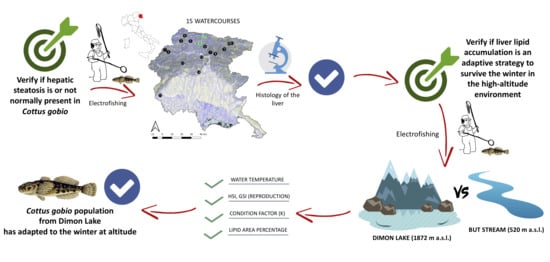
Liver Lipid Accumulation in European Bullhead (Cottus cobio) from a High-Mountain Lake: An Adaptive Strategy to Survive the Adverse Winter Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.3. Histological, Cytological Analysis and Lipid Content
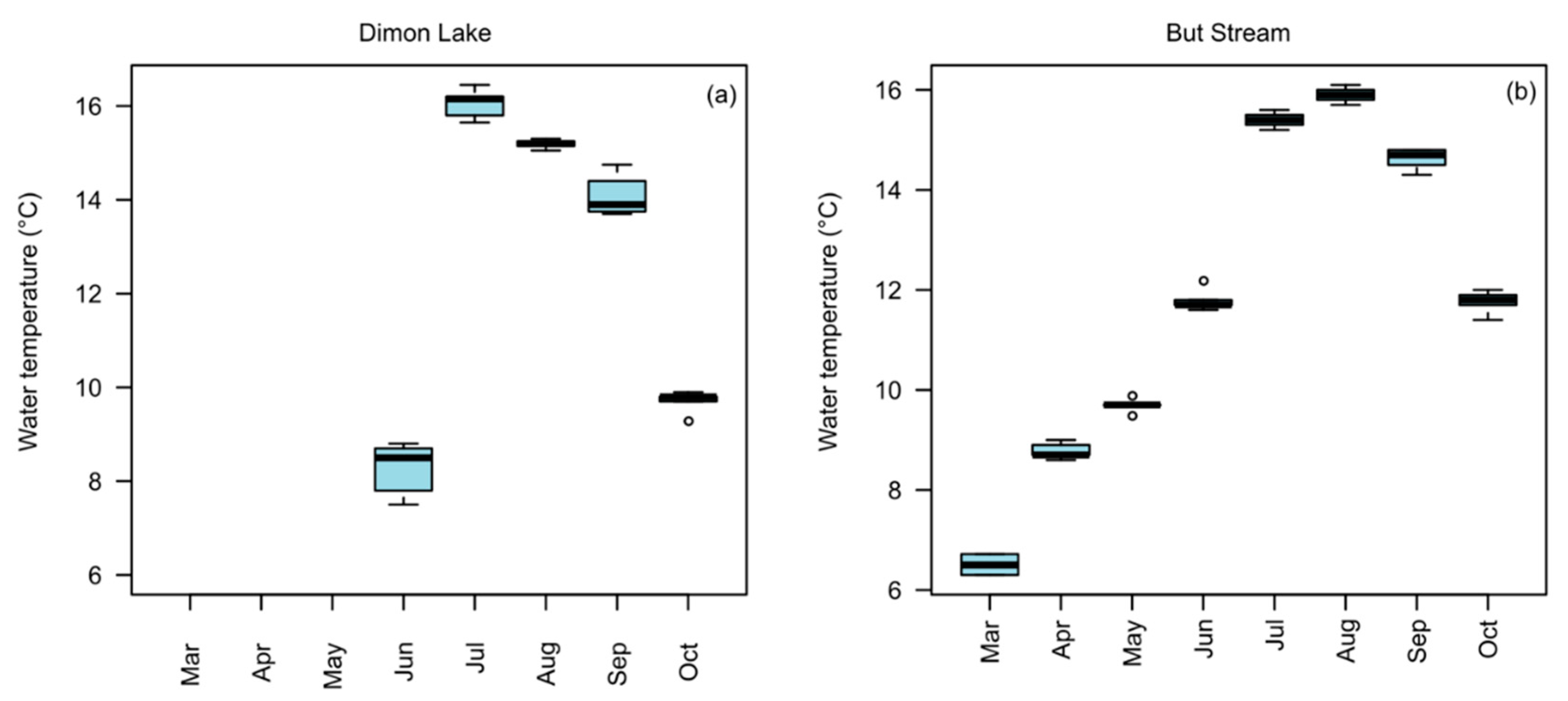
2.4. Determination of Water Temperature in Dimon Lake and But Stream
2.5. Statistical Analysis
2.6. Ethical Statement
3. Results
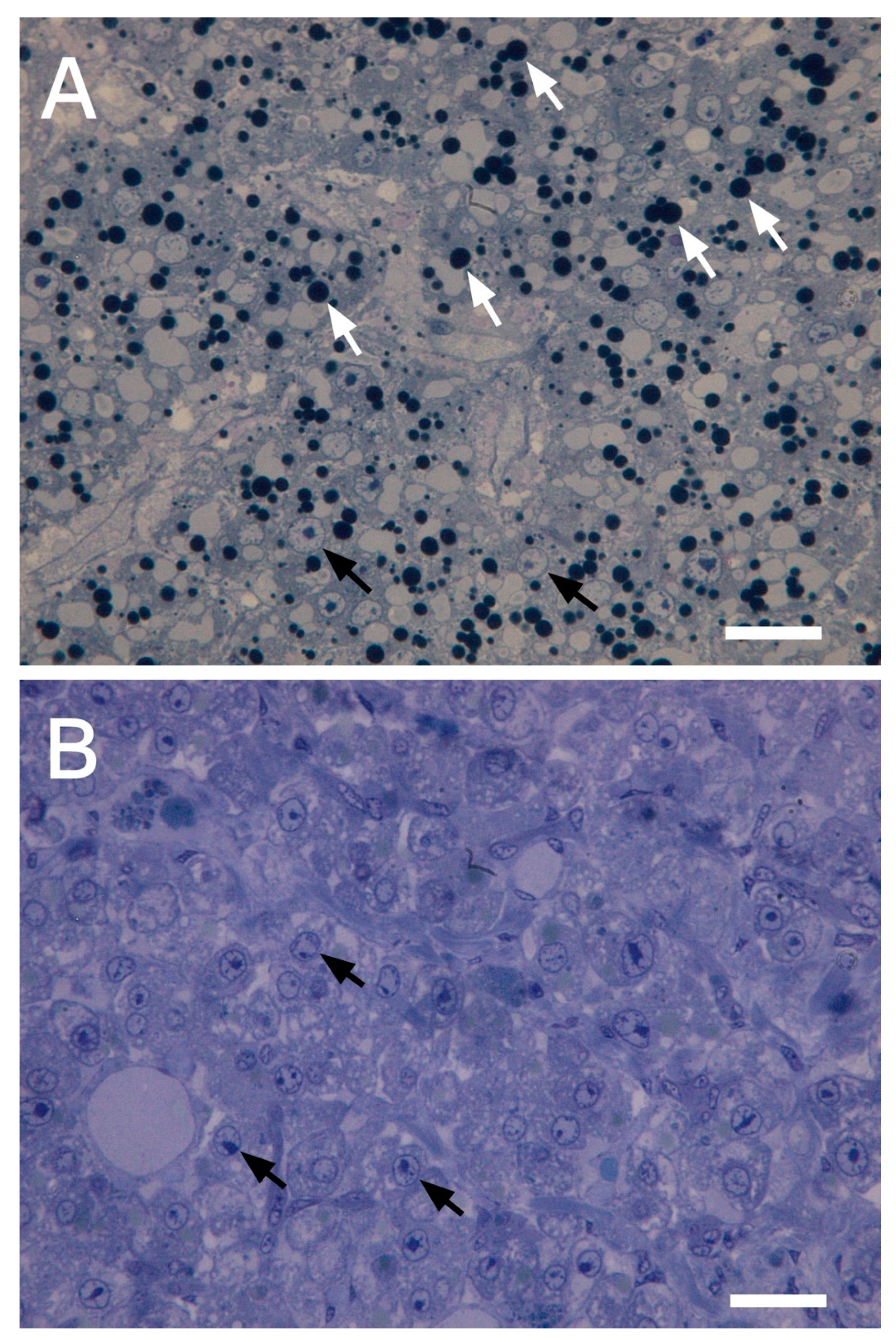
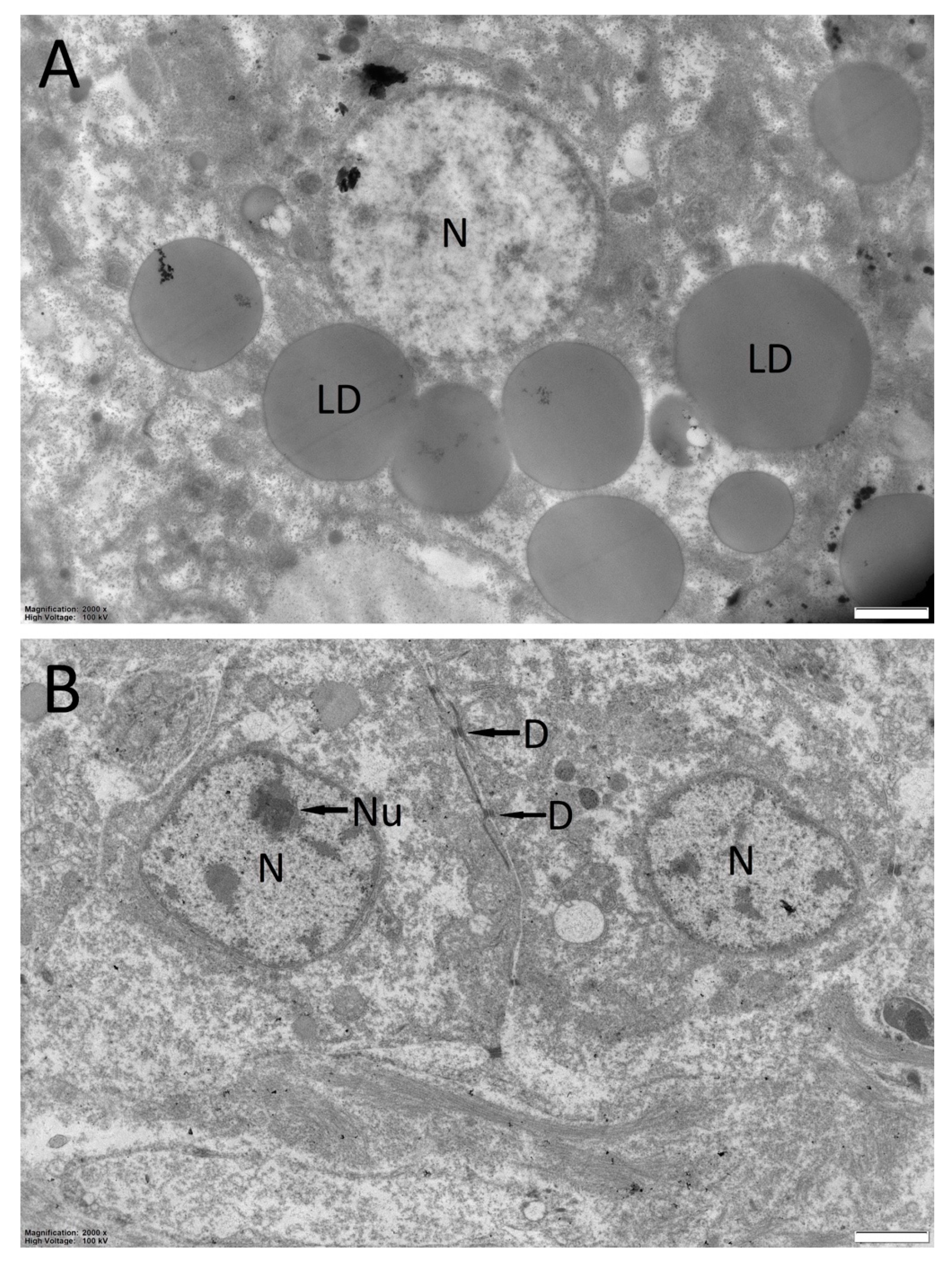
3.1. Hepatic Steatosis in Specimens of Bullhead from Watercourses
3.2. Water temperature in Dimon Lake and But Stream
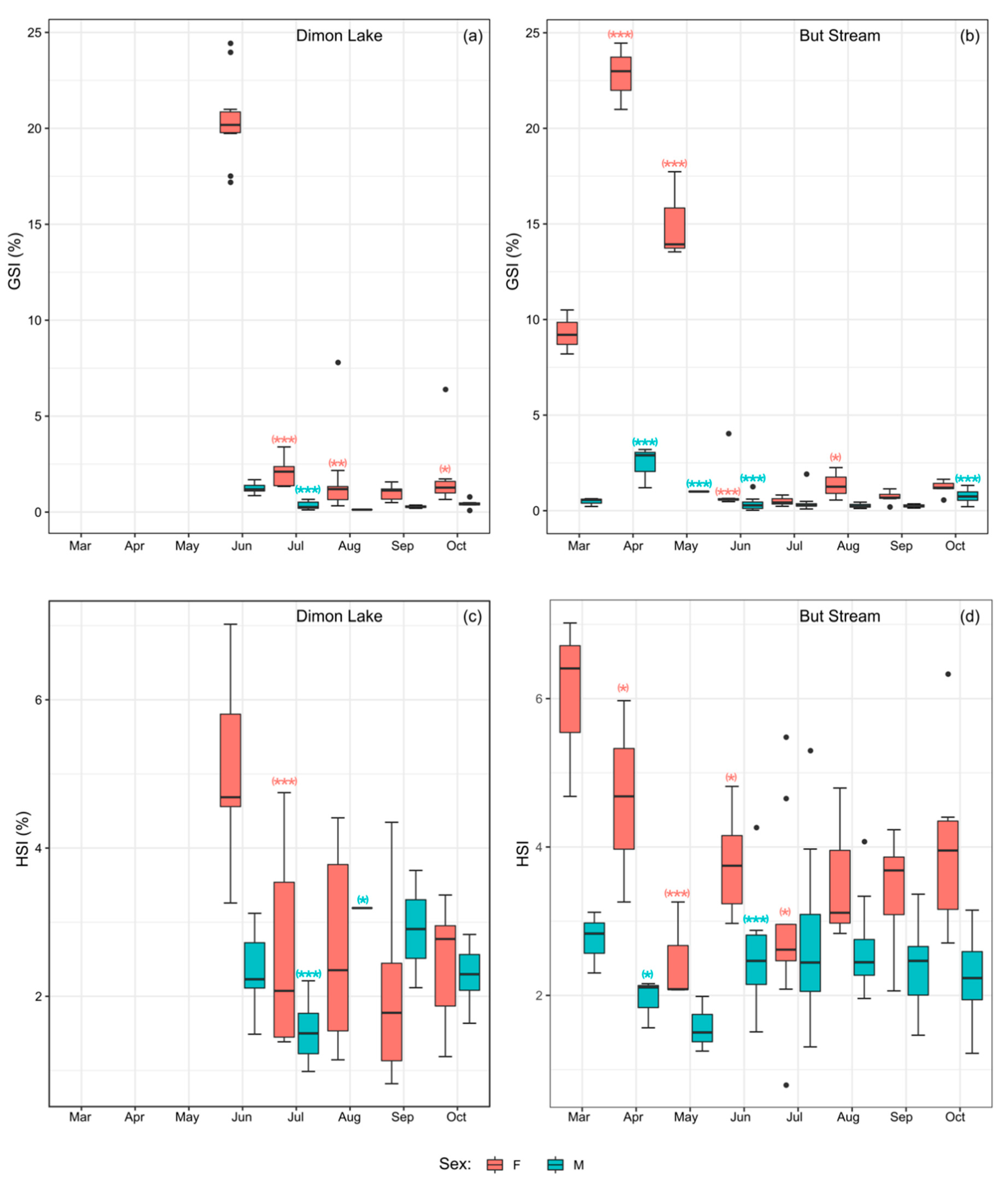
3.3. Biometric Features, Gonadosomatic Index (GSI), Hepatosomatic Index (HSI) and K Index of Bullhead from Dimon Lake and But Stream
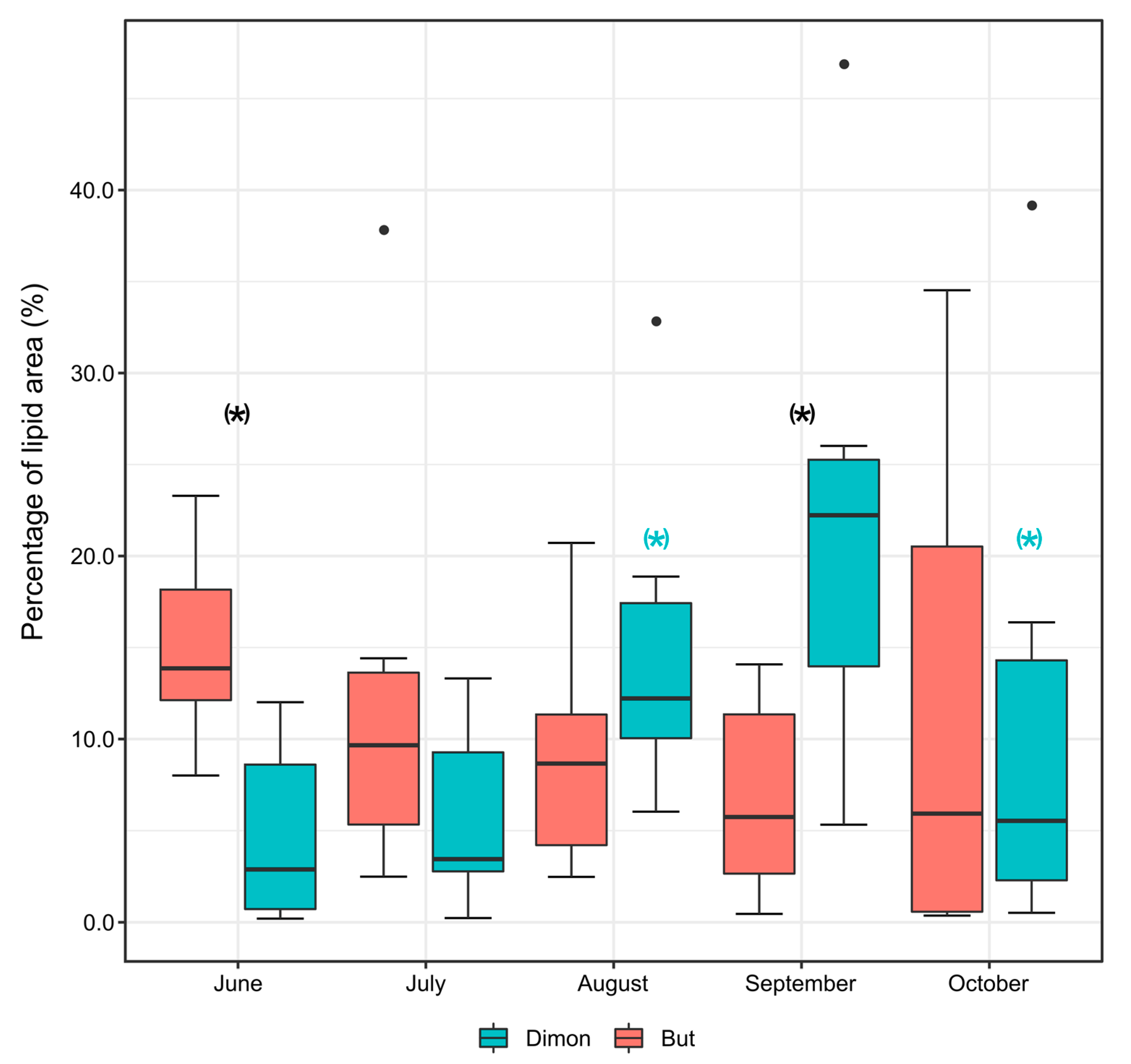
3.4. Histological Analysis and Lipid Content in Bullhead from Dimon Lake and But Stream
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tao, Y.F.; Qiang, J.; Bao, J.W.; Chen, D.J.; Yin, G.J.; Xu, P.; Zhu, H.J. Changes in physiological parameters, lipid metabolism, and expression of MicroRNAs in genetically improved farmed Tilapia (Oreochromis niloticus) with fatty liver induced by a high-fat diet. Front. Physiol. 2018, 9, 1521. [Google Scholar] [CrossRef]
- Sheridan, M.A. Lipid dynamics in fish: Aspects of absorption, transportation, deposition and mobilization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1988, 90, 679–690. [Google Scholar] [CrossRef]
