Respiration by the Opportunistic Spionid Polychaete Streblospio gynobranchiata during Adjustment to and Recovery from Moderate Hypoxia
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Culture
2.2. Experimental Conditions
2.3. Data Analysis
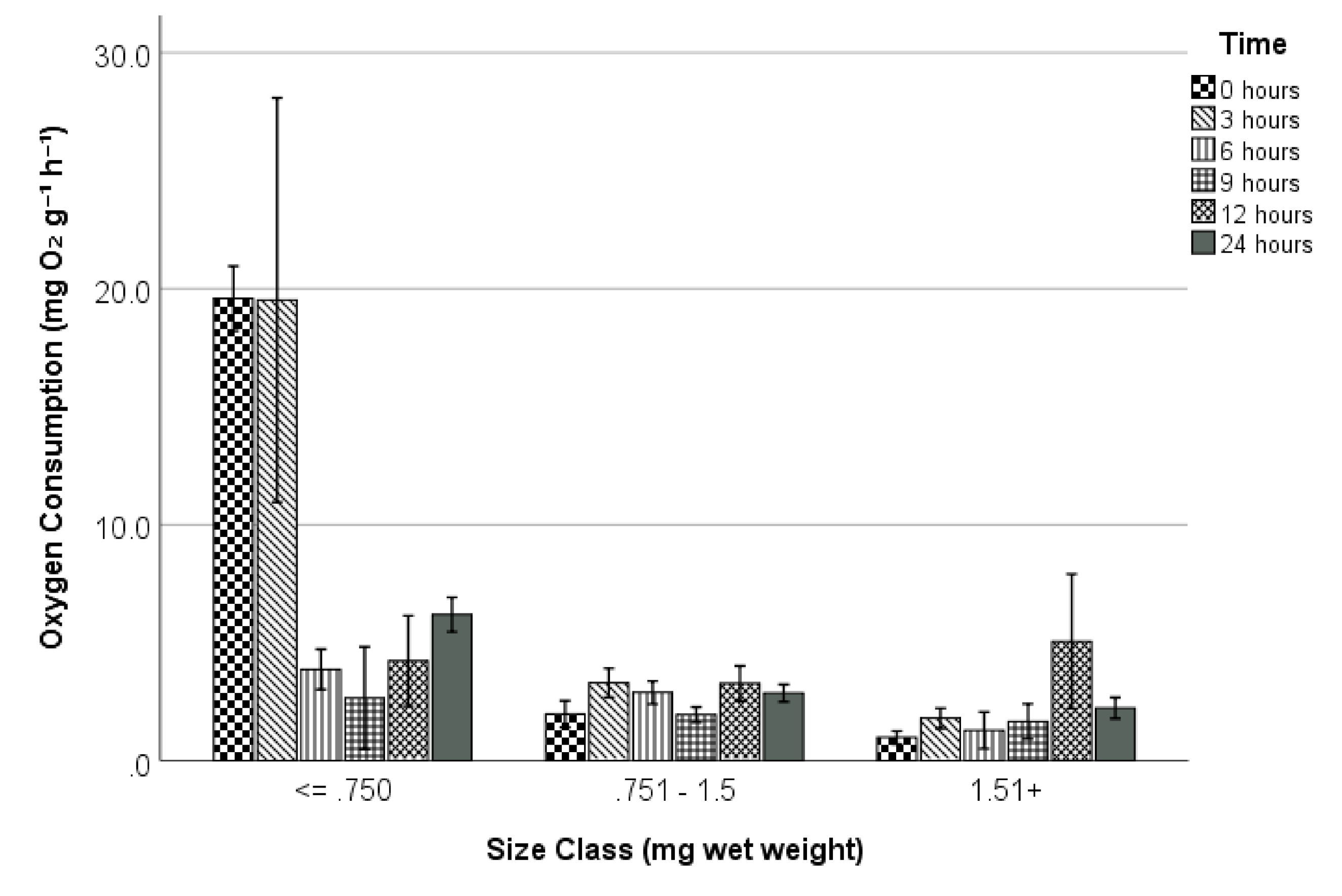
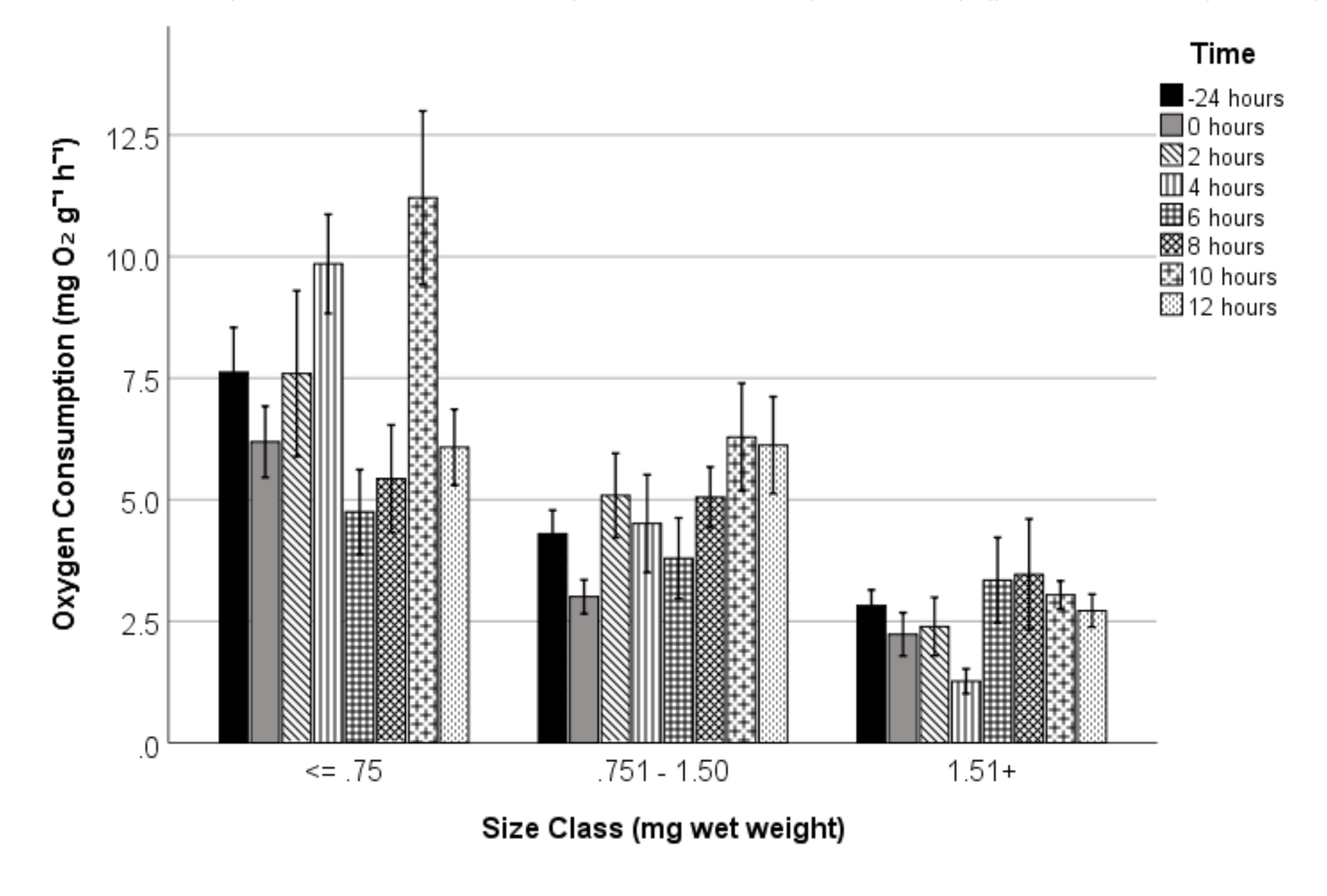
3. Results
3.1. Exposure
3.2. Recovery
4. Discussion
4.1. Exposure
4.2. Recovery
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burnett, L.E.; Stickle, W.B. Physiological responses to hypoxia. In Coastal and Estuarine Studies; American Geophysical Union (AGU): Malden, MA, USA, 2001; Volume 58, pp. 101–114. [Google Scholar]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Diaz, R.J.; Neubauer, R.J.; Schaffner, L.C.; Pihl, L.; Baden, S.P. Continuous monitoring of dissolved oxygen in an estuary experiencing periodic hypoxia and the effect of hypoxia on macrobenthos and fish. In Marine Coastal Eutrophication; Vollenweider, R.A., Marchetti, R., Viviani, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1055–1068. [Google Scholar]
- Wu, R.S.S. Hypoxia: From molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002, 45, 35–45. [Google Scholar] [CrossRef]
- Wells, R.; Jarvis, P.; Shumway, S. Oxygen uptake, the circulatory system, and haemoglobin function in the intertidal polychaete Terebella haplochaeta (Ehlers). J. Exp. Mar. Biol. Ecol. 1980, 46, 255–277. [Google Scholar] [CrossRef]
- Sturdivant, S.K.; Perchik, M.; Brill, R.W.; Bushnell, P.G. Metabolic responses of the nereid polychaete, Alitta succinea, to hypoxia at two different temperatures. J. Exp. Mar. Biol. Ecol. 2015, 473, 161–168. [Google Scholar] [CrossRef]
- Rakocinski, C.F.; Gillam, K.B. Temperature-modulated expression of allometric respiration strategies supports a metabolic scaling rule. Front. Mar. Sci. 2017, 4, 261. [Google Scholar] [CrossRef]
- Gonzalez, R.; Quiñones, R. Pyruvate oxidoreductases involved in glycolytic anaerobic metabolism of polychaetes from the continental shelf off central-south Chile. Estuar. Coast. Shelf Sci. 2000, 51, 507–519. [Google Scholar] [CrossRef]
- Ellington, W.R. The recovery from anaerobic metabolism in invertebrates. J. Exp. Zool. 1983, 228, 431–444. [Google Scholar] [CrossRef]
