Effects of Short-Duration and Diel-Cycling Hypoxia on Predation of Mussels and Oysters in Two Tributaries of the Chesapeake Bay
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
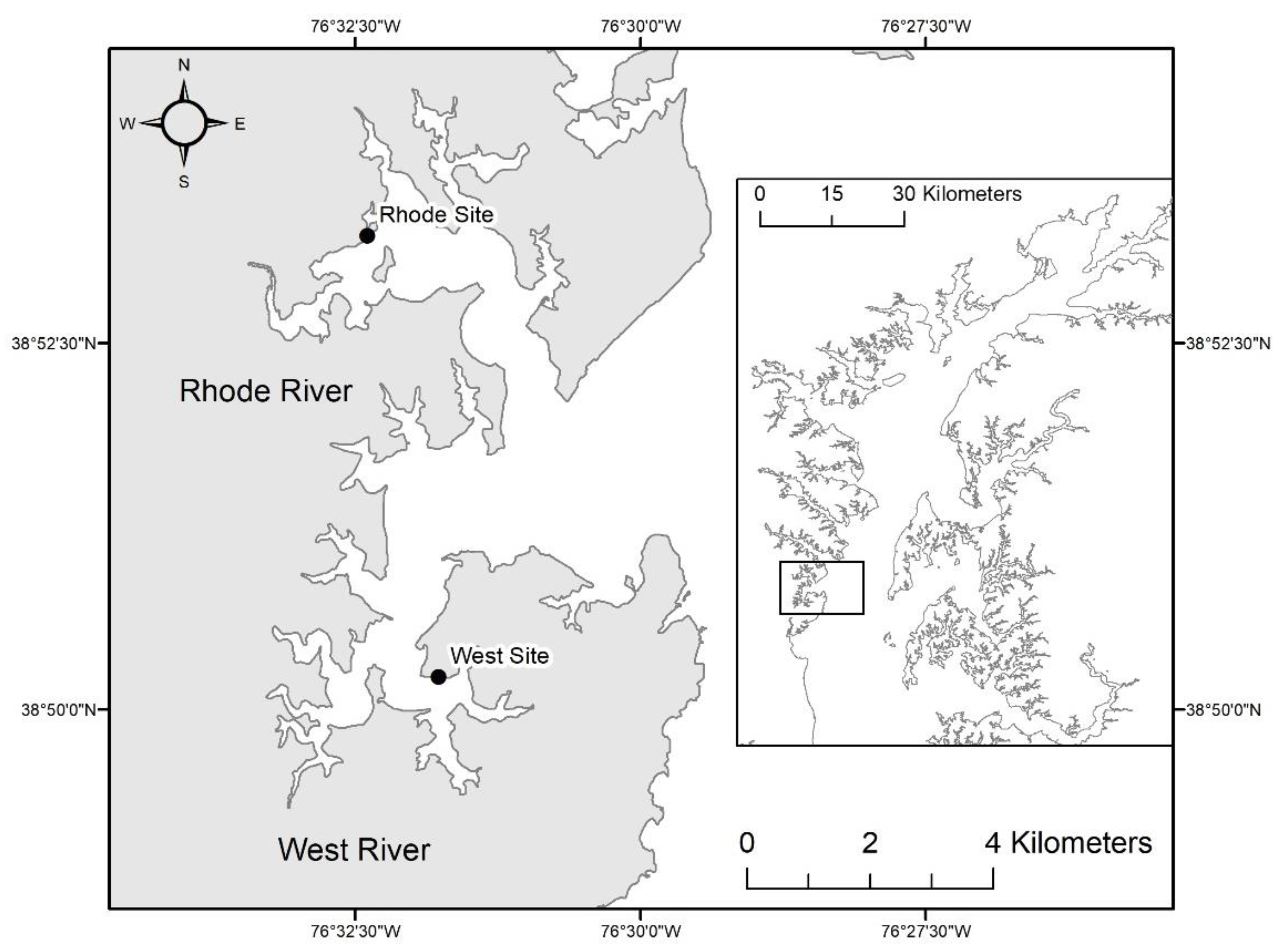
2.2. Field Sites
2.3. Field Experiment
2.4. Laboratory Experiment
3. Results
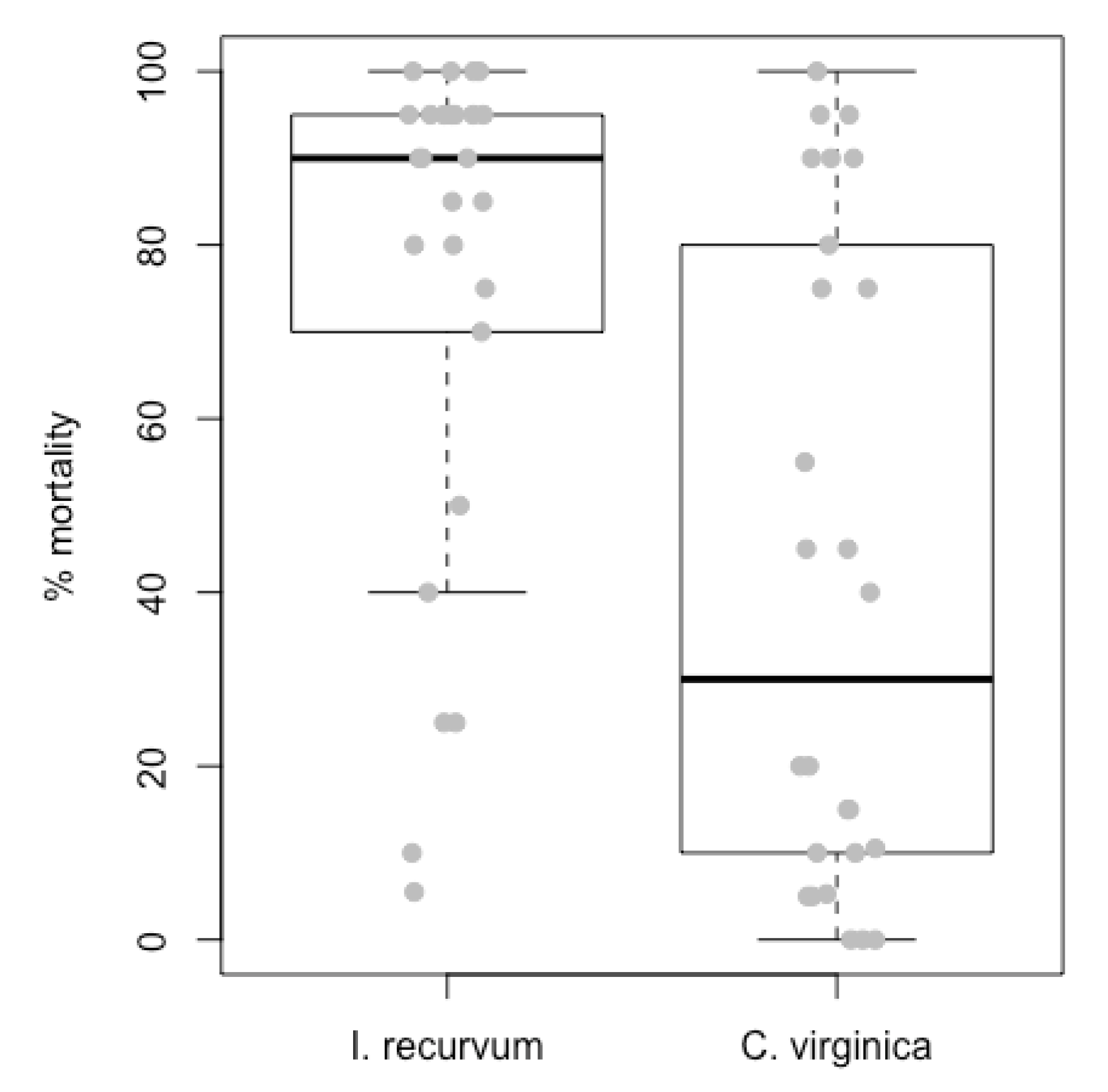
3.1. Field Experiment
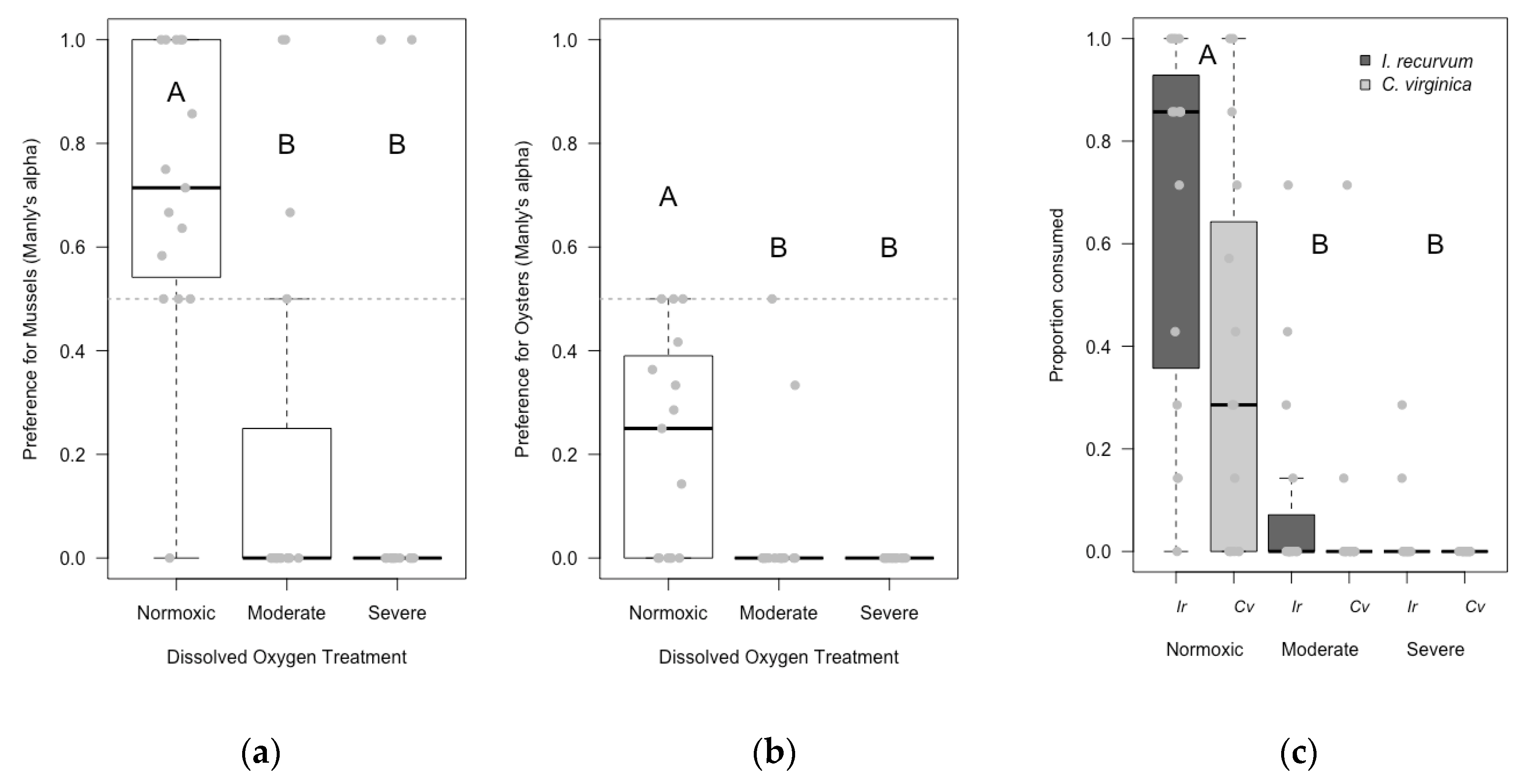
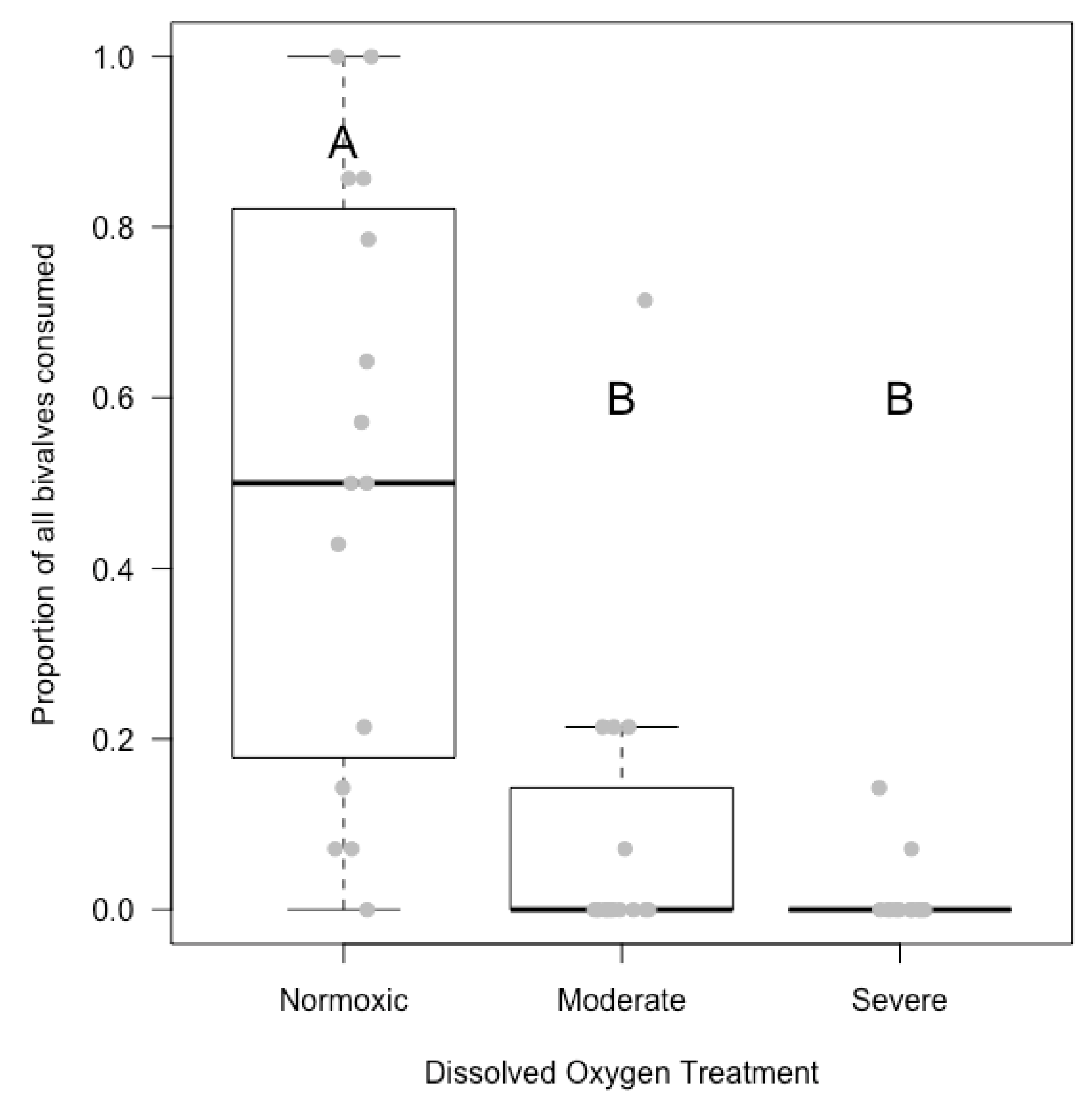
3.2. Laboratory Experiment
4. Discussion
4.1. Hypoxia Lowered Predation Rates in the Laboratory; Inconclusive in the Field
4.2. Blue Crab Preference for Hooked Mussels over Eastern Oysters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baird, D.; Christian, R.R.; Peterson, C.H.; Johnson, G.A. Consequences of hypoxia on estuarine ecosystem function: Energy diversion from consumers to microbes. Ecol. Appl. 2004, 14, 805–822. [Google Scholar] [CrossRef]
- Tyler, R.M.; Brady, D.C.; Targett, T.E. Temporal and Spatial Dynamics of Diel-Cycling Hypoxia in Estuarine Tributaries. Estuaries Coasts 2009, 32, 123–145. [Google Scholar] [CrossRef]
- Kemp, W.M.; Boynton, W.R.; Adolf, J.E.; Boesch, D.F.; Boicourt, W.C.; Brush, G.; Cornwell, J.C.; Fisher, T.R.; Glibert, P.M.; Hagy, J.D.; et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005, 303, 1–29. [Google Scholar] [CrossRef]
- Hagy, J.D.; Boynton, W.R.; Keefe, C.W.; Wood, K.V. Hypoxia in Chesapeake Bay, 1950–2001: Long-term change in relation to nutrient loading and river flow. Estuaries 2004, 27, 634–658. [Google Scholar] [CrossRef]
- Breitburg, D.L. Near-shore hypoxia in the Chesapeake Bay: Patterns and relationships among physical factors. Estuar. Coast. Shelf Sci. 1990, 30, 593–609. [Google Scholar] [CrossRef]
