ± 0.2 | 5.0 ± 0.3 | 5.2 ± 1 | ns | |
| Ammonia-oxidizing archea (107 copy per g of dry soil) | 8.6 ± 0.5 | 9.2 ± 0.6 | 6.7 ± 1.3 | ns | |
| nirK (107 copy per g of dry soil) | 2.6 ± 0.2 | 2.6 ± 0.2 | 3.4 ± 0.5 | ns | |
| nirS (106 copy per g of dry soil) | 5.4 ± 0.2 | 6.5 ± 0.6 | 9.5 ± 2.7 | ns | |
| FF | FS | SS | p-value | ||
| Ammonia-oxidizing bacteria (106 copy per g of dry soil) | 4.2 ± 0.2 | 4.5 ± 0.2 | 4.3 ± 0.5 | ns | |
| Ammonia-oxidizing archea (107 copy per g of dry soil) | 8.6 ± 0.5 | 10 ± 0.4 | 6.6 ± 0.3 | 0.023 | |
| nirK (107 copy per g of dry soil) | 2.6 ± 0.2 | 3.2 ± 0.1 | 2.4 ± 0.2 | 0.033 | |
| nirS (106 copy per g of dry soil) | 5.4 ± 0.2 | 6.7 ± 0.2 | 5.6 ± 0.3 | 0.004 | |
| FF | FU | UU | p-value | ||
| Ammonia-oxidizing bacteria (106 copy per g of dry soil) | 4.2 ± 0.2 | 3.7 ± 0.2 | 4.1 ± 0.3 | ns | |
| Ammonia-oxidizing archea (107 copy per g of dry soil) | 8.6 ± 0.5 | 8.9 ± 0.4 | 9.3 ± 0.7 | ns | |
| nirK (107 copy per g of dry soil) | 2.6 ± 0.2 | 2.4 ± 0.1 | 2.6 ± 0.1 | ns | |
| nirS (106 copy per g of dry soil) | 5.4 ± 0.2 | 7.2 ± 0.9 | 5.6 ± 0.2 | ns | |
| (b) High nitrate | FF | FH | HH | p-value | |
| Ammonia-oxidizing bacteria (106 copy per g of dry soil) | 8.0 ± 0.4 | 7.3 ± 0.6 | 5.5 ± 1.4 | ns | |
| Ammonia-oxidizing archea (107 copy per g of dry soil) | 2.5 ± 0.1 | 2.3 ± 0.1 | 1.6 ± 0.4 | ns | |
| nirK (107 copy per g of dry soil) | 5.1 ± 0.5 | 4.3 ± 0.1 | 3.7 ± 0.7 | ns | |
| nirS (106 copy per g of dry soil) | 24.4 ± 1.3 | 21.1 ± 1.1 | 18.2 ± 4.5 | ns | |
| FF | FS | SS | p-value | ||
| Ammonia-oxidizing bacteria (106 copy per g of dry soil) | 8.0 ± 0.4 | 6.6 ± 0.4 | 5.9 ± 0.5 | 0.013 | |
| Ammonia-oxidizing archea (107 copy per g of dry soil) | 2.5 ± 0.1 | 6.6 ± 0.5 | 5.9 ± 0.6 | ns | |
| nirK (107 copy per g of dry soil) | 5.1 ± 0.5 | 6.6 ± 0.6 | 5.9 ± 0.7 | ns | |
| nirS (106 copy per g of dry soil) | 24.4 ± 1.3 | 6.6 ± 0.7 | 5.9 ± 0.8 | ns | |
| FF | FU | UU | p-value | ||
| Ammonia-oxidizing bacteria (106 copy per g of dry soil) | 8.0 ± 0.4 | 5.7 ± 0.4 | 5.7 ± 0.5 | 0.005 | |
| Ammonia-oxidizing archea (107 copy per g of dry soil) | 2.5 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.01 | |
| nirK (107 copy per g of dry soil) | 5.1 ± 0.5 | 4.5 ± 0.4 | 4.8 ± 0.2 | ns | |
| nirS (106 copy per g of dry soil) | 24.4 ± 1.3 | 20.0 ± 1.6 | 21.2 ± 0.9 | ns | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantarel, A.A.M.; Rouifed, S.; Simon, L.; Bourg, J.; Gervaix, J.; Blazère, L.; Poussineau, S.; Creuzé des Châtelliers, C.; Piola, F. In Nitrate-Rich Soil, Fallopia x bohemica Modifies Functioning of N Cycle Compared to Native Monocultures. Diversity 2020, 12, 156. https://doi.org/10.3390/d12040156
Cantarel AAM, Rouifed S, Simon L, Bourg J, Gervaix J, Blazère L, Poussineau S, Creuzé des Châtelliers C, Piola F. In Nitrate-Rich Soil, Fallopia x bohemica Modifies Functioning of N Cycle Compared to Native Monocultures. Diversity. 2020; 12(4):156. https://doi.org/10.3390/d12040156
Chicago/Turabian StyleCantarel, Amélie A. M., Soraya Rouifed, Laurent Simon, Julien Bourg, Jonathan Gervaix, Leslie Blazère, Sophie Poussineau, Charline Creuzé des Châtelliers, and Florence Piola. 2020. "In Nitrate-Rich Soil, Fallopia x bohemica Modifies Functioning of N Cycle Compared to Native Monocultures" Diversity 12, no. 4: 156. https://doi.org/10.3390/d12040156
APA StyleCantarel, A. A. M., Rouifed, S., Simon, L., Bourg, J., Gervaix, J., Blazère, L., Poussineau, S., Creuzé des Châtelliers, C., & Piola, F. (2020). In Nitrate-Rich Soil, Fallopia x bohemica Modifies Functioning of N Cycle Compared to Native Monocultures. Diversity, 12(4), 156. https://doi.org/10.3390/d12040156