- Fernandes, T.; McMeans, B.C. Coping with the cold: Energy storage strategies for surviving winter in freshwater fish. Ecography 2019, 42, 2037–2052. [Google Scholar] [CrossRef]
- Shuter, B.J.; Finstad, A.G.; Helland, I.P.; Zweimüller, I.; Hölker, F. The role of winter phenology in shaping the ecology of freshwater fish and their sensitivities to climate change. Aquat. Sci. 2012, 74, 637–657. [Google Scholar] [CrossRef]
- Shimeno, S.; Kheyyali, D.; Takeda, M. Metabolic adaptation to prolonged starvation in carp. Nippon Suisan Gakkaishi 1990, 56, 35–41. [Google Scholar] [CrossRef][Green Version]
- Coker, G.A.; Portt, C.B.; Minns, C.K. Morphological and ecological characteristics of Canadian freshwater fishes. Can. Manuscr. Rep. Fish. Aquat. Sci. 2001, 2554. Available online: https://waves-vagues.dfo-mpo.gc.ca/Library/254364.pdf (accessed on 12 November 2020).
- Evans, D.O. Temperature independence of the annual cycle of standard metabolism in the pumpkinseed. Trans. Am. Fish. Soc. 1984, 113, 494–512. [Google Scholar] [CrossRef]
- Heermann, L.; Borderding, J. Winter short-distance migration of juvenile fish between two floodplain water bodies of the Lower River Rhine. Ecol. Freshw. Fish. 2006, 15, 161–168. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Secor, S.M.; Carey, H.V. Integrative physiology of fasting. Compr. Physiol 2016, 6, 773–825. [Google Scholar]
- Pastorino, P.; Prearo, M.; Pizzul, E.; Bertoli, M.; Francese, D.R.; Menconi, V.; Mugetti, D.; Bozzetta, E.; Varello, K. Hepatic steatosis in a bullhead (Cottus gobio) population from a high-mountain lake (Carnic Alps): Adaptation to an extreme ecosystem? Water 2019, 11, 2570. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Bertoli, M.; Abete, M.C.; Dondo, A.; Salvi, G.; Zaccaroni, A.; Abete, M.C.; Elia, A.C.; Pizzul, E. Accumulation of As, Cd, Pb, and Zn in sediment, chironomids and fish from a high-mountain lake: First insights from the Carnic Alps. Sci. Total Environ. 2020, 729, 139007. [Google Scholar] [CrossRef] [PubMed]
- Venturini, C. Il Friuli nel Quaternario: L’evoluzione del territorio. In Catalogo Alla Mostra: Glacies. L’età dei ghiacci in Friuli: Ambienti, Climi e Vita Negli Ultimi 100.000 Anni; Muscio, G., Ed.; Museo Friulano di Storia Naturale: Udine, Italy, 2003; pp. 23–106. [Google Scholar]
- Venturini, C. Un viaggio nel tempo tra cause ed effetti. In Glacies L’età Dei Ghiacci in Friuli: Ambienti, Climi e Vita Negli Ultimi 100.000 Anni; Museo Friulano di Storia Naturale: Udine, Italy, 2003; pp. 1–63. [Google Scholar]
- Venturini, C. Evoluzione geologica delle Alpi Carniche. In Carta Geologica Delle Alpi Carniche. 2 Fogli 1:25.000; Museo Friulano di Storia Naturale, Comune di Udine: Udine, Italy, 2006; pp. 1–2. [Google Scholar]
- Pastorino, P.; Polazzo, F.; Bertoli, M.; Santi, M.; Righetti, M.; Pizzul, E.; Prearo, M. Consequences of fish introduction in fishless Alpine lakes: Preliminary notes from a sanitary point of view. TrJFAS 2020, 20, 1–8. [Google Scholar]
- Mosetti, F. Sintesi sull’idrologia del Friuli-Venezia Giulia. Quad. ETP Riv. Di Limnol. 1983, 6, 1–295. [Google Scholar]
- Topic Popovic, N.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Persin Berakovic, A.; Sauerborn Klobucar, R. Tricaine methane-sulfonate (MS-222) application in fish anesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef]