- Herreid, C.F. Hypoxia in invertebrates. Comp. Biochem. Physiol. Part A Physiol. 1980, 67, 311–320. [Google Scholar] [CrossRef]
- Pörtner, H.-O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef]
- Calow, P. Proximate and ultimate responses to stress in biological systems. Biol. J. Linn. Soc. 1989, 37, 173–181. [Google Scholar] [CrossRef]
- Bayne, B.L.; Livingstone, D.R. Responses of Mytilus edulis L. to low oxygen tension: Acclimation of the rate of oxygen consumption. J. Comp. Physiol. B 1977, 114, 129–142. [Google Scholar] [CrossRef]
- Fleddum, A.; Cheung, S.G.; Hodgson, P.; Shin, P. Impact of hypoxia on the structure and function of benthic epifauna in Tolo Harbour, Hong Kong. Mar. Pollut. Bull. 2011, 63, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Llansó, R.J. Tolerance of low dissolved oxygen and hydrogen sulfide by the polychaete Streblospio benedicti (Webster). J. Exp. Mar. Biol. Ecol. 1991, 153, 165–178. [Google Scholar] [CrossRef]
- Rice, S.A.; Levin, L.A. Streblospio gynobranchiata, a new spionid polychaete species (Annelida: Polychaeta) from Florida and the Gulf of Mexico with an analysis of phylogenetic relationships within the genus Streblospio. Proc. Biol. Soc. Wash. 1998, 111, 694–707. [Google Scholar]
- Ferguson, H.J.; Rakocinski, C.F. Tracking marsh restoration using macrobenthic metrics: Implementing a functional approach. Wetl. Ecol. Manag. 2008, 16, 277–289. [Google Scholar] [CrossRef]
- Ritter, C.; Montagna, P.A. Seasonal hypoxia and models of benthic response in a Texas Bay. Estuaries 1999, 22, 7. [Google Scholar] [CrossRef]
- Çinar, M.E.; Ergen, Z.; Dağli, E.; Petersen, M.E. Alien species of spionid polychaetes (Streblospio gynobranchiata and Polydora cornuta) in Izmir Bay, eastern Mediterranean. J. Mar. Biol. Assoc. 2005, 85, 821–827. [Google Scholar] [CrossRef]
- Dean, D.; Mazurkiewicz, M. Methods of Culturing Polychaetes. In Culture of Marine Invertebrate Animals; Springer Science and Business Media LLC: New York, NY, USA, 1975; pp. 177–197. [Google Scholar]
- Schulze, S.R.; Rice, S.A.; Simon, J.L.; Karl, S.A. Evolution of poecilogony and the biogeography of North American populations of the polychaete Streblospio. Evolution 2000, 54, 1247–1259. [Google Scholar] [CrossRef]
