Diversity of Olfactory Responses and Skills in Astyanax Mexicanus Cavefish Populations Inhabiting different Caves
Abstract
1. Introduction
2. Materials and Methods
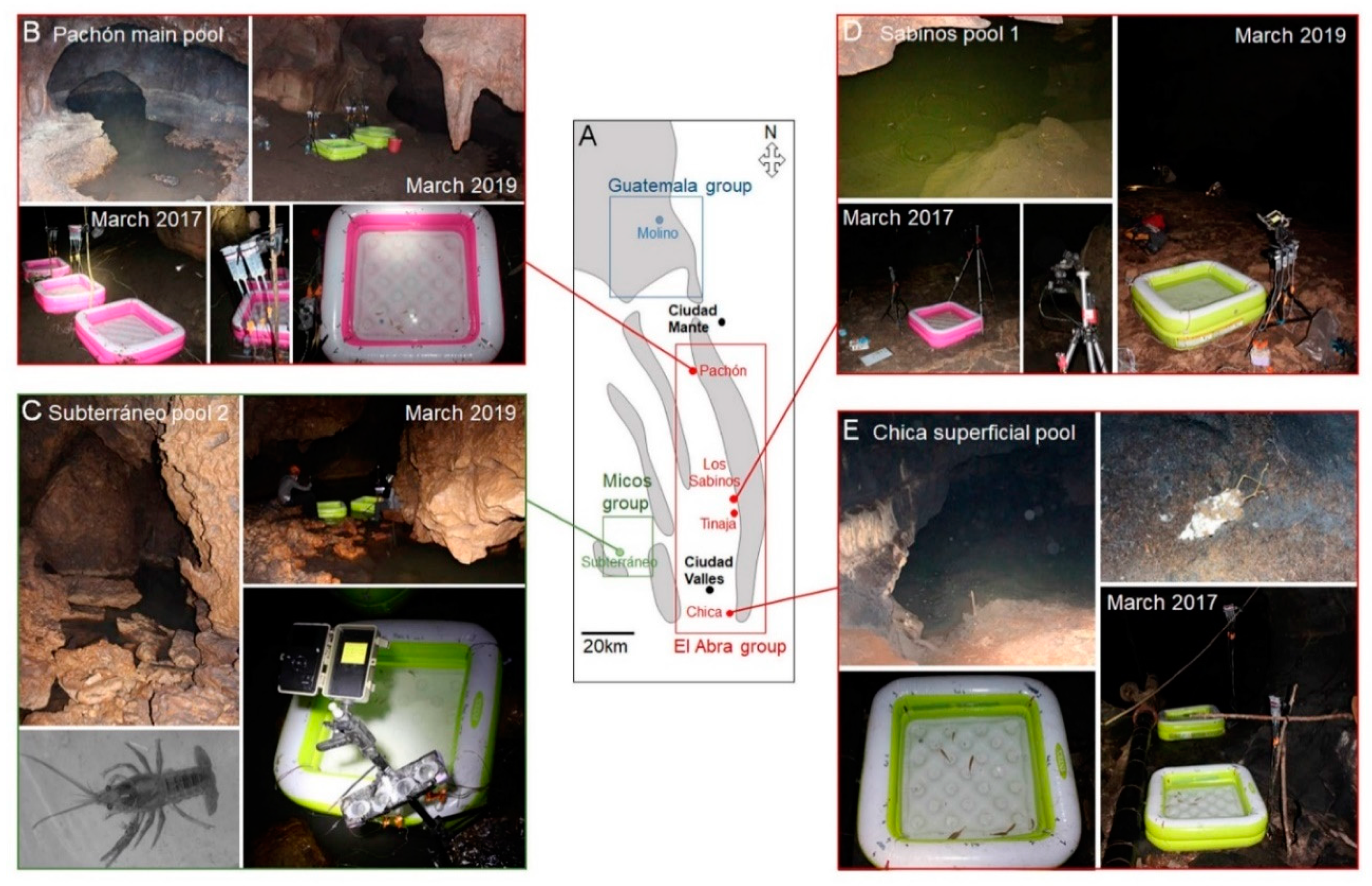
2.1. Field Experimentation in Five Different Caves: Constraints and Criteria for Choice
2.2. Cavefishes in the Wild
2.3. Fishes from the Lab Facility
2.4. Sampling and Photography
2.5. Odor Choice
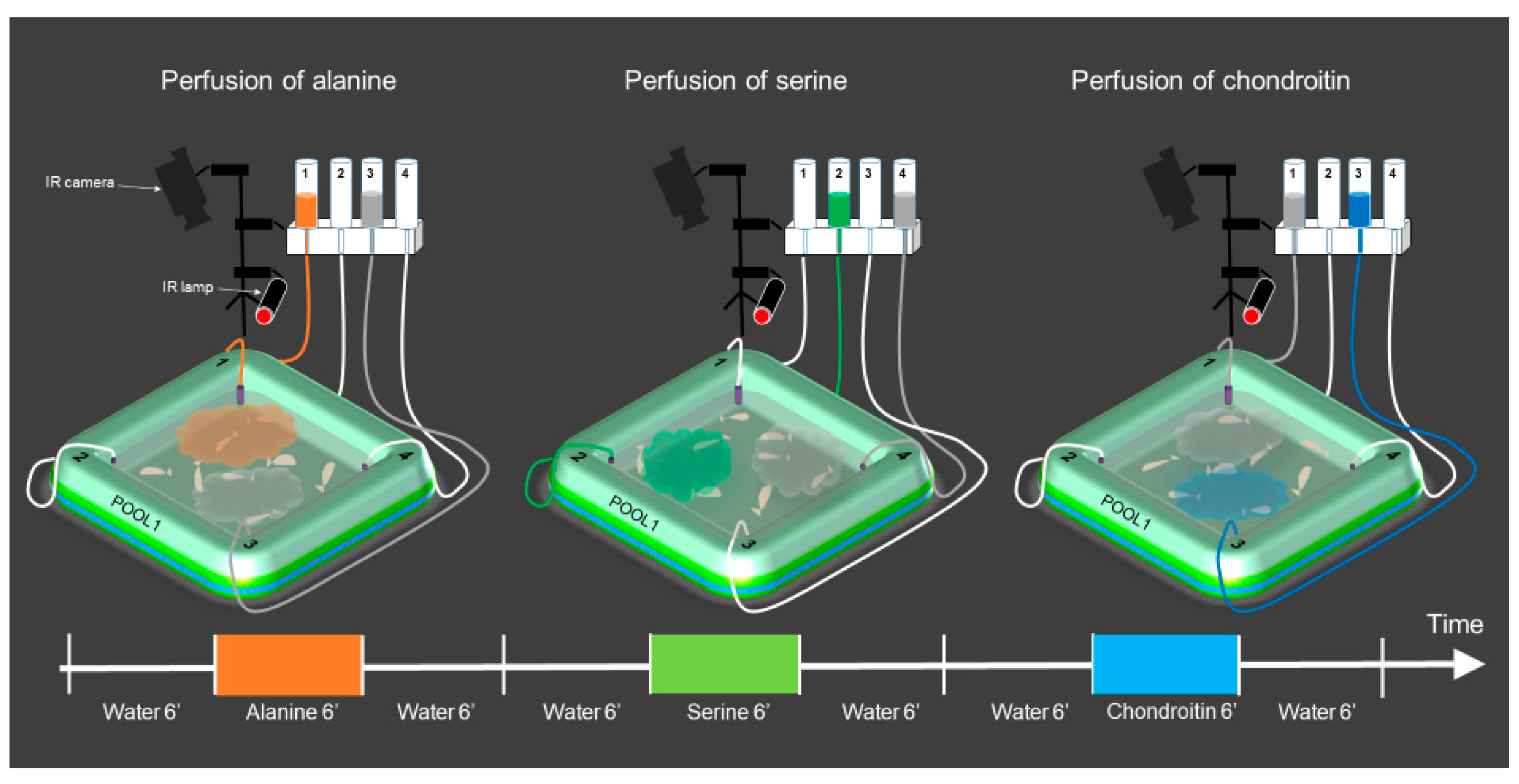
2.6. Setup Design
2.7. Procedure
2.8. Video Scoring
2.9. Data Analyses and Statistics
3. Results
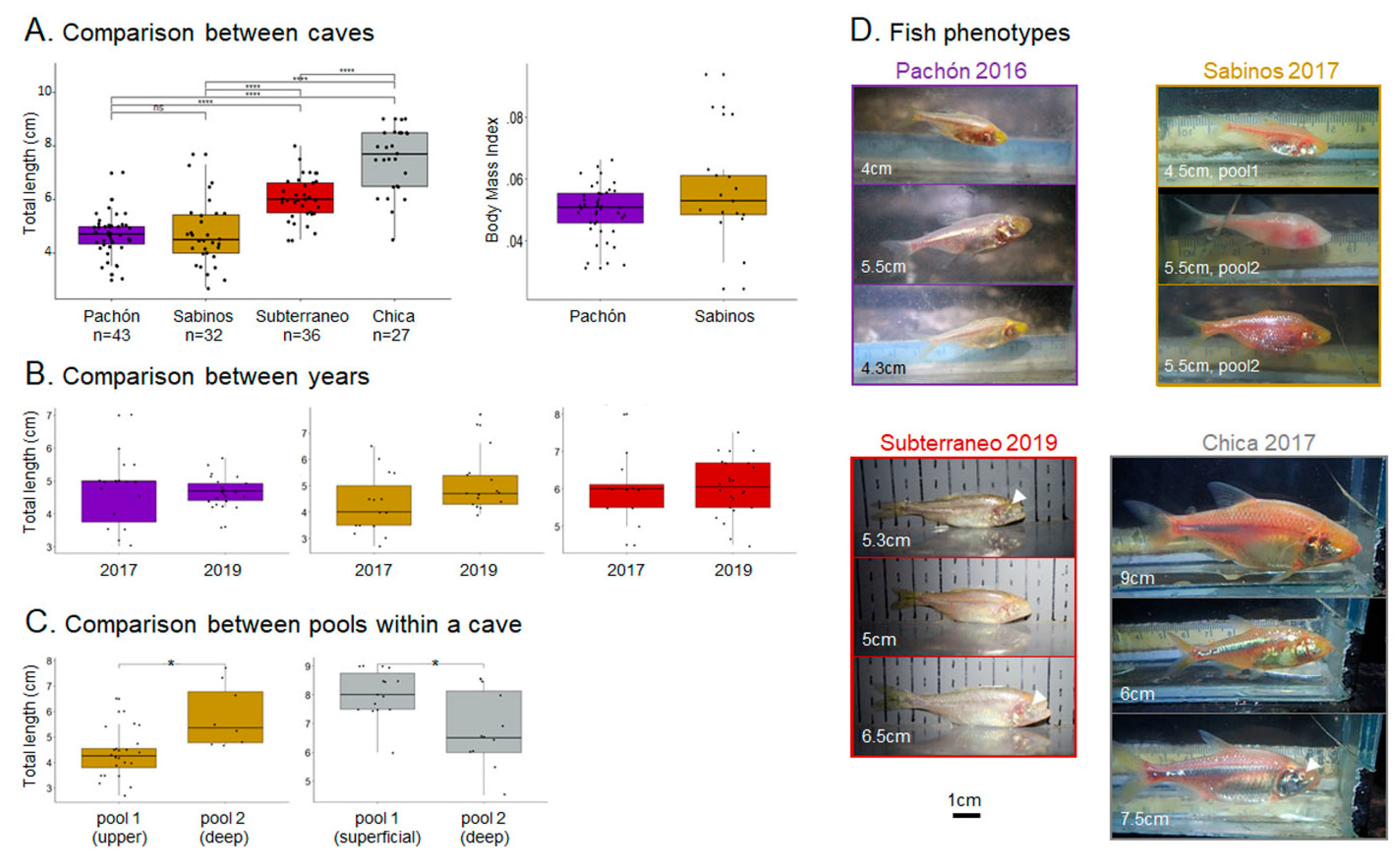
3.1. Methodological Considerations: Testing Olfactory Responses in the Lab versus in the Wild
3.2. Responses to Odors in Laboratory-Raised A. mexicanus
3.3. Responses to Odors in Caves, in Wild A. mexicanus Cavefish Populations
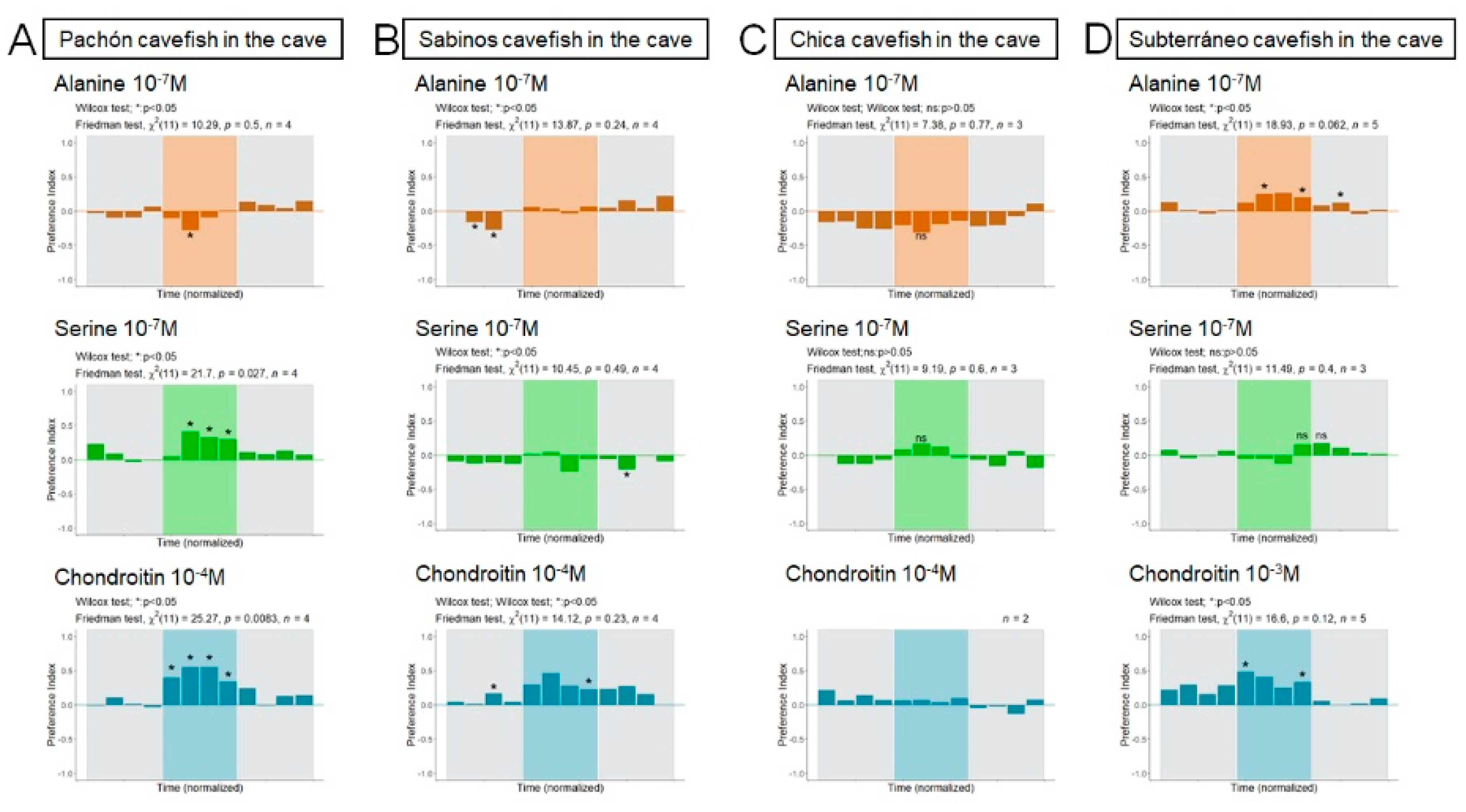
3.3.1. Pachón Cave
3.3.2. Sabinos Cave
3.3.3. Subterráneo Cave
3.3.4. Chica Cave
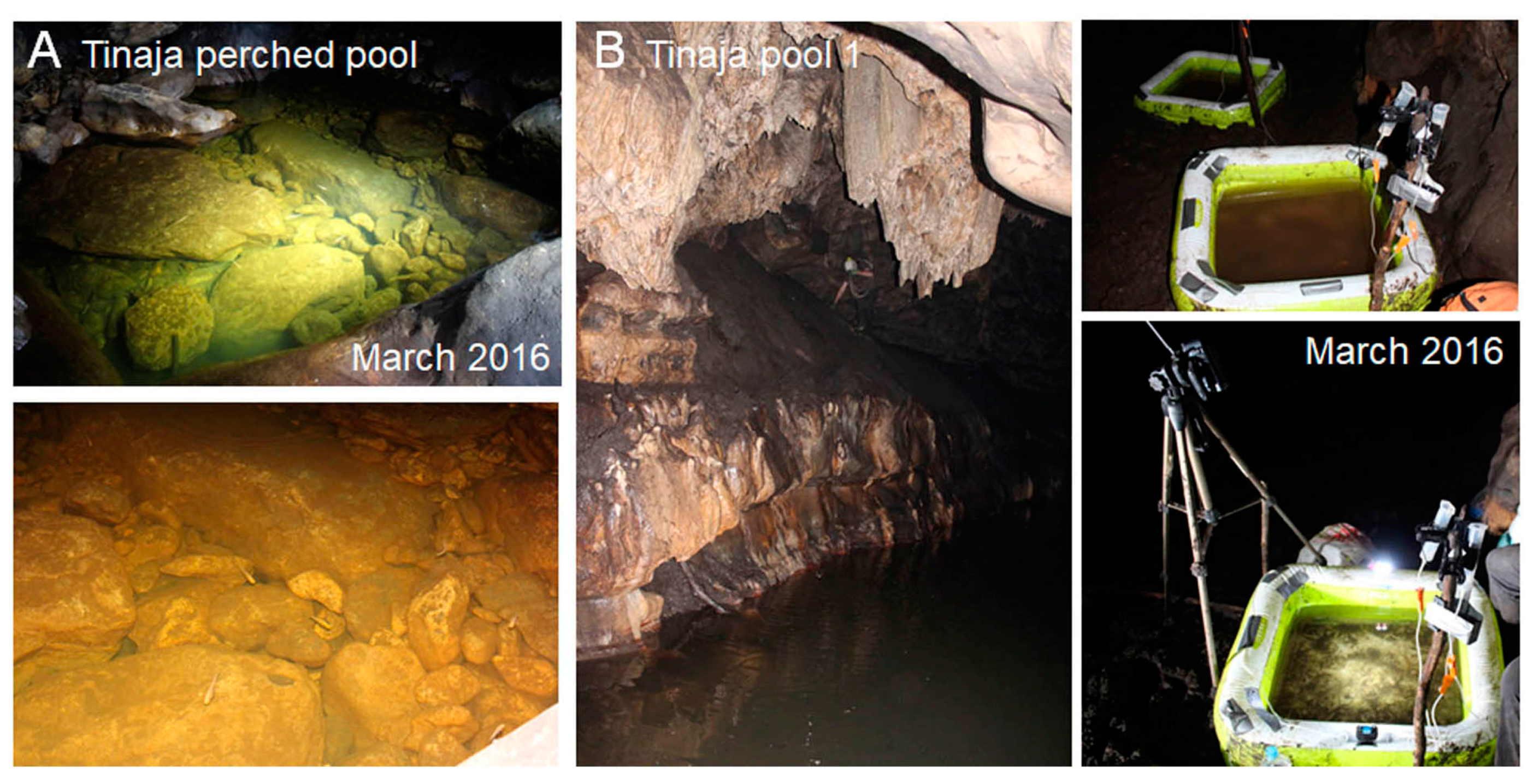
3.3.5. Tinaja Cave
4. Discussion
4.1. Testing Cavefish Olfaction in the Field: A Challenge
4.2. Alanine, Serine and Chondroitin Elicit Variable Behavioral Responses in Wild and Lab-Raised Adult A. mexicanus
4.3. Not All Cavefish Respond the Same
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats, 2nd ed.; Biology of Habitats Series; Oxford University Press: Oxford, UK, 2019; ISBN 978-0-19-882077-2. [Google Scholar]
- Rétaux, S.; Casane, D. Evolution of eye development in the darkness of caves: Adaptation, drift, or both? EvoDevo 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Romero, A. Cave Biology: Life in Darkness. Integr. Comp. Biol. 2010, 50, 689–691. [Google Scholar] [CrossRef][Green Version]