- Zerunian, S. Pesci Delle Acque Interne D’italia. Quaderni di Conservazione Della Natura n° 20; Ministero dell’Ambiente: Roma, Italy, 2004; pp. 1–265. [Google Scholar]
- Dorts, J.; Grenouillet, G.; Douxfils, J.; Mandiki, S.N.M.; Milla, S.; Silvestre, F.; Kestemont, P. Evidence that elevated water temperature affects the reproductive physiology of the European bullhead Cottus gobio. Fish. Physiol. Biochem. 2011, 38, 389–399. [Google Scholar] [CrossRef]
- Bertoli, M.; Pizzul, E.; Devescovi, V.; Franz, F.; Pastorino, P.; Giulianini, P.G.; Ferrari, C.; Nonnis Marzano, F. Biology and distribution of Danube barbel (Barbus balcanicus) (Osteichthyes: Cyprinidae) at the Northwestern limit of its range. Eur. ZoolJ. 2019, 86, 280–293. [Google Scholar] [CrossRef]
- Caballero, M.J.; Izquierdo, M.S.; Kjørsvik, E.; Fernandez, A.J.; Rosenlund, G. Histological alterations in the liver of sea bream, Sparus aurata L., caused by short-or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. J. Fish. Dis. 2004, 27, 531–541. [Google Scholar] [CrossRef]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Ermak, T.H.; Eakin, R.M. Fine structure of the cerebral and pygidial ocelli in Chone ecaudata (Polychaeta: Sabellidae). J. Ultrastruct. Res. 1976, 54, 243–260. [Google Scholar] [CrossRef]
- Conover, W.J.; Iman, R.L. On multiple-comparisons procedures. Los Alamos Sci. Lab. Tech. Rep. 1979, 1, 14. [Google Scholar]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; Wiley & Sons, Inc.: New York, NY, USA, 1999; pp. 1–608. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.L.; Perrow, M.R. Ecology of the Bullhead Cottus Gobio; Conserving Natura 2000 Rivers, Ecology Series No. 4; Natural England: Peterborough, UK, 2003; pp. 1–19. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; UICN: Delemont, Switzerland, 2007; pp. 1–646. [Google Scholar]
