Genetic Structure and Connectivity of the Red Mangrove at Different Geographic Scales through a Complex Transverse Hydrological System from Freshwater to Marine Ecosystems
Abstract
1. Introduction
2. Materials and Methods
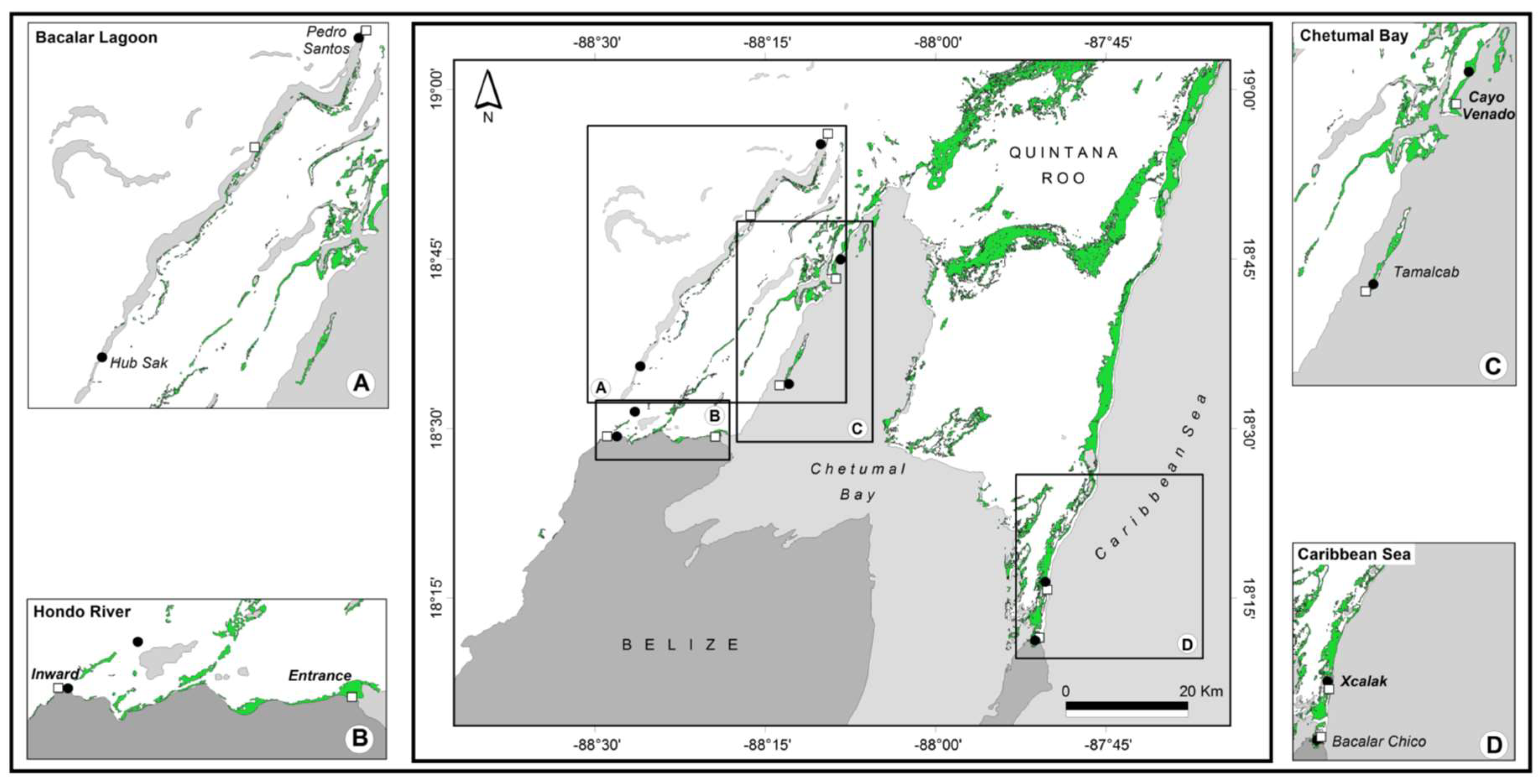
2.1. Sampling Sites and Collection
2.2. DNA Extraction and ISSR-PCR Amplification
2.3. Data Analysis
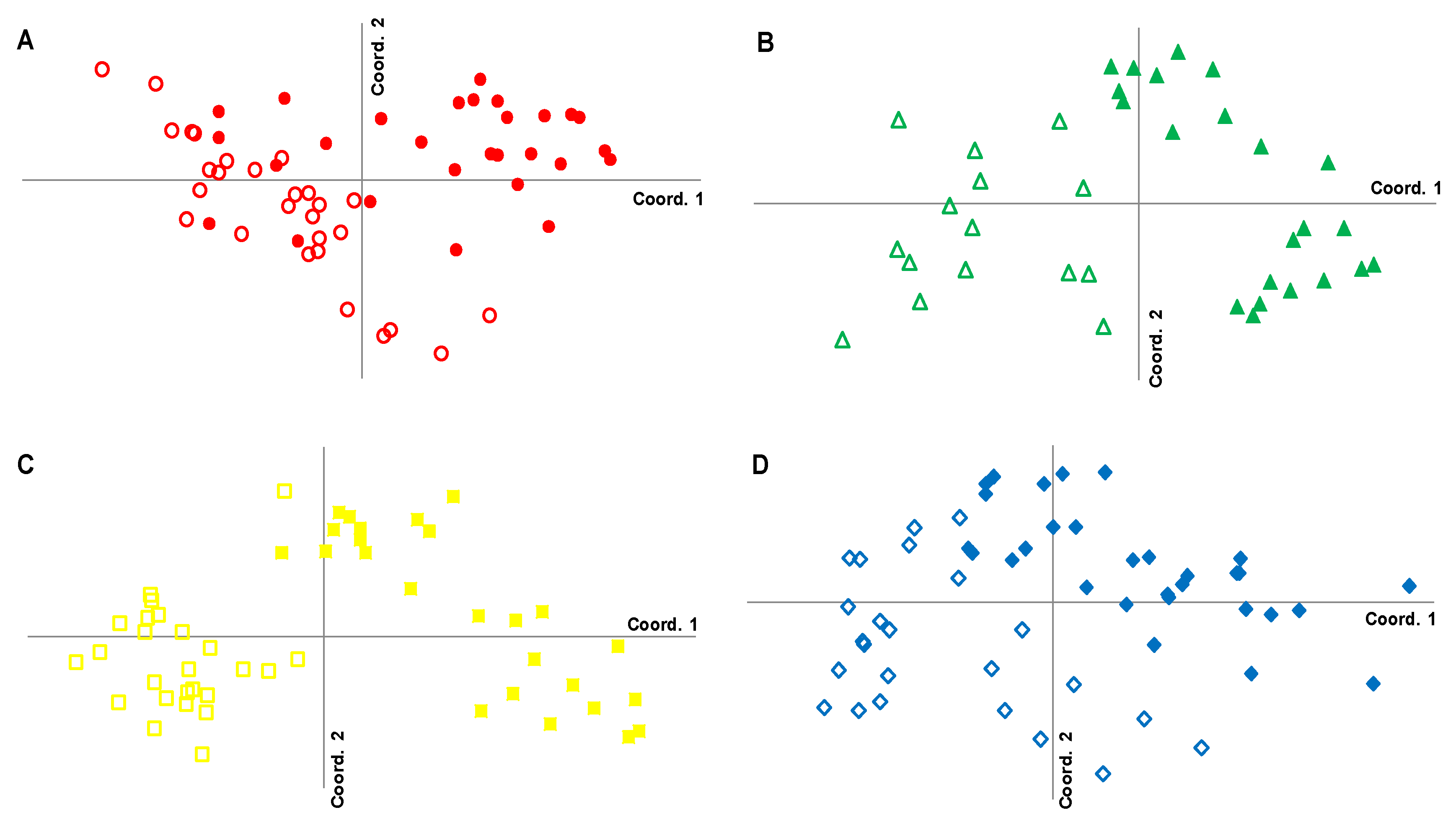
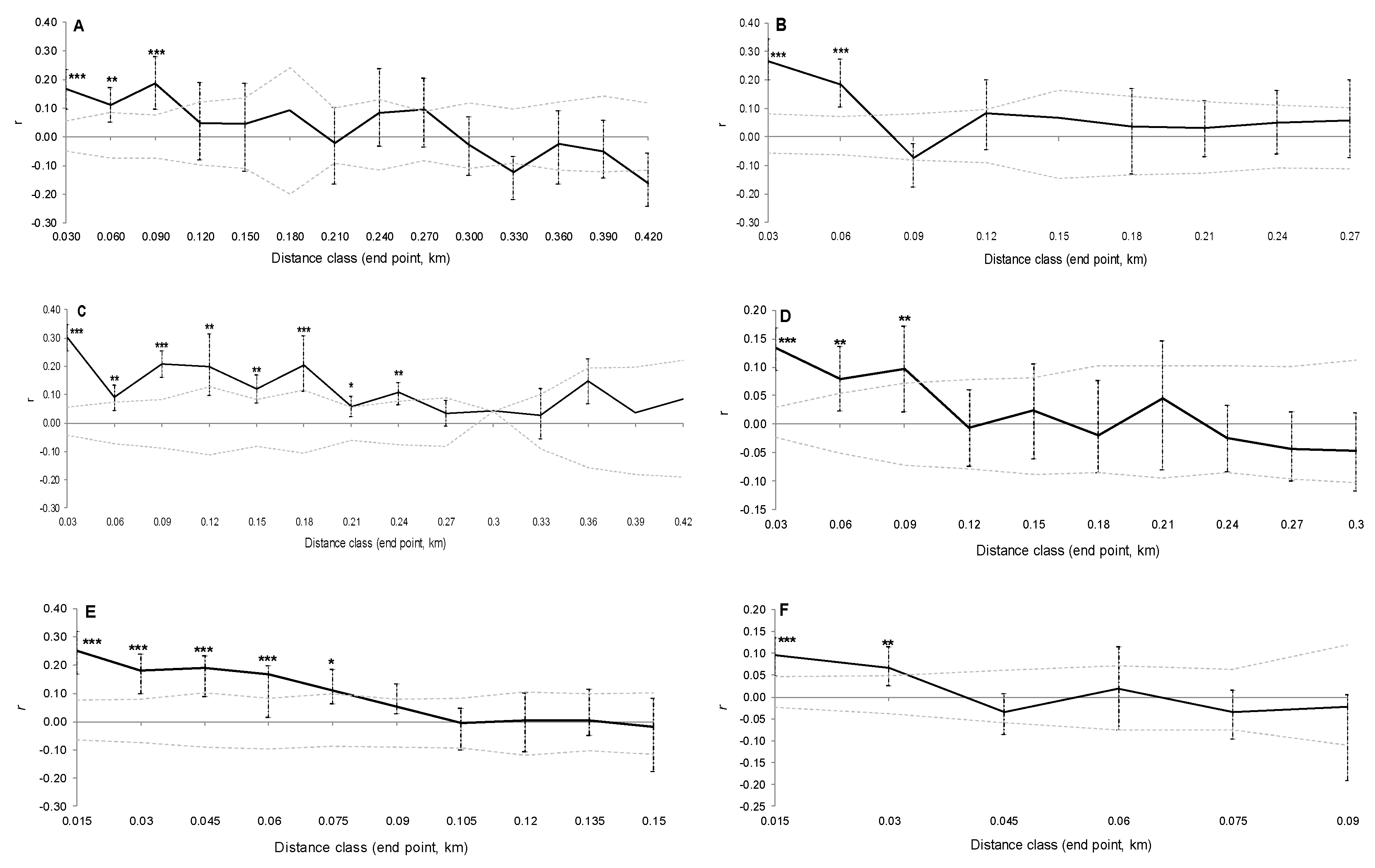
3. Results
3.1. Genetic Differentiation of Dwarf and Fringe Mangrove Trees at A Local Geographical Scale (< 100 km)
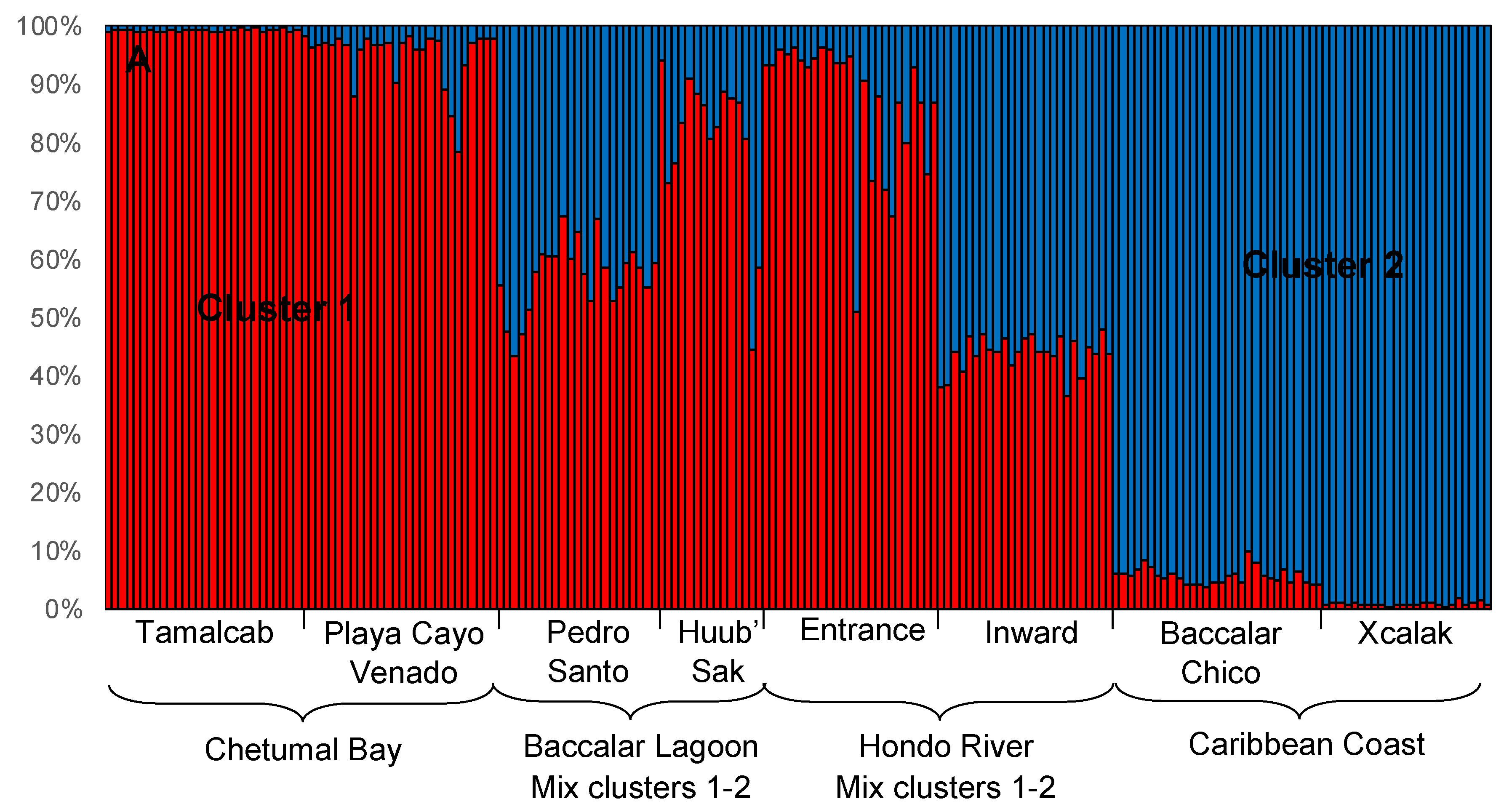
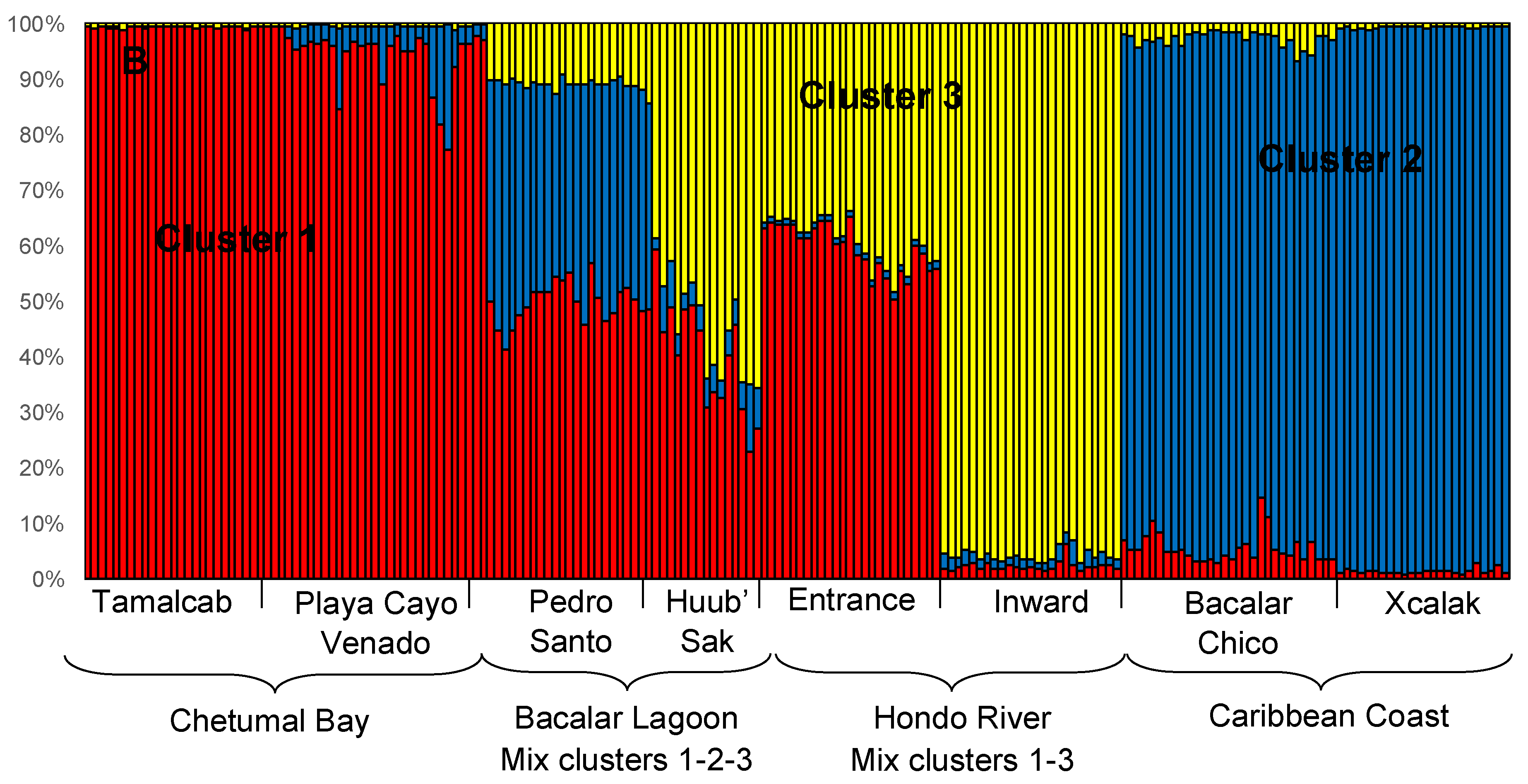
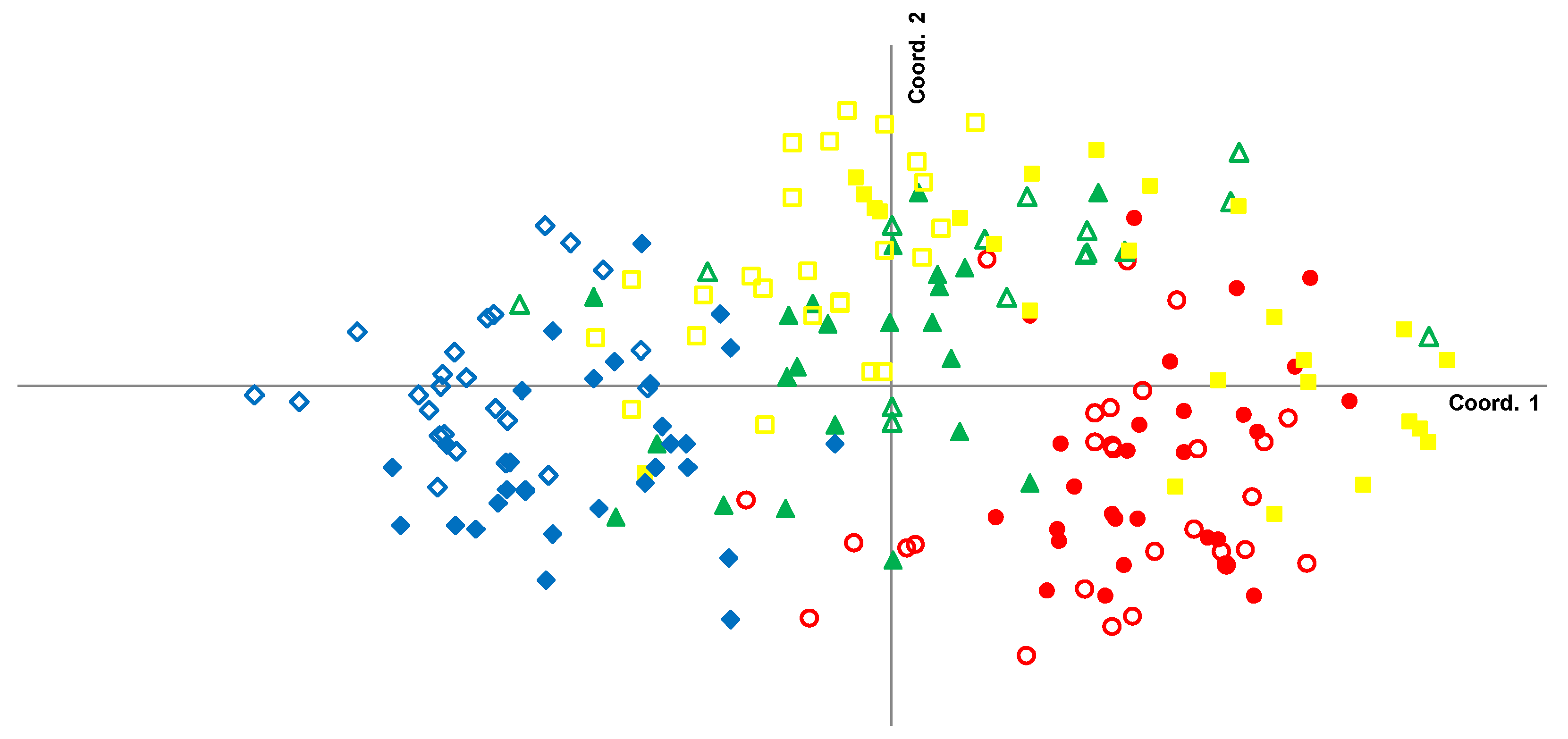
3.2. Local-Scale (10 km–100 km) Genetic Diversity and Structure of Rhizophora Mangle
3.3. Fine-Scale (<10 km) Genetic Structure of Rhizophora Mangle
3.4. Genetic Characterization of Fringe and Dwarf Physiognomic Types of Mangroves at a Fine Geographic Scale
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zaldívar-Jiménez, M.A.; Herrera-Silveira, J.A.; Teutli-Hernández, C.; Comín, F.A.; Andrade, J.L.; Molina, C.C.; Pérez Ceballos, R. Conceptual framework for mangrove restoration in the Yucatán Peninsula. Ecol. Restor. 2010, 28, 333–342. [Google Scholar] [CrossRef]
- Hernández-Arana, H.A.; Vega-Zepeda, A.; Ruiz-Zárate, M.A.; Falcón-Álvarez, L.I.; López-Adame, H.; Herrera-Silveira, J.; Kaster, J. Transverse Coastal Corridor: from freshwater lakes to coral reefs ecosystems. In Biodiversity and Conservation of the Yucatán Peninsula; Islebe, G.A., Calmé, S., Léon-Córtez, J.L., Schmook, B., Eds.; Springer: Cham, Switzerland, 2015; pp. 355–376. [Google Scholar]
- Alonji, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Schaeffer-Novelli, Y.; Cintron-Molero, G.; Adaime, R.R.; de Camargo, T.M. Variability of Mangrove Ecosystems Along the Brazilian Coast. Estuaries 1990, 13, 204–218. [Google Scholar] [CrossRef]
- Medina, E.; Cuevas, E.; Lugo, A.E. Nutrient relations of dwarf Rhizophora mangle L. mangroves on peat in eastern Puerto Rico. Plant. Ecol. 2010, 207, 13–24. [Google Scholar] [CrossRef]
- Valderrama-Landeros, L.H.; Rodríguez-Zúñiga, M.T.; Troche-Souza, C.; Velázquez-Salazar, S.; Villeda-Chávez, E.; Alcántara Maya, J.A.; Vázquez-Balderas, B.; Cruz-López, M.I.; Ressl, R. Manglares de México: actualización y exploración de los datos del sistema de monitoreo 1970/1980–2015; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2017. [Google Scholar]
- Gonçalves Reis, C.R.; Nardoto, G.B.; Casarin Rochelle, A.L.; Vieira, S.A.; Oliveira, R.S. Nitrogen dynamics in subtropical fringe and basin mangrove forests inferred from stable isotopes. Oecologia 2017, 183, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Torres, J.C.; Duran, R.; Olmsted, I. Manglares de la Península de Yucatán. In Biodiversidad Marina y Costera de México; Salazar-Vallejo, S., González, N.E., Eds.; Comisión Nacional de Biodiversidad (CONABIO) and Centro de Investigaciones de Quintana Roo (CIQRO): Quintana Roo, Mexico, 1993; pp. 660–672. [Google Scholar]