- Simon, V.; Hyacinthe, C.; Rétaux, S. Breeding behavior in the blind Mexican cavefish and its river-dwelling conspecific. PLoS ONE 2019, 14, e0212591. [Google Scholar] [CrossRef]
- Mitchell, R.; Russell, W.; Elliott, W. Mexican eyeless characin fishes, genus Astyanax: Environment, distribution, and evolution. Spec. Publ. Mus. Texas Tech. Univ. 1977, 12, 1–89. [Google Scholar]
- Elliott, W.R. Cave Biodiversity and Ecology of the Sierra de El Abra Region. In Biology and Evolution of the Mexican Cavefish; Elsevier: Amsterdam, The Netherlands, 2016; pp. 59–76. ISBN 978-0-12-802148-4. [Google Scholar]
- Casane, D.; Rétaux, S. Evolutionary Genetics of the Cavefish Astyanax mexicanus. Adv. Genet. 2016, 95, 117–159. [Google Scholar] [CrossRef]
- Jeffery, W.R. Chapter 8 Evolution and Development in the Cavefish Astyanax. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 86, pp. 191–221. ISBN 978-0-12-374455-5. [Google Scholar]
- Keene, A.; Yoshizawa, M.; Mcgaugh, S.E. Biology and Evolution of the Mexican Cavefish; Elsevier Science: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-802148-4. [Google Scholar]
- Espinasa, L.; Legendre, L.; Fumey, J.; Blin, M.; Rétaux, S.; Espinasa, M. A new cave locality for Astyanax cavefish in Sierra de El Abra, Mexico. Subterr. Biol. 2018, 26, 39–53. [Google Scholar] [CrossRef]
- Elliott, W.R. The Astyanax Caves of Mexico Cavefishes of Tamaulipas, San Luis Potosí, and Guerrero; Association for Mexican Cave Studies: Austin, TX, USA, 2018. [Google Scholar]
- Şadoğlu, P. A mendelian gene for albinism in natural cavefish. Experientia 1957, 13, 394. [Google Scholar] [CrossRef]