- Wang, L.N.; Liu, W.B.; Lu, K.L.; Xu, W.N.; Cai, D.S.; Zhang, C.N.; Qian, Y. Effects of dietary carbohydrate/lipid ratios on non-specific immune responses, oxidative status and liver histology of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2014, 426, 41–48. [Google Scholar] [CrossRef]
- Gandolfi, G.; Zerunian, S.; Torricelli, P.; Marconato, A. I Pesci Delle Acque Interne Italiane; Istituto Poligrafico e Zecca dello Stato: Roma, Italy, 1991; pp. 1–616. [Google Scholar]
- Brasfield, S.M.; Tetreault, G.R.; McMaster, M.E.; Bennett, J.; Munkittrick, K.R. Seasonal patterns of gonad size, liver size, and in vitro gonadal steroidogenic capacity in slimy sculpin (Cottus cognatus). Water Qual. Res. J. Can. 2013, 48, 243–254. [Google Scholar] [CrossRef]
- Qiao, Q.; Le Manach, S.; Sotton, B.; Huet, H.; Duvernois-Berthet, E.; Paris, A.; Duval, C.; Ponger, L.; Marie, A.; Blond, A.; et al. Deep sexual dimorphism in adult medaka fish liver highlighted by multi-omic approach. Sci. Rep. 2016, 6, 32459. [Google Scholar] [CrossRef] [PubMed]
- Tagarao, S.M.; Solania, C.L.; Jumawan, J.C.; Masangcay, S.G.; Calagui, L.B. Length-Weight Relationship (LWR), Gonadosomatic Index (GSI) and fecundity of Johnius borneensis (Bleeker, 1850) from Lower Agusan River basin, Butuan City, Philippines. J. Aquac. Res. Dev. 2020, 11, 598. [Google Scholar]