- Adame, M.F.; Zaldívar-Jimenez, A.; Teutli, C.; Caamal, J.P.; Andueza, M.T.; López-Adame, H.; Cano, R.; Hernández-Arana, H.A.; Torres-Lara, R.; Herrera-Silveira, J.A. Drivers of Mangrove Litterfall within a Karstic Region Affected by Frequent Hurricanes. Biotropica 2013, 45, 147–154. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Ellis, J.; Schwarz, A.M.; Hancock, N.; Nichols, P.; Sorrel, P. Mangrove growth in New Zealand estuaries: The role of nutrient enrichment at sites with contrasting rates of sedimentation. Oecologia 2007, 153, 633–641. [Google Scholar] [CrossRef]
- Naidoo, G. Ecophysiological differences between fringe and dwarf Avicennia marina mangroves. Trees 2010, 24, 667–673. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M.; Wachowiak, M.P.; Flores-Verdugo, F. Relationship between hyperspectral measurements and mangrove leaf nitrogen concentrations. Remote Sens. 2013, 5, 891–908. [Google Scholar] [CrossRef]
- Cerón-Souza, I.; Turner, B.L.; Winter, K.; Medina, E.; Bermingham, E.; Feliner, G.N. Reproductive phenology and physiological traits in the red mangrove hybrid complex (Rhizophora mangle and R. racemosa) across a natural gradient of nutrients and salinity. Plant. Ecol. 2014, 215, 481–493. [Google Scholar] [CrossRef]
- Cisneros-de la Cruz, D.J.; Martínez-Castillo, J.; Herrera-Silveira, J.; Yáñez-Espinosa, L.; Ortiz-García, M.; Us-Santamaria, R.; Andrade, J.L. Short-distance barriers affect genetic variability of Rhizophora mangle L. in the Yucatan Peninsula. Ecol. Evol. 2018, 8, 11083–11099. [Google Scholar] [CrossRef] [PubMed]
- Lira-Medeiros, C.F.; Parisod, C.; Fernandes, R.A.; Mata, C.S.; Cardoso, M.A.; Ferreira, P.C.G. Epigenetic variation in mangrove plants occurring in contrasting natural environment. PLoS ONE 2010, 5, e10326. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Middleton, B.; Yan, C.; Zuro, M.; Hartman, H. Landscape characteristics of Rhizophora mangle forests and propagule deposition in coastal environments of Florida (USA). Landsc. Ecol. 2005, 20, 63–72. [Google Scholar] [CrossRef]
- Sandoval-Castro, E.; Dodd, R.S.; Riosmena-Rodríguez, R.; Enríquez-Paredes, L.M.; Tovilla-Hernández, C.; López-Vivas, J.M.; Aguilar-May, B.; Muñiz-Salazar, R. Post-glacial expansion and population genetic divergence of mangrove species Avicennia germinans (L.) Stearn and Rhizophora mangle L. along the Mexican coast. PLoS ONE 2014, 9, e93358. [Google Scholar] [CrossRef] [PubMed]