- Borowsky, R. Restoring sight in blind cavefish. Curr. Biol. CB 2008, 18, R23–R24. [Google Scholar] [CrossRef]
- Bibliowicz, J.; Alié, A.; Espinasa, L.; Yoshizawa, M.; Blin, M.; Hinaux, H.; Legendre, L.; Père, S.; Rétaux, S. Differences in chemosensory response between eyed and eyeless Astyanax mexicanus of the Rio Subterráneo cave. EvoDevo 2013, 4, 25. [Google Scholar] [CrossRef]
- Fumey, J.; Hinaux, H.; Noirot, C.; Thermes, C.; Rétaux, S.; Casane, D. Evidence for late Pleistocene origin of Astyanax mexicanus cavefish. BMC Evol. Biol. 2018, 18, 43. [Google Scholar] [CrossRef]
- Policarpo, M.; Fumey, J.; Lafargeas, P.; Naquin, D.; Thermes, C.; Naville, M.; Dechaud, C.; Volff, J.-N.; Cabau, C.; Klopp, C.; et al. Contrasting gene decay in subterranean vertebrates: Insights from cavefishes and fossorial mammals. Mol. Biol. Evol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Byerly, M.S.; Jackman, W.R.; Jeffery, W.R. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev. Biol. 2009, 330, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Gorički, Š.; Soares, D.; Jeffery, W.R. Evolution of a Behavioral Shift Mediated by Superficial Neuromasts Helps Cavefish Find Food in Darkness. Curr. Biol. 2010, 20, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Blin, M.; Tine, E.; Meister, L.; Elipot, Y.; Bibliowicz, J.; Espinasa, L.; Rétaux, S. Developmental evolution and developmental plasticity of the olfactory epithelium and olfactory skills in Mexican cavefish. Dev. Biol. 2018, 441, 242–251. [Google Scholar] [CrossRef]