- Pastorino, P.; Pizzul, E.; Bertoli, M.; Perilli, S.; Brizio, P.; Salvi, G.; Esposito, G.; Abete, M.C.; Prearo, M.; Squadrone, S. Macrobenthic invertebrates as bioindicators of trace elements in high-mountain lakes. Environ. Sci. Pollut. Res. 2019, 27, 5958–5970. [Google Scholar] [CrossRef]
- Brown, R.S.; Hubert, W.A.; Daly, S.F. A primer on winter, ice, and fish: What fisheries biologists should know about winter ice processes and stream-dwelling fish. Fisheries 2011, 36, 8–26. [Google Scholar] [CrossRef]
- Thompson, J.M.; Bergersen, E.P.; Carlson, C.A.; Kaeding, L.R. Role of size, condition, and lipid content in the overwinter survival of age-0 Colorado Squawfish. Trans. Am. Fish. Soc. 1991, 120, 346–353. [Google Scholar] [CrossRef]
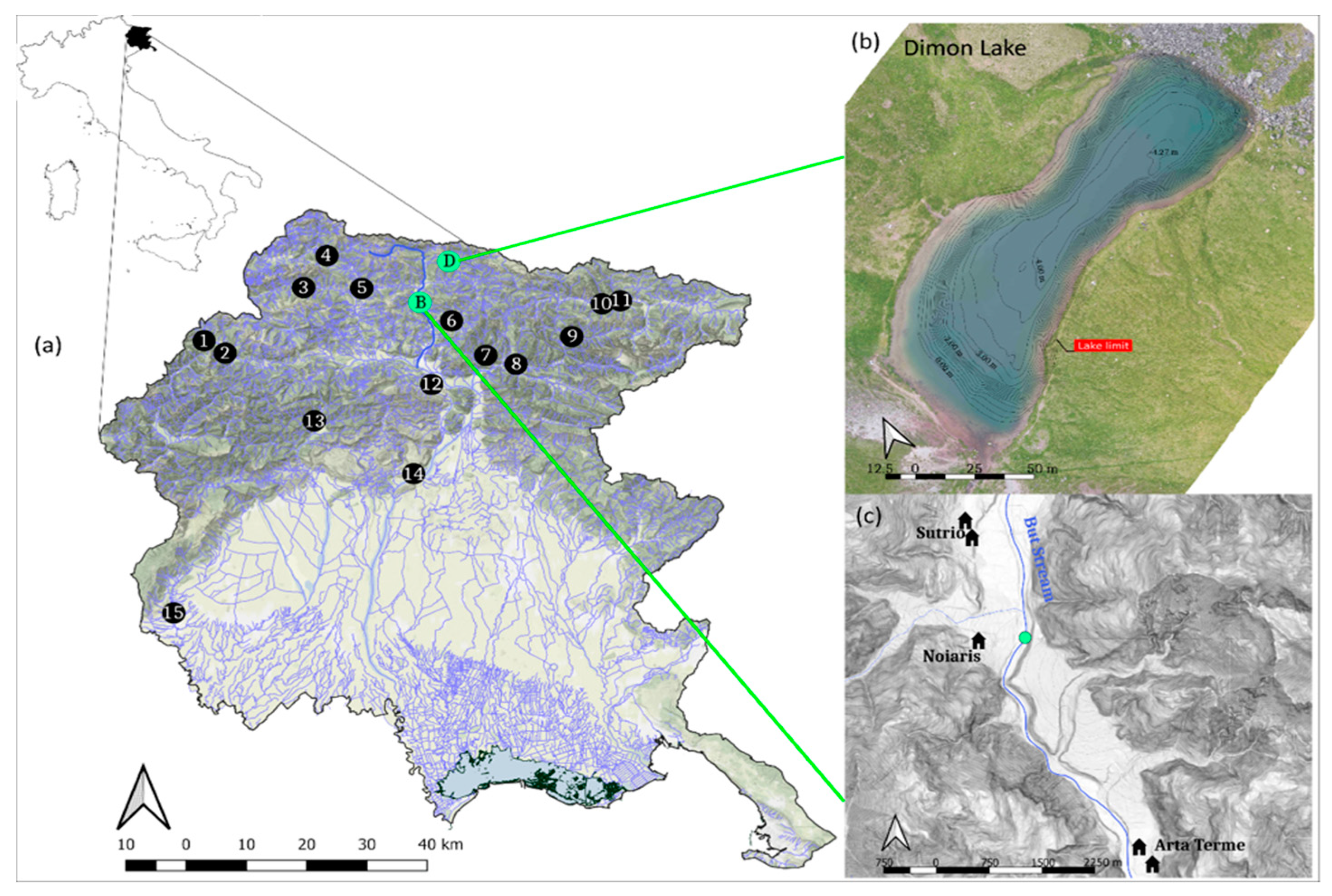
| Map Code | Watercourse | Latitude | Longitude | Elevation (m a.s.l.) | Liver Alteration Score | |
|---|---|---|---|---|---|---|
| Min | Max | |||||
| 1 | Giaf Creek | 46°25’38″ N | 12°32’19″ E | 1050 | 2 | 2 |
| 2 | Tagliamento River | 46°24’29″ N | 12°35’07″ E | 870 | 0 | 1 |
| 3 | Pesarina Stream | 46°31’09″ N | 12°44’59″ E | 820 | 0 | 1 |
| 4 | Alpo Creek | 46°34’20″ N | 12°47’53″ E | 815 | 0 | 1 |
| 5 | Margò Stream | 46°31’12″ N | 12°52’32″ E | 555 | 0 | 0 |
| 6 | Ambruseit Creek | 46°28’12″ N | 13°04’17″ E | 458 | 2 | 3 |
| 7 | Glagnò Stream | 46°24’54″ N | 13°08’50″ E | 334 | 0 | 1 |
| 8 | Alba Creek | 46°24’05″ N | 13°12’46″ E | 316 | 0 | 1 |
| 9 | Dogna Stream | 46°26’55″ N | 13°19’54″ E | 453 | 1 | 3 |
| 10 | Bianco Creek | 46°30’11″ N | 13°23’45″ E | 767 | 0 | 3 |
| 11 | Malborghetto Creek | 46°30’31″ N | 13°26’06″ E | 713 | 0 | 2 |
| 12 | Faeit Stream | 46°21’55″ N | 13°01’56″ E | 290 | 0 | 1 |