- Ngeve, M.N.; Van der Stocken, T.; Menemenlis, D.; Koedam, N.; Triest, L. Hidden founders? Strong bottlenecks and fine-scale genetic structure in mangrove populations of the Cameroon Estuary complex. Hydrobiologia 2017, 803, 189–207. [Google Scholar] [CrossRef]
- Francisco, P.M.; Mori, G.M.; Alves, F.M.; Tambarussi, E.V.; de Souza, A.P. Population genetic structure; introgression; and hybridization in the genus Rhizophora along the Brazilian coast. Ecol. Evol. 2018, 8, 3491–3504. [Google Scholar] [CrossRef]
- Nilsson, C.; Brown, R.L.; Jansson, R.; Merritt, D.M. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 2010, 85, 837–858. [Google Scholar] [CrossRef]
- Nettel, A.; Dodd, R.S. Drifting propagules and receding swamps: Genetic footprints of mangrove recolonization and dispersal along tropical coasts. Evolution Int. J. Org. Evolution 2007, 61, 958–971. [Google Scholar] [CrossRef]
- Cerón-Souza, I.; Bermingham, E.; McMillan, W.O.; Jones, F.A. Comparative genetic structure of two mangrove species in Caribbean and Pacific estuaries of Panama. BMC Evol. Biol. 2012, 12, 205. [Google Scholar] [CrossRef]
- Mori, G.M.; Zucchi, M.I.; Souza, A.P. Multiple-geographic-scale genetic structure of two mangrove tree species: the roles of mating system; hybridization; limited dispersal and extrinsic factors. PLoS ONE 2015, 10, e0118710. [Google Scholar] [CrossRef] [PubMed]
- Hodel, R.G.; Cortez, M.B.; Soltis, P.S.; Soltis, D.E. Comparative phylogeography of black mangroves (Avicennia germinans) and red mangroves (Rhizophora mangle) in Florida: Testing the maritime discontinuity in coastal plants. Am. J. Bot. 2016, 103, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Pil, M.W.; Boeger, M.R.; Muschner, V.C.; Pie, M.R.; Ostrensky, A.; Boeger, W.A. Postglacial north–south expansion of populations of Rhizophora mangle (Rhizophoraceae) along the Brazilian coast revealed by microsatellite analysis. Am. J. Bot. 2011, 98, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Arbeláez-Cortes, E.; Castillo-Cárdenas, M.F.; Toro-Perea, N.; Cárdenas-Henao, H. Genetic structure of the red mangrove (Rhizophora mangle L.) on the Colombian Pacific detected by microsatellite molecular markers. Hydrobiologia 2007, 583, 321–330. [Google Scholar] [CrossRef]
- Núñez-Farfán, J.; Domínguez, C.A.; Eguiarte, L.E.; Cornejo, A.; Quijano, M.; Vargas, J.; Dirzo, R. Genetic divergence among Mexican populations of red mangrove (Rhizophora mangle): Geographic and historic effects. Evol. Ecol. Res. 2002, 4, 1049–1064. [Google Scholar]
- Sandoval-Castro, E.; Muniz-Salazar, R.; Enríquez-Paredes, L.M.; Riosmena-Rodríguez, R.; Dodd, R.S.; Tovilla-Hernández, C.; Arredondo-García, M.C. Genetic population structure of red mangrove (Rhizophora mangle L.) along the northwestern coast of Mexico. Aquat. Bot. 2012, 99, 20–26. [Google Scholar] [CrossRef]