- Hinaux, H.; Devos, L.; Blin, M.; Elipot, Y.; Bibliowicz, J.; Alié, A.; Rétaux, S. Sensory evolution in blind cavefish is driven by early embryonic events during gastrulation and neurulation. Development 2016, 143, 4521–4532. [Google Scholar] [CrossRef]
- Torres-Paz, J.; Hyacinthe, C.; Pierre, C.; Rétaux, S. Towards an integrated approach to understand Mexican cavefish evolution. Biol. Lett. 2018, 14. [Google Scholar] [CrossRef]
- Parzefall, J. Field observation in epigean and cave populations of the Mexican characid Astyanax mexicanus (Pisces, Characidae). Mémoires de Biospéologie 1983, 10, 171–176. [Google Scholar]
- Espinasa, L.; Bonaroti, N.; Wong, J.; Pottin, K.; Queinnec, E.; Rétaux, S. Contrasting feeding habits of post-larval and adult Astyanax cavefish. Subterr. Biol. 2017, 21, 1–17. [Google Scholar] [CrossRef]
- Simon, V.; Elleboode, R.; Mahé, K.; Legendre, L.; Ornelas-Garcia, P.; Espinasa, L.; Rétaux, S. Comparing growth in surface and cave morphs of the species Astyanax mexicanus: Insights from scales. EvoDevo 2017, 8. [Google Scholar] [CrossRef]
- Pierre, C.; Pradère, N.; Froc, C.; Ornelas-García, P.; Callebert, J.; Rétaux, S. A mutation in monoamine oxidase (MAO) affects the evolution of stress behavior in the blind cavefish Astyanax mexicanus. J. Exp. Biol. 2020. [Google Scholar] [CrossRef]
- Strecker, U.; Bernatchez, L.; Wilkens, H. Genetic divergence between cave and surface populations of Astyanax in Mexico (Characidae, Teleostei). Mol. Ecol. 2003, 12, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Mathuru, A.S.; Kibat, C.; Cheong, W.F.; Shui, G.; Wenk, M.R.; Friedrich, R.W.; Jesuthasan, S. Chondroitin fragments are odorants that