| 13 | Meduna Stream | 46°18’03″ N | 12°46’54″ E | 365 | 1 | 3 |
| 14 | Molino Millrace | 46°13’04″ N | 12°59’56″ E | 170 | 1 | 3 |
| 15 | Bodegan Creek | 45°58’45″ N | 12°39’37″ E | 20 | 0 | 2 |
| But Stream | ||||||||||||
| Female | Male | |||||||||||
| Total Length (cm) | Total Weight (g) | K Value | Total Length (cm) | Total Weight (g) | K Value | |||||||
| March | 11.57 | ±2.24 | 18.14 | ±8.75 | 1.19 a | ±0.18 | 13.83 | ±0.71 | 31.42 | ±4.48 | 1.18 a | ±0.02 |
| April | 10.87 | ±2.53 | 19.12 | ±4.48 | 1.39 a | ±0.13 | 11.30 | ±1.65 | 22.07 | ±4.69 | 1.44 a | ±0.08 |
| May | 12.40 | ±1.49 | 25.98 | ±9.70 | 1.31 a | ±0.08 | 10.97 | ±0.99 | 18.15 | ±4.70 | 1.36 a | ±0.02 |
| June | 8.03 | ±1.14 | 6.34 | ±0.97 | 1.23 a | ±0.17 | 9.82 | ±1.14 | 12.40 | ±3.22 | 1.31 a | ±0.20 |
| July | 8.87 | ±3.62 | 9.66 | ±3.62 | 1.34 a | ±0.10 | 9.24 | ±1.27 | 11.69 | ±4.33 | 1.42 a | ±0.11 |
| August | 11.40 | ±2.91 | 17.91 | ±9.95 | 1.15 a | ±0.19 | 16.00 | ±1.20 | 16.00 | ±4.54 | 1.30 a | ±0.16 |
| September | 9.23 | ±2.89 | 9.93 | ±2.89 | 1.22 a | ±0.06 | 11.15 | ±0.98 | 19.04 | ±4.60 | 1.35 a | ±0.09 |
| October | 10.05 | ±2.03 | 13.60 | ±6.86 | 1.22 a | ±0.06 | 11.35 | ±2.03 | 20.77 | ±8.32 | 1.31 a | ±0.11 |
| Dimon Lake | ||||||||||||
| Female | Male | |||||||||||
| Total Length (cm) | Total Weight (g) | K Value | Total Length (cm) | Total Weight (g) | K Value | |||||||
| June | 11.61 | ±2.14 | 20.68 | ±9.91 | 1.23 a | ±0.14 | 12.36 | ±1.47 | 26.44 | ±8.07 | 1.32 a | ±0.12 |
| July | 11.08 | ±1.66 | 14.69 | ±6.31 | 1.07 ab | ±0.09 | 12.37 | ±1.65 | 21.05 | ±7.74 | 1.08 ab | ±0.11 |