- Reyes Medina, I.; Salazar, R.M.; Arango, G.H.; Vivas, J.L.; Rodríguez, R.R. Genetic analysis for red mangrove reforestation (Rhizophora mangle L.). In The Arid Mangrove Forest from Baja California Peninsula; Riosmena Rodríguez, R., López Vivas, J.M., Hinojosa Arango, G., Eds.; Nova Science Pub Inc.: London, UK, 2016; pp. 35–51. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Murray, G. Constructing paradise: The impacts of big tourism in the Mexican coastal zone. Coast. Manag. 2007, 35, 339–355. [Google Scholar] [CrossRef]
- Hirales-Cota, M.; Espinoza-Avalos, J.; Schmook, B.; Ruiz-Luna, A.; Ramos-Reyes, R. Drivers of mangrove deforestation in Mahahual-Xcalak; Quintana Roo; southeast Mexico. Cienc. Mar. 2010, 36, 147–159. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, F.; Su, G.; Deng, S.; He, H.; Shi, S. Population genetic structure of three tree species in the mangrove genus Ceriops (Rhizophoraceae) from the Indo West Pacific. Genetica 2008, 133, 47–56. [Google Scholar] [CrossRef]
- Takayama, K.; Tamura, M.; Tateishi, Y.; Webb, E.L.; Kajita, T. Strong genetic structure over the American continents and transoceanic dispersal in the mangrove genus Rhizophora (Rhizophoraceae) revealed by broad-scale nuclear and chloroplast DNA analysis. Am. J. Bot. 2013, 100, 1191–1201. [Google Scholar] [CrossRef]
- Maguire, T.L.; Peakall, R.; Saenger, P. Comparative analysis of genetic diversity in the mangrove species Avicennia marina (Forsk.) Vierh. (Avicenniaceae) detected by AFLPs and SSRs. Theor. Appl. Genet. 2002, 104, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Nandy, P.; Sengupta, C.; Das, S. RAPD and ISSR marker mediated genetic polymorphism of two mangroves Bruguiera gymnorrhiza and Heritiera fomes from Indian Sundarbans in relation to their sustainability. Physiol. Mol. Biol. Pla. 2015, 21, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.J.; Sun, M. Population genetic structure of Ceriops tagal (Rhizophoraceae) in Thailand and China. Wetl. Ecol. Manag. 2001, 9, 213–219. [Google Scholar] [CrossRef]
- Su, G.; Huang, Y.; Tan, F.; Ni, X.; Tang, T.; Shi, S. Conservation genetics of Lumnitzera littorea (Combretaceae); an endangered mangrove; from the Indo-West Pacific. Mar. Biol. 2007, 150, 321–328. [Google Scholar] [CrossRef]
- Kader, A.; Sinha, S.N.; Ghosh, P. Evaluation of genetic diversity of Avicenniaceae family in Indian sundarban by using RAPD and ISSR markers. Iran. J. Genet. Plant. Breed 2012, 1, 22–27. [Google Scholar]
- Machkour-M’Rabet, S.; Cruz-Medina, J.; García-De León, F.J.; De Jesús-Navarrete, A.; Hénaut, Y. Connectivity and genetic structure of the queen conch on the Mesoamerican Reef. Coral Reefs 2017, 36, 535–548. [Google Scholar] [CrossRef]