trigger fear behavior in fish. Curr. Biol. CB 2012, 22, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, W. [On the heredity of the fear reaction in Astyanax (Characidae, pisces)]. Zeitschrift fur Vererbungslehre 1966, 98, 97–105. [Google Scholar] [PubMed]
- Parzefall, J.; Fricke, D. Alarm reaction and schooling in population hybrids of Astyanax fasciatus (Pisces, Characidae). Memoires e Biospeologie 1991, 18, 29–32. [Google Scholar]
- VideoLan. VLC Media Player. 2006. Available online: https://www.videolan.org/vlc/index.html (accessed on 1 February 2010).
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; de Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 1 February 2018).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA; Springer: New York, NY, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 1 February 2018).
- Alboukadel Kassambara Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.5.0. 2020. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 1 February 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 1 February 2020).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Sour. Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Elipot, Y.; Legendre, L.; Père, S.; Sohm, F.; Rétaux, S. Astyanax transgenesis and husbandry: How cavefish enters the laboratory. Zebrafish 2014, 11, 291–299. [Google Scholar] [CrossRef]
- Hara, T.J. Olfaction and gustation in fish: An overview. Acta Physiol. Scand. 1994, 152, 207–217. [Google Scholar] [CrossRef]
- Hara, T.J. Feeding behaviour in some teleosts is triggered by single amino acids primarily through olfaction. J. Fish. Biol. 2006, 68, 810–825. [Google Scholar] [CrossRef]