- Burrell, R.B.; Keppel, A.G.; Clark, V.M.; Breitburg, D.L. An automated monitoring and control system for flow-through co-cycling hypoxia and pH experiments. Limnol. Oceanogr. Methods 2016, 14, 168–185. [Google Scholar] [CrossRef]
- Bever, A.J.; Friedrichs, M.A.; Friedrichs, C.T.; Scully, M.E. Estimating hypoxic volume in the Chesapeake Bay using two continuously sampled oxygen profiles. J. Geophys. Res. Ocean. 2018, 123, 6392–6407. [Google Scholar] [CrossRef]
- Officer, C.B.; Biggs, R.B.; Taft, J.L.; Cronin, L.E.; Tyler, M.A.; Boynton, W.R. Chesapeake Bay anoxia: Origin, development, and significance. Science 1984, 223, 22–27. [Google Scholar] [CrossRef]
- Garlo, E.V.; Milstein, C.B.; Jahn, A.E. Impact of hypoxic conditions in the vicinity of Little Egg Inlet, New Jersey in summer 1976. Estuar. Coast. Mar. Sci. 1979, 8, 421–432. [Google Scholar] [CrossRef]
- Breitburg, D.L. Episodic hypoxia in Chesapeake Bay: Interacting effects of recruitment, behavior, and physical disturbance. Ecol. Monogr. 1992, 62, 525–546. [Google Scholar] [CrossRef]
- Brady, D.C.; Targett, T.E.; Tuzzolino, D.M. Behavioral responses of juvenile weakfish (Cynoscion regalis) to diel-cycling hypoxia: Swimming speed, angular correlation, expected displacement, and effects of hypoxia acclimation. Can. J. Fish. Aquat. Sci. 2009, 66, 415–424. [Google Scholar] [CrossRef]
- Cheek, A.O.; Landry, C.A.; Steele, S.L.; Manning, S. Diel hypoxia in marsh creeks impairs the reproductive capacity of estuarine fish populations. Mar. Ecol. Prog. Ser. 2009, 392, 211–221. [Google Scholar] [CrossRef]