- Tanya, P.; Taeprayoon, P.; Hadkam, Y.; Srinives, P. Genetic diversity among Jatropha and Jatropha-related species based on ISSR markers. Plant Mol. Biol. Rep. 2011, 29, 252–264. [Google Scholar] [CrossRef]
- Kumar, H.; Priya, P.; Singh, N.; Kumar, M.; Kumar Choudhary, B.; Kumar, L. RAPD and ISSR marker-based comparative evaluation of genetic diversity among Indian germplasms of Euryale ferox: An aquatic food plant. App. Biochem. Biotechnol. 2016, 180, 1345–1360. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic homogeneity revealed using SCoT; ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle-an endemic and endangered medicinal plant. PLoS ONE 2016, 11, e0159050. [Google Scholar] [CrossRef]
- Hernández-Arana, H.A.; Rowden, A.A.; Attril, M.J.; Warwick, R.M.; Gold Bouchot, G. Large-scale environmental influences on the benthic macroinfauna of the Southern Gulf of Mexico. Estuar. Coast. Shelf. Sci. 2003, 58, 825–841. [Google Scholar] [CrossRef]
- Torrescano-Valle, N.; Folan, W.J. Physical settings, environmental history with an outlook on global change. In Biodiversity and Conservation of the Yucatan Peninsula; Islebe, G., Calmè, S., León-Cortes, J.L., Schmook, B., Eds.; Springer: Cham, Switzerland, 2015; pp. 9–37. [Google Scholar]
- Calderon-Aguilera, L.E.; Rivera-Monroy, V.H.; Porter-Bolland, L.; Martínez-Yrízar, A.; Ladah, L.B.; Martínez-Ramos, M.; Alcocer, J.; Santiago-Pérez, A.L.; Hernandez-Arana, H.A.; Reyes-Gómez, V.M.; et al. An assessment of natural and human disturbance effects on Mexican ecosystems: Current trends and research gaps. Biodivers Conserv. 2012, 21, 589–617. [Google Scholar] [CrossRef]
- Martínez-Castillo, J.; Camacho-Pérez, L.; Villanueva-Viramontes, S.; Andueza-Noh, R.H.; Chacón-Sánchez, M.I. Genetic structure within the Mesoamerican gene pool of wild Phaseolus lunatus (Fabaceae) from Mexico as revealed by microsatellite markers: Implications for conservation and the domestication of the species. Am. J. Bot. 2014, 101, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, K.E.; Lewis, P.O.; Dey, D.K. A Bayesian method for analysis of genetic population structure with dominant marker data. Mol. Ecol. 2002, 11, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, K.E.; Lewis, P.O. Hickory: A package for analysis of population genetic data v1. 0. Department of Ecology and Evolutionary Biology; University of Connecticut: Storrs, CT, USA, 2003. [Google Scholar]
- Manrique-Poyato, M.I.; López-León, M.D.; Gómez, R.; Perfectti, F.; Camacho, J.P.M. Population genetic structure of the grasshopper Eyprepocnemis plorans in the south and east of the Iberian Peninsula. PLoS ONE 2013, 8, e59041. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P.J. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef]