- Friedrich, R.W.; Korsching, S.I. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 1997, 18, 737–752. [Google Scholar] [CrossRef]
- Frisch, K. Die Bedeutung des Geruchsinnes im Leben der Fische. Süffert F Ed. Naturwissenschaften 1941, 321–333. [Google Scholar] [CrossRef]
- Døving, K.B.; Lastein, S. The alarm reaction in fishes-odorants, modulations of responses, neural pathways. Ann. N. Y. Acad. Sci. 2009, 1170, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Fricke, D. Reaction to Alarm Substance in Cave Populations of Astyanax fasciatus (Characidae, Pisces). Ethology 1987, 76, 305–308. [Google Scholar] [CrossRef]
- Kowalko, J. Utilizing the blind cavefish Astyanax mexicanus to understand the genetic basis of behavioral evolution. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef]
- Vitebsky, A.; Reyes, R.; Sanderson, M.J.; Michel, W.C.; Whitlock, K.E. Isolation and characterization of the laure olfactory behavioral mutant in the zebrafish, Danio rerio. Dev. Dyn. 2005, 234, 229–242. [Google Scholar] [CrossRef]
- Braubach, O.R.; Wood, H.-D.; Gadbois, S.; Fine, A.; Croll, R.P. Olfactory conditioning in the zebrafish (Danio rerio). Behav. Brain Res. 2009, 198, 190–198. [Google Scholar] [CrossRef]
- Valentinčič, T.; Wegert, S.; Caprio, J. Learned olfactory discrimination versus innate taste responses to amino acids in channel catfish (Ictalurus punctatus). Physiol. Behav. 1994, 55, 865–873. [Google Scholar] [CrossRef]