- Taylor, J.C.; Miller, J.M. Physiological performance of juvenile southern flounder, Paralichthys lethostigma (Jordan and Gilbert, 1884), in chronic and episodic hypoxia. J. Exp. Mar. Biol. Ecol. 2001, 258, 195–214. [Google Scholar] [CrossRef]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef]
- Bell, G.W.; Eggleston, D.B. Species-specific avoidance responses by blue crabs and fish to chronic and episodic hypoxia. Mar. Biol. 2005, 146, 761–770. [Google Scholar] [CrossRef]
- Stickle, W.B.; Kapper, M.A.; Liu, L.-L.; Gnaiger, E.; Wang, S.Y. Metabolic adaptations of several species of crustaceans and molluscs to hypoxia: Tolerance and microcalorimetric studies. Biol. Bull. 1989, 177, 303–312. [Google Scholar] [CrossRef]
- Greenway, S.C.; Storey, K.B. The effect of prolonged anoxia on enzyme activities in oysters (Crassostrea virginica) at different seasons. J. Exp. Mar. Biol. Ecol. 1999, 242, 259–272. [Google Scholar] [CrossRef]
- David, E.; Tanguy, A.; Pichavant, K.; Moraga, D. Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J. 2005, 272, 5635–5652. [Google Scholar] [CrossRef]
- Menge, B.A.; Sutherland, J.P. Community regulation: Variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am. Nat. 1987, 130, 730–757. [Google Scholar] [CrossRef]
- Mistri, M. Effects of hypoxia on predator-prey interactions between juvenile Carcinus aestuarii and Musculista senhousia. Mar. Ecol. Prog. Ser. 2004, 275, 211–217. [Google Scholar] [CrossRef]
- Altieri, A.H. Dead zones enhance key fisheries species by providing predation refuge. Ecology 2008, 89, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A.; Olson, A.M. Role of scale and environmental factors in regulation of community structure. Trends Ecol. Evol. 1990, 5, 52–57. [Google Scholar] [CrossRef]