- Bennett, A.D. Combined effects of dissolved oxygen and temperature on aerobic respiration and respiratory recovery responses of the spioniform polychaete, Streblospio gynobranchiata, in relation to body size. Master’s Thesis, University of Southern Mississippi, Hattiesburg, MS, USA, 2017. [Google Scholar]
- Shumway, S.E. Factors affecting oxygen consumption in the marine pulmonate Amphibola crenata (Gmelin, 1791). Biol. Bull. 1981, 160, 332–347. [Google Scholar] [CrossRef]
- Hervant, F.; Mathieu, J.; Garin, D.; Fréminet, A. Behavioral, ventilatory, and metabolic responses of the hypogean amphipod Niphargus virei and the epigean isopod Asellus aquaticus to severe hypoxia and subsequent recovery. Physiol. Zool. 1996, 69, 1277–1300. [Google Scholar] [CrossRef]
- Shumway, S.E. The effects of body size, oxygen tension and mode of life on the oxygen uptake rates of polychaetes. Comp. Biochem. Physiol. Part A Physiol. 1979, 64, 273–278. [Google Scholar] [CrossRef]
- Bridges, C.R.; Brand, A.R. The effect of hypoxia on oxygen consumption and blood lactate levels of some marine crustaceans. Comp. Biochem. Physiol. Part A Comp Physiol. 1980, 65, 399–409. [Google Scholar] [CrossRef]
- Kristensen, E. Oxygen and carbon dioxide exchange in the polychaete Nereis virens: Influence of ventilation activity and starvation. Mar. Biol. 1989, 101, 381–388. [Google Scholar] [CrossRef]
- Sagasti, A.; Schaffner, L.C.; Duffy, J.E. Epifaunal communities thrive in an estuary with hypoxic episodes. Estuaries 2000, 23, 474. [Google Scholar] [CrossRef]
- Vismann, B.; Hagerman, L. Recovery from hypoxia with and without sulfide in Saduria entomon: Oxygen debt, reduced sulfur and anaerobic metabolites. Mar. Ecol. Prog. Ser. 1996, 143, 131–139. [Google Scholar] [CrossRef]
- Linke-Gamenick, I.; Vismann, B.; Forbes, V. Effects of fluoranthene and ambient oxygen levels on survival and metabolism in three sibling species of Capitella (Polychaeta). Mar. Ecol. Prog. Ser. 2000, 194, 169–177. [Google Scholar] [CrossRef]
- Christensen, A.; Nguyen, H.; Byrne, M. Thermotolerance and the effects of hypercapnia on the metabolic rate of the ophiuroid Ophionereis schayeri: Inferences for survivors in a changing ocean. J. Exp. Mar. Biol. Ecol. 2011, 403, 31–38. [Google Scholar] [CrossRef]
- Dales, R.P. Survival of anaerobic periods by two intertidal polychaetes, Arenicola marina (L.) and Owenia fusiformis Delle Chiaje. J. Mar. Biol. Assoc. 1958, 37, 521. [Google Scholar] [CrossRef]