- Bradic, M.; Beerli, P.; García-de León, F.J.; Esquivel-Bobadilla, S.; Borowsky, R.L. Gene flow and population structure in the Mexican blind cavefish complex (Astyanax mexicanus). BMC Evol. Biol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Avise, J.C.; Selander, R.K. Evolutionary genetics of cave-dwelling fishes of the genus astyanax. Evol. Int. J. Org. Evol. 1972, 26, 1–19. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Jeffery, W.R. Evolutionary tuning of an adaptive behavior requires enhancement of the neuromast sensory system. Commun. Integr. Biol. 2011, 4, 89–91. [Google Scholar] [CrossRef]
- Espinasa, L.; Heintz, C.; Rétaux, S.; Yoshisawa, M.; Agnès, F.; Ornelas-Garcia, P.; Balogh-Robinson, R. Vibration Attraction Response (VAB) is a plastic trait in Blind Mexican tetra (Astyanax mexicanus), variable within subpopulations inhabiting the same cave. J. Fish. Biol. 2020. [Google Scholar] [CrossRef]
- Breder, C.M. Descriptive ecology of La Cueva Chica, with especial reference to the blind fish, Anoptichthys. Zoologica 1942, 27, 7–15. [Google Scholar]
- Hyacinthe, C.; Attia, J.; Rétaux, S. Evolution of acoustic communication in blind cavefish. Nat. Commun. 2019, 10, 4231. [Google Scholar] [CrossRef]
- Peuß, R.; Box, A.C.; Chen, S.; Wang, Y.; Tsuchiya, D.; Persons, J.L.; Kenzior, A.; Maldonado, E.; Krishnan, J.; Scharsack, J.P.; et al. Adaptation to low parasite abundance affects immune investment and immunopathological responses of cavefish. Nat. Ecol. Evol. 2020. [Google Scholar] [CrossRef]


| Fish | Cave Fishes | Surface Fishes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Attraction: (+) No response: (0) | Lab-Pachón | CF-Pachón | CF-SubT | CF-Sabinos | CF-Chica | Lab-SF | Lab-SF | Lab-Hyb | |
| Alanine | + | 0 | + | 0 | 0 | + | 0 | 0 | |