- Pihl, L.; Baden, S.P.; Diaz, R.J.; Schaffner, L.C. Hypoxia-induced structural changes in the diet of bottom-feeding fish and Crustacea. Mar. Biol. 1992, 112, 349–361. [Google Scholar] [CrossRef]
- Long, W.C.; Seitz, R.D. Trophic interactions under stress: Hypoxia enhances foraging in an estuarine food web. Mar. Ecol. Prog. Ser. 2008, 362, 59–68. [Google Scholar] [CrossRef]
- Tallqvist, M. Burrowing behaviour of the Baltic clam Macoma balthica: Effects of sediment type, hypoxia and predator presence. Mar. Ecol. Prog. Ser. 2001, 212, 183–191. [Google Scholar] [CrossRef]
- Saloom, M.E.; Scot Duncan, R. Low dissolved oxygen levels reduce anti-predation behaviours of the freshwater clam Corbicula fluminea. Freshw. Biol. 2005, 50, 1233–1238. [Google Scholar] [CrossRef]
- Hines, A.H.; Haddon, A.M.; Wiechert, L.A. Guild structure and foraging impact of blue crabs and epibenthic fish in a subestuary of Chesapeake Bay. Mar. Ecol. Prog. Ser. 1990. [Google Scholar] [CrossRef]
- Sheridan, P.F. Trophic resource utilization by three species of sciaenid fishes in a northwest Florida estuary. Gulf Mex. Sci. 1979, 3, 1. [Google Scholar] [CrossRef]
- Das, T.; Stickle, W.B. Detection and avoidance of hypoxic water by juvenile Callinectes sapidus and C. similis. Mar. Biol. 1994, 120, 593–600. [Google Scholar] [CrossRef]
- Micheli, F. Effects of predator foraging behavior on patterns of prey mortality in marine soft bottoms. Ecol. Monogr. 1997, 67, 203–224. [Google Scholar] [CrossRef]
- Alexander, S.K. Diet of the blue crab, Callinectes sapidus, from nearshore habitats of Galveston Island, Texas, USA. Tex. J. Sci. 1986, 38, 85–89. [Google Scholar]