- Hoffman, J.I.; Peck, L.S.; Linse, K.; Clarke, A. Strong population genetic structure in a broadcast-spawning Antarctic marine invertebrate. J. Hered. 2011, 102, 55–66. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Wen, X.; Falush, D. Documentation for STRUCTURE software, version 2.3, University of Chicago: Chicago, IL, USA, 2010.
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Smouse, P.E.; Peakall, R. Spatial autocorrelation analysis of multi-allele and multi-locus genetic microstructure. Heredity 1999, 82, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Ruibal, M.; Lindenmayer, D.B. Spatial autocorrelation analysis offers new insights into gene flow in the Australian bush rat, Rattus fuscipes. Evolution 2003, 57, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Carriconde, F.; Gryta, H.; Jargeat, P.; Mouhamadou, B.; Gardes, M. High sexual reproduction and limited contemporary dispersal in the ectomycorrhizal fungus Tricholoma scalpturatum: New insights from population genetics and spatial autocorrelation analysis. Mol. Ecol. 2005, 17, 4433–4445. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Arana, H.A.; Espinoza-Avalos, J.; Islebe, G.A. Introducción y perspectivas. In El sistema ecológico de la bahía de Chetumal/Corozal: costa occidental del Mar Caribe; Espinoza-Avalos, J., Islebe, G.A., Hernández-Arana, H.A., Eds.; ECOSUR: Chetumal, México, 2009; pp. 1–4. [Google Scholar]
- Hernández-Arana, H.A.; Ameneyro-Angeles, B. Benthic biodiversity changes due to the opening of an artificial channel in a tropical coastal lagoon (Mexican Caribbean). J. Mar. Biol. Assoc. U. K. 2011, 91, 969–978. [Google Scholar] [CrossRef]
- Ngeve, M.N.; Van der Stocken, T.; Menemenlis, D.; Koedam, N.; Triest, L. Contrasting effects of historical sea level rise and contemporary ocean currents on regional gene flow of Rhizophora racemosa in Eastern Atlantic mangroves. PLoS ONE 2016, 11, e0150950. [Google Scholar] [CrossRef]
- Torrescano, N.; Islebe, G.A. Tropical forest and mangrove history from southeastern Mexico: A 5000 yr pollen record and implications for sea level rise. Veg. Hist. Archaeobot. 2006, 15, 191–195. [Google Scholar] [CrossRef]
- Aragón-Moreno, A.A.; Islebe, G.A.; Roy, P.D.; Torrescano-Valle; Mueller, A.D. Climate forcings on vegetation of the southeastern Yucatán Peninsula (Mexico) during the middle to late Holocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 495, 214–226. [Google Scholar]
- Ruiz-Ramírez, J.D.; Euán-Ávila, J.I.; Torres-Irineo, E. Tendencias del nivel del mar en las costas del Caribe mexicano. Eur. Sci. J. 2014, 10, 86–96. [Google Scholar]
- Ezer, T.; Thattai, D.V.; Kjerfve, B.; Heyman, W.D. On the variability of the flow along the Meso-American Barrier Reef system: a numerical model study of the influence of the Caribbean current and eddies. Ocean. Dynam. 2005, 55, 458–475. [Google Scholar] [CrossRef]