- Leung, Y.; Shin, P.; Qiu, J.-W.; Ang, P.; Chiu, J.; Thiyagarajan, V.; Cheung, S.G. Physiological and behavioural responses of different life stages of a serpulid polychaete to hypoxia. Mar. Ecol. Prog. Ser. 2013, 477, 135–145. [Google Scholar] [CrossRef][Green Version]
- Mangum, C. Respiratory physiology in annelids: Adaptations of marine segmented worms to the low oxygen conditions encountered in sand exemplify alternative solutions to problems that also confront higher animals. Am. Sci. 1970, 58, 641–647. [Google Scholar]
- Ali, S.S.; Hsiao, M.; Zhao, H.W.; Dugan, L.L.; Haddad, G.G.; Zhou, D. Hypoxia-adaptation involves mitochondrial metabolic depression and decreased ROS leakage. PLoS ONE 2012, 7, e36801. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Fraser, K.P.P. Why does metabolism scale with temperature? Funct. Ecol. 2004, 18, 243–251. [Google Scholar] [CrossRef]
- Gray, J.; Wu, R.; Or, Y. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 2002, 238, 249–279. [Google Scholar] [CrossRef]
- Lim, H.-S.; Diaz, R.J.; Hong, J.-S.; Schaffner, L.C. Hypoxia and benthic community recovery in Korean coastal waters. Mar. Pollut. Bull. 2006, 52, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.B. Seasonal oxygen depletion in the bottom waters of a Danish fjord and its effect on the benthic community. Oikos 2008, 34, 68–76. [Google Scholar] [CrossRef]
- Seitz, R.D.; Dauer, D.M.; Llansó, R.J.; Long, W.C. Broad-scale effects of hypoxia on benthic community structure in Chesapeake Bay, USA. J. Exp. Mar. Biol. Ecol. 2009, 381, S4–S12. [Google Scholar] [CrossRef]
- Rosenberg, R. Effect of oxygen deficiency on benthic macrofauna in fjords. In Proceedings of the Fjord Oceanography; Springer Science and Business Media LLC: New York, NY, USA, 1980; Volume 4, pp. 499–514. [Google Scholar]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef]
- Baird, D.; Christian, R.R.; Peterson, C.H.; Johnson, G.A. Consequences of hypoxia on estuarine ecosystem function: Energy diversion from consumers to microbes. Ecol. Appl. 2004, 14, 805–822. [Google Scholar] [CrossRef]