- Ebersole, E.L.; Kennedy, V.S. Prey preferences of blue crabs Callinectes sapidus feeding on three bivalve species. Mar. Ecol. Prog. Ser. Oldendorf 1995, 118, 167–177. [Google Scholar] [CrossRef]
- Bell, G.W.; Eggleston, D.B.; Wolcott, T.G. Behavioral responses of free-ranging blue crabs to episodic hypoxia. II. Feeding. Mar. Ecol. Prog. Ser. 2003, 259, 227–235. [Google Scholar] [CrossRef]
- Brante, A.; Hughes, R.N. The effect of hypoxia on the prey-handling behaviour of Carcinus maenas feeding on Mytilus edulis. Mar. Ecol. Prog. Ser. 2001, 209, 301–305. [Google Scholar] [CrossRef]
- Eggleston, D.B. Foraging behavior of the blue crab, Callinectes sapidus, on juvenile oysters, Crassostrea virginica: Effects of prey density and size. Bull. Mar. Sci. 1990, 46, 62–82. [Google Scholar]
- Brumbaugh, R.D.; Sorabella, L.A.; Johnson, C.; Goldsborough, W.J. Small scale aquaculture as a tool for oyster restoration in Chesapeake Bay. Mar. Technol. Soc. J. 2000, 34, 79–86. [Google Scholar] [CrossRef]
- Schulte, D.M.; Burke, R.P.; Lipcius, R.N. Unprecedented restoration of a native oyster metapopulation. Science 2009, 325, 1124–1128. [Google Scholar] [CrossRef]
- Kennedy, V.S.; Breitburg, D.L.; Christman, M.C.; Luckenbach, M.W.; Paynter, K.; Kramer, J.; Sellner, K.G.; Dew-Baxter, J.; Keller, C.; Mann, R. Lessons learned from efforts to restore oyster populations in Maryland and Virginia, 1990 to 2007. J. Shellfish Res. 2011, 30, 719–732. [Google Scholar] [CrossRef]
- Rodney, W.S.; Paynter, K.T. Comparisons of macrofaunal assemblages on restored and non-restored oyster reefs in mesohaline regions of Chesapeake Bay in Maryland. J. Exp. Mar. Biol. Ecol. 2006, 335, 39–51. [Google Scholar] [CrossRef]