- Chérubin, L.M.; Kuchinke, C.P.; Paris, C.B. Ocean circulation and terrestrial runoff dynamics in the Mesoamerican region from spectral optimization of SeaWiFS data and a high resolution simulation. Coral Reefs 2008, 27, 503–519. [Google Scholar] [CrossRef]
- Avalos-Cueva, D.; Palacios-Hernandez, E.; Carrillo, L.; Gonzalez-Vivanco, L.A. Numerical models as tools to understand the dynamics in Bays: case of study Chetumal Bay; Quintana Roo. Ra Ximhai 2017, 13, 267–290. [Google Scholar]
- Chen, S.B.; Ding, W.Y.; Qiu, J.B.; Wang, G.Y.; Zhou, Z.M.; Chen, J.F.; Ai, W.M.; Wang, C.Y.; Xie, Q.L. The genetic diversity of the mangrove Kandelia obovata in China revealed by ISSR analysis. Pak. J. Bot. 2010, 42, 3755–3764. [Google Scholar]
- Jena, S.N.; Verma, S.; Nair, K.N.; Srivastava, A.K.; Misra, S.; Rana, T.S. Genetic diversity and population structure of the mangrove lime (Merope angulata) in India revealed by AFLP and ISSR markers. Aquat. Bot. 2015, 120, 260–267. [Google Scholar] [CrossRef]
- Jian, S.; Ban, J.; Ren, H.; Yan, H. Low genetic variation detected within the widespread mangrove species Nypa fruticans (Palmae) from Southeast Asia. Aquat. Bot. 2010, 92, 23–27. [Google Scholar] [CrossRef]
- Li, H.S.; Chen, G.Z. Genetic diversity of mangrove plant Sonneratia caseolaris in Hainan Island based on ISSR analysis. Acta Ecol. Sin 2004, 24, 1657–1663. [Google Scholar]
- Van der Stocken, T.; De Ryck, D.J.R.; Vanschoenwinkel, B.; Deboelpaep, E.; Bouma, T.J.; Dahdouh-Guebas, F. Impact of landscape structure on propagule dispersal in mangrove forests. Mar. Ecol. Progr. Ser. 2015, 524, 95–106. [Google Scholar] [CrossRef]
- Alleman, L.K.; Hester, M.W. Reproductive ecology of black mangrove (Avicennia germinans) along the Louisiana coast: Propagule production cycles, dispersal limitations, and establishment elevations. Estuaries Coast. 2011, 34, 1068. [Google Scholar] [CrossRef]
- Schwarzbach, A.E.; Ricklefs, R.E. The use of molecular data in mangrove plant research. Wetl. Ecol. Manag. 2001, 9, 205–211. [Google Scholar] [CrossRef]
- Twilley, R.R.; Rivera-Monroy, V.H. Developing performance measures of mangrove wetlands using simulation models of hydrology; nutrient biogeochemistry; and community dynamics. J. Coast. Res. 2005, 40, 79–93. [Google Scholar]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
| Hydrological System | Locality | Physiognomic Type | n | PPL | He | hs |
|---|---|---|---|---|---|---|
| Chetumal Bay | Tamalcaba | Fringe | 15 | 48.15 | 0.182 (0.023) | 0.228 (0.012) |
| Dwarf | 13 | 55.56 | 0.209 (0.023) | 0.244 (0.011) | ||
| Playa Cayo Venado | Fringe | 15 | 58.02 | 0.222 (0.024) | 0.251 (0.010) | |
| Dwarf | 13 | 58.02 | 0.211 (0.023) | 0.249 (0.012) | ||