- Conley, D.J.; Carstensen, J.; Ærtebjerg, G.; Christensen, P.B.; Dalsgaard, T.; Hansen, J.L.S.; Josefson, A.B. Long-term changes and impacts of hypoxia in Danish coastal waters. Ecol. Appl. 2007, 17, S165–S184. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Wiseman, W.J., Jr. Gulf of Mexico hypoxia, aka “The dead zone”. Annu. Rev. Ecol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
| Exposure-Custom Contrasts | t-Value | df | p |
|---|---|---|---|
| (1) 0 vs 3 h for all 3 sizes | 1.054 | 33.7 | 0.299 |
| (2) 6 and 9 h vs 12 h for all 3 sizes | 0.324 | 41.0 | 0.748 |
| (3) 9 h vs 12 h for all 3 sizes | 2.313 | 33.7 | 0.027 |
| (4) 3 h vs 6, 9, and 12 h for small vs med and large | 2.835 | 32.1 | 0.008 |
| (5) 0 h vs 6, 9, and 12 h for small vs med and large | 4.113 | 29.5 | <0.001 |
| (6) 0 and 3 h vs 6, 9, and 12 h for small vs large | 3.594 | 74.4 | 0.001 |
| Recovery-Custom Contrasts | t-Value | df | p |
|---|---|---|---|
| (1) −24 h vs 0 h for all 3 sizes | −2.784 | 25.9 | 0.01 |
| (2) −24 h vs 2, 4, 6, 8, 10, and 12 h for all 3 sizes | −0.574 | 45.8 | 0.569 |
| (3) 0 h vs 2, 4, 6, 8, 10, and 12 h for all 3 sizes | 2.074 | 53.6 | 0.043 |
| (4) 2 and 4 h vs 10 and 12 h for small vs large | 1.509 | 45.4 | 0.138 |
| (5) 2 and 4 h vs 6 and 8 h for small vs large | 2.865 | 51.1 | 0.006 |
| (6) 6 and 8 h vs 10 and 12 h for small vs large | −1.640 | 48.2 | 0.107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennett, A.D.; Rakocinski, C.F. Respiration by the Opportunistic Spionid Polychaete Streblospio gynobranchiata during Adjustment to and Recovery from Moderate Hypoxia. Diversity 2020, 12, 73. https://doi.org/10.3390/d12020073
Bennett AD, Rakocinski CF. Respiration by the Opportunistic Spionid Polychaete Streblospio gynobranchiata during Adjustment to and Recovery from Moderate Hypoxia. Diversity. 2020; 12(2):73. https://doi.org/10.3390/d12020073
Chicago/Turabian StyleBennett, Alyssa D., and Chet F. Rakocinski. 2020. "Respiration by the Opportunistic Spionid Polychaete Streblospio gynobranchiata during Adjustment to and Recovery from Moderate Hypoxia" Diversity 12, no. 2: 73. https://doi.org/10.3390/d12020073
APA StyleBennett, A. D., & Rakocinski, C. F. (2020). Respiration by the Opportunistic Spionid Polychaete Streblospio gynobranchiata during Adjustment to and Recovery from Moderate Hypoxia. Diversity, 12(2), 73. https://doi.org/10.3390/d12020073