| August | 10.05 | ±1.41 | 13.55 | ±10.41 | 1.04 b | ±0.16 | 7.25 | ±0.35 | 3.91 | ±1.05 | 1.01 b | ±0.13 |
| September | 10.75 | ±2.59 | 15.43 | ±9.50 | 1.06 ab | ±0.18 | 9.20 | ±3.25 | 8.47 | ±7.71 | 0.99 b | ±0.05 |
| October | 9.82 | ±2.12 | 10.68 | ±6.39 | 0.98 b | ±0.11 | 9.30 | ±0.91 | 7.99 | ±2.00 | 0.97 b | ±0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorino, P.; Bertoli, M.; Kušće, M.; Giulianini, P.G.; Menconi, V.; Prearo, M.; Pizzul, E. Liver Lipid Accumulation in European Bullhead (Cottus cobio) from a High-Mountain Lake: An Adaptive Strategy to Survive the Adverse Winter Season. Diversity 2020, 12, 442. https://doi.org/10.3390/d12120442
Pastorino P, Bertoli M, Kušće M, Giulianini PG, Menconi V, Prearo M, Pizzul E. Liver Lipid Accumulation in European Bullhead (Cottus cobio) from a High-Mountain Lake: An Adaptive Strategy to Survive the Adverse Winter Season. Diversity. 2020; 12(12):442. https://doi.org/10.3390/d12120442
Chicago/Turabian StylePastorino, Paolo, Marco Bertoli, Manuel Kušće, Piero Giulio Giulianini, Vasco Menconi, Marino Prearo, and Elisabetta Pizzul. 2020. "Liver Lipid Accumulation in European Bullhead (Cottus cobio) from a High-Mountain Lake: An Adaptive Strategy to Survive the Adverse Winter Season" Diversity 12, no. 12: 442. https://doi.org/10.3390/d12120442
APA StylePastorino, P., Bertoli, M., Kušće, M., Giulianini, P. G., Menconi, V., Prearo, M., & Pizzul, E. (2020). Liver Lipid Accumulation in European Bullhead (Cottus cobio) from a High-Mountain Lake: An Adaptive Strategy to Survive the Adverse Winter Season. Diversity, 12(12), 442. https://doi.org/10.3390/d12120442