| Mean | 56 | 79.01 | 0.271 (0.022) | 0.266 (0.011) | ||
| Bacalar Lagoon | Pedro Santos | Fringe | 11 | 54.32 | 0.197 (0.023) | 0.248 (0.012) |
| Dwarf | 12 | 60.49 | 0.246 (0.024) | 0.266 (0.012) | ||
| Huub’ Sak | Fringe | 6 | 49.38 | 0.184 (0.023) | 0.245 (0.013) | |
| Dwarf | 9 | 56.79 | 0.224 (0.024) | 0.268 (0.012) | ||
| Mean | 38 | 81.48 | 0.296 (0.020) | 0.288 (0.011) | ||
| Hondo River | Entrance | Fringe | 13 | 54.32 | 0.177 (0.023) | 0.228 (0.010) |
| Dwarf | 12 | 65.43 | 0.233 (0.023) | 0.259 (0.011) | ||
| Inward | Fringe | 13 | 60.49 | 0.191 (0.022) | 0.251 (0.013) | |
| Dwarf | 12 | 61.73 | 0.231 (0.023) | 0.262 (0.011) | ||
| Mean | 50 | 88.89 | 0.292 (0.021) | 0.288 (0.009) | ||
| Caribbea Coasts | Xcalak | Fringe | 12 | 46.91 | 0.188 (0.024) | 0.252 (0.013) |
| Dwarf | 12 | 54.32 | 0.221 (0.024) | 0.268 (0.011) | ||
| Bacalar Chico | Fringe | 15 | 66.67 | 0.241 (0.023) | 0.263 (0.011) | |
| Dwarf | 15 | 61.73 | 0.223 (0.022) | 0.257 (0.011) | ||
| Mean | 54 | 81.48 | 0.291 (0.021) | 0.287 (0.012) |
| Chetumal Bay | Bacalar Lagoon | Hondo River | Caribbean Coast | |
|---|---|---|---|---|
| Chetumal Bay | 0.0001 | 0.0001 | 0.0001 | |
| Bacalar Lagoon | 0.119 | 0.0001 | 0.0001 | |
| Hondo River | 0.132 | 0.113 | 0.0001 | |
| Caribbean Coast | 0.192 | 0.143 | 0.164 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chablé Iuit, L.R.; Machkour-M’Rabet, S.; Espinoza-Ávalos, J.; Hernández-Arana, H.A.; López-Adame, H.; Hénaut, Y. Genetic Structure and Connectivity of the Red Mangrove at Different Geographic Scales through a Complex Transverse Hydrological System from Freshwater to Marine Ecosystems. Diversity 2020, 12, 48. https://doi.org/10.3390/d12020048
Chablé Iuit LR, Machkour-M’Rabet S, Espinoza-Ávalos J, Hernández-Arana HA, López-Adame H, Hénaut Y. Genetic Structure and Connectivity of the Red Mangrove at Different Geographic Scales through a Complex Transverse Hydrological System from Freshwater to Marine Ecosystems. Diversity. 2020; 12(2):48. https://doi.org/10.3390/d12020048
Chicago/Turabian StyleChablé Iuit, Landy R., Salima Machkour-M’Rabet, Julio Espinoza-Ávalos, Héctor A. Hernández-Arana, Haydée López-Adame, and Yann Hénaut. 2020. "Genetic Structure and Connectivity of the Red Mangrove at Different Geographic Scales through a Complex Transverse Hydrological System from Freshwater to Marine Ecosystems" Diversity 12, no. 2: 48. https://doi.org/10.3390/d12020048
APA StyleChablé Iuit, L. R., Machkour-M’Rabet, S., Espinoza-Ávalos, J., Hernández-Arana, H. A., López-Adame, H., & Hénaut, Y. (2020). Genetic Structure and Connectivity of the Red Mangrove at Different Geographic Scales through a Complex Transverse Hydrological System from Freshwater to Marine Ecosystems. Diversity, 12(2), 48. https://doi.org/10.3390/d12020048