- Gregalis, K.C.; Powers, S.P.; Heck, K.L. Restoration of oyster reefs along a bio-physical gradient in Mobile Bay, Alabama. J. Shellfish Res. 2008, 27, 1163–1170. [Google Scholar] [CrossRef]
- Gedan, K.B.; Kellogg, L.; Breitburg, D.L. Accounting for multiple foundation species in oyster reef restoration benefits. Restor. Ecol. 2014, 22, 517–524. [Google Scholar] [CrossRef]
- Brown, K.M.; Aronhime, B.; Wang, X. Predatory blue crabs induce byssal thread production in hooked mussels. Invertebr. Biol. 2011, 130, 43–48. [Google Scholar] [CrossRef]
- Maryland Department of Natural Resources. Eyes on the Bay Program. Available online: http://eyesonthebay.dnr.maryland.gov/ (accessed on 9 February 2020).
- Bell, G.; Eggleston, D.; Wolcott, T. Behavioral responses of free-ranging blue crabs to episodic hypoxia. I. Movement. Mar. Ecol. Prog. Ser. 2003, 259, 215–225. [Google Scholar] [CrossRef]
- Brill, R.W.; Bushnell, P.G.; Elton, T.A.; Small, H.J. The ability of blue crab (Callinectes sapidus, Rathbun 1886) to sustain aerobic metabolism during hypoxia. J. Exp. Mar. Biol. Ecol. 2015, 471, 126–136. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Manly, B.F.J.; Miller, P.; Cook, L.M. Analysis of a selective predation experiment. Am. Nat. 1972, 106, 719–736. [Google Scholar] [CrossRef]
- Clark, K.L.; Ruiz, G.M.; Hines, A.H. Diel variation in predator abundance, predation risk and prey distribution in shallow-water estuarine habitats. J. Exp. Mar. Biol. Ecol. 2003, 287, 37–55. [Google Scholar] [CrossRef]
- Seitz, R.D.; Lipcius, R.N.; Hines, A.H. Consumer versus resource control and the importance of habitat heterogeneity for estuarine bivalves. Oikos 2017, 126, 121–135. [Google Scholar] [CrossRef]