| 10−5 M | 10−7 M | ||||||||
| Serine | + | ++ | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10−5 M | 10−7 M | ||||||||
| Chondroitin | +++ | +++ | ++ | + | 0 | ++ | + | + | |
| 10−3 M | 10−4 M | ||||||||
| Olfactory performance summary | 3/3 | 2/3 | 2/3 | 1/3 | 0/3 | 2/3 | 1/3 | 1/3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blin, M.; Fumey, J.; Lejeune, C.; Policarpo, M.; Leclercq, J.; Père, S.; Torres-Paz, J.; Pierre, C.; Imarazene, B.; Rétaux, S. Diversity of Olfactory Responses and Skills in Astyanax Mexicanus Cavefish Populations Inhabiting different Caves. Diversity 2020, 12, 395. https://doi.org/10.3390/d12100395
Blin M, Fumey J, Lejeune C, Policarpo M, Leclercq J, Père S, Torres-Paz J, Pierre C, Imarazene B, Rétaux S. Diversity of Olfactory Responses and Skills in Astyanax Mexicanus Cavefish Populations Inhabiting different Caves. Diversity. 2020; 12(10):395. https://doi.org/10.3390/d12100395
Chicago/Turabian StyleBlin, Maryline, Julien Fumey, Camille Lejeune, Maxime Policarpo, Julien Leclercq, Stéphane Père, Jorge Torres-Paz, Constance Pierre, Boudjema Imarazene, and Sylvie Rétaux. 2020. "Diversity of Olfactory Responses and Skills in Astyanax Mexicanus Cavefish Populations Inhabiting different Caves" Diversity 12, no. 10: 395. https://doi.org/10.3390/d12100395
APA StyleBlin, M., Fumey, J., Lejeune, C., Policarpo, M., Leclercq, J., Père, S., Torres-Paz, J., Pierre, C., Imarazene, B., & Rétaux, S. (2020). Diversity of Olfactory Responses and Skills in Astyanax Mexicanus Cavefish Populations Inhabiting different Caves. Diversity, 12(10), 395. https://doi.org/10.3390/d12100395