- Clark, V. The effects of diel-cycling hypoxia and hypercapnia on eastern oyster, Crassostrea virginica (Gmelin), clearance rates and hemolymph pH. Coll. Park MD Univ. Md. Cent. Environ. Sci. 2014. [Google Scholar] [CrossRef]
- McGaw, I.J. Gastric processing in the Dungeness crab, Cancer magister, during hypoxia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 150, 458–463. [Google Scholar] [CrossRef]
- Bernatis, J.L.; Gersternberger, S.L.; McGaw, I.J. Behavioural responses of the Dungeness crab, Cancer magister, during feeding and digestion in hypoxic conditions. Mar. Biol. 2007, 150, 941–951. [Google Scholar] [CrossRef]
- Aronhime, B.R. Predator-Prey Interaction in Estuarine Bivalves: Size Selection, Effects of Salinity, and Indirect Interactions. Ph.D. Thesis, Louisiana State University, Baton, LA, USA, 2010; p. 91. [Google Scholar]
- Lipcius, R.N.; Burke, R.P. Abundance, Biomass and Size Structure of Eastern Oyster and Hooked Mussel on a Modular Artificial Reef in the Rappahannock River, Chesapeake Bay; Virginia Institute of Marine Science: Gloucester Point, VA, USA, 2006. [Google Scholar] [CrossRef]
- Milke, L.M.; Kennedy, V.S. Mud crabs (Xanthidae) in Chesapeake Bay: Claw characteristics and predation on epifaunal bivalves. Invertebr. Biol. 2001, 120, 67–77. [Google Scholar] [CrossRef]
- Aronhime, B.R.; Brown, K.M. The roles of profit and claw strength in determining mussel size selection by crabs. J. Exp. Mar. Biol. Ecol. 2009, 379, 28–33. [Google Scholar] [CrossRef]

| Predictor | During Trial (Immediate Effect) | Pre-Trial (Delayed Effect) | Pre & During (Cumulative Effect) | |||
|---|---|---|---|---|---|---|
| Model Statistics | Adj R2 = 0.86 F5,46 = 3.331 | Adj R2 = 0.22 F5,44 = 3.703 | Adj R2 = 0.20 F5,44 = 3.405 | |||
| t | p | t | p | t | p | |
| bivalve species | −2.461 | 0.0177 | −2.528 | 0.0152 | −2.497 | 0.0163 |
| site | −1.099 | 0.2776 | −0.067 | 0.9467 | −0.463 | 0.6457 |
| bivalve species × site | −0.327 | 0.7448 | −0.596 | 0.5545 | −0.588 | 0.5592 |
| moderate hypoxia | 0.108 | 0.9142 | 0.234 | 0.8162 | −0.253 | 0.8016 |
| severe hypoxia | 0.664 | 0.5101 | −1.06 | 0.2948 | −0.033 | 0.9734 |
| Predictor | Degrees of Freedom | F | p |
|---|---|---|---|
| bivalve species | 1 | 13.82 | <0.001 |
| site | 1 | 2.823 | 0.099 |
| bivalve species × site | 1 | 0.111 | 0.741 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neff, E.; MacGregor, J.; Gedan, K.B. Effects of Short-Duration and Diel-Cycling Hypoxia on Predation of Mussels and Oysters in Two Tributaries of the Chesapeake Bay. Diversity 2020, 12, 87. https://doi.org/10.3390/d12030087
Neff E, MacGregor J, Gedan KB. Effects of Short-Duration and Diel-Cycling Hypoxia on Predation of Mussels and Oysters in Two Tributaries of the Chesapeake Bay. Diversity. 2020; 12(3):87. https://doi.org/10.3390/d12030087
Chicago/Turabian StyleNeff, Ellen, Jessica MacGregor, and Keryn B. Gedan. 2020. "Effects of Short-Duration and Diel-Cycling Hypoxia on Predation of Mussels and Oysters in Two Tributaries of the Chesapeake Bay" Diversity 12, no. 3: 87. https://doi.org/10.3390/d12030087
APA StyleNeff, E., MacGregor, J., & Gedan, K. B. (2020). Effects of Short-Duration and Diel-Cycling Hypoxia on Predation of Mussels and Oysters in Two Tributaries of the Chesapeake Bay. Diversity, 12(3), 87. https://doi.org/10.3390/d12030